Study on Microstructure and Properties of Bimodal Structured Ultrafine-Grained Ferrite Steel
Abstract
:1. Introduction
2. Materials and Experimental Methods
3. Results and Discussion
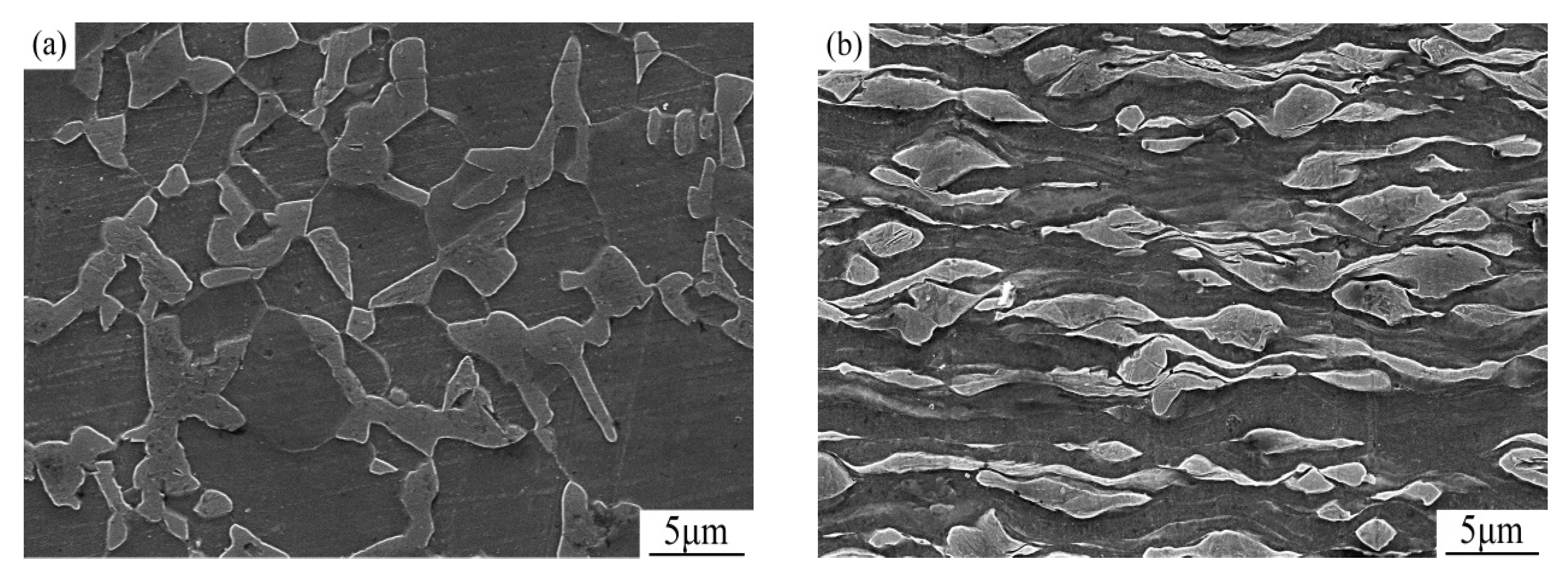
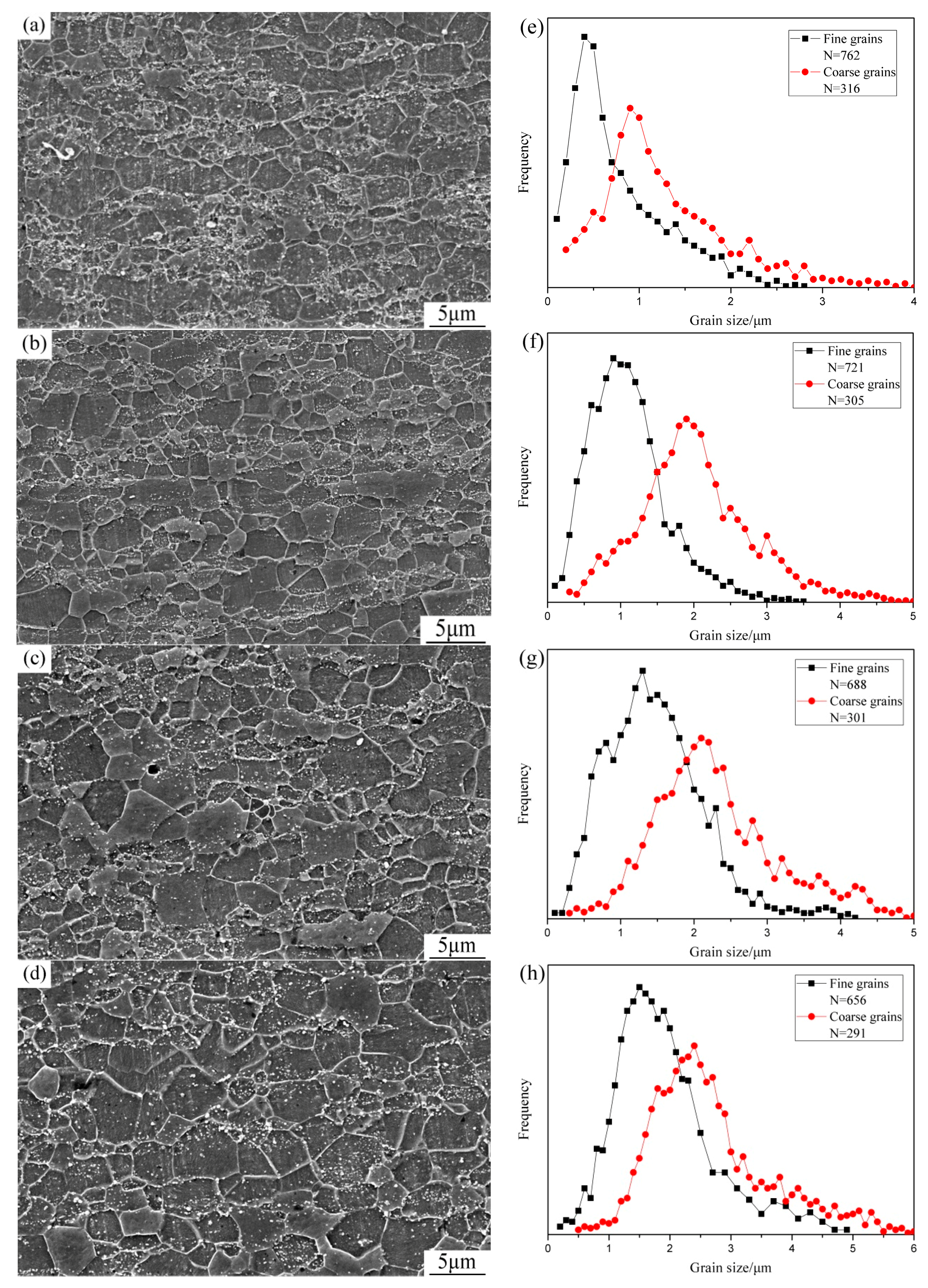
3.1. Effect of Annealing on the Microstructure Evolution of Cold-Rolled Dual-Phase Steel
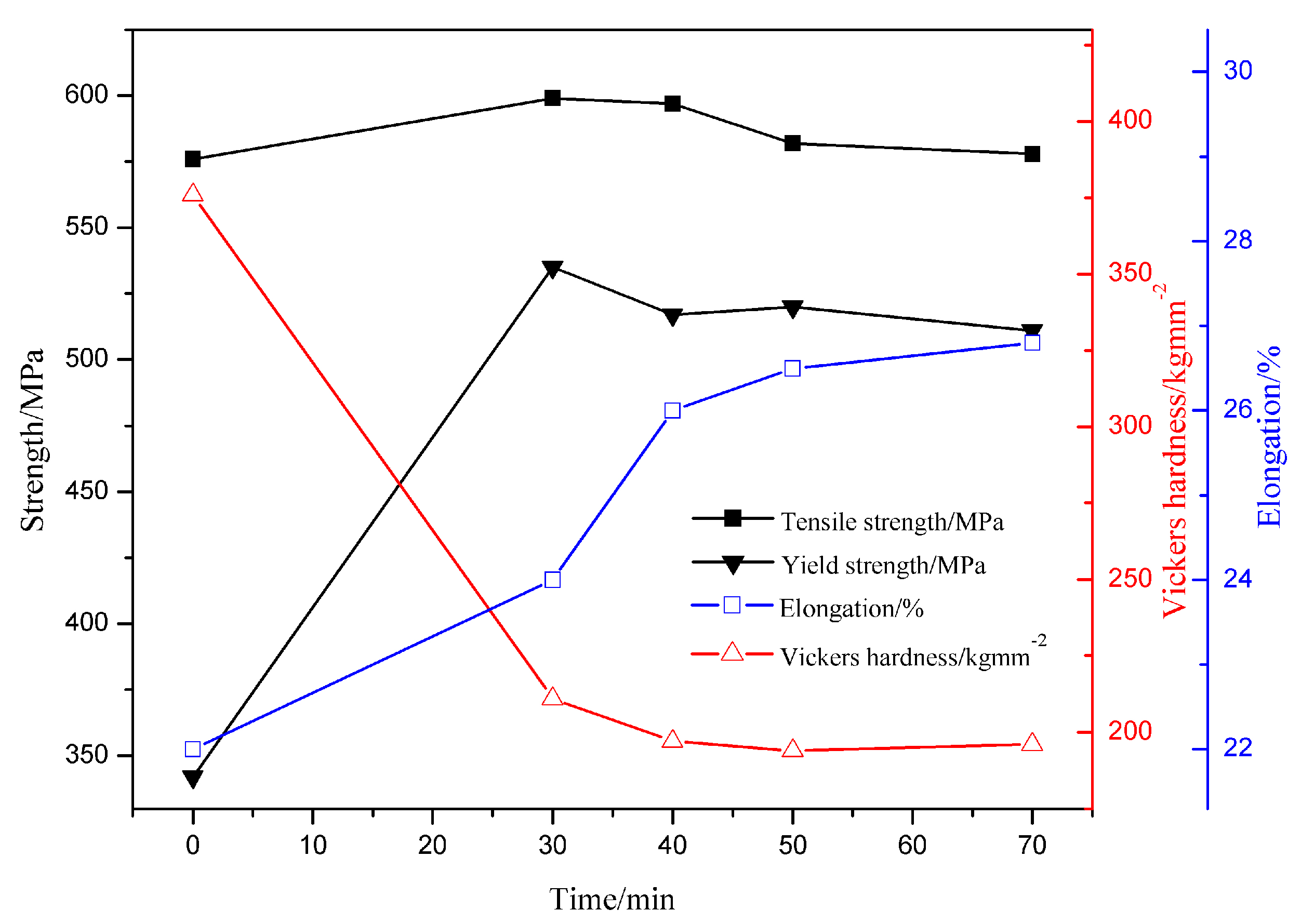
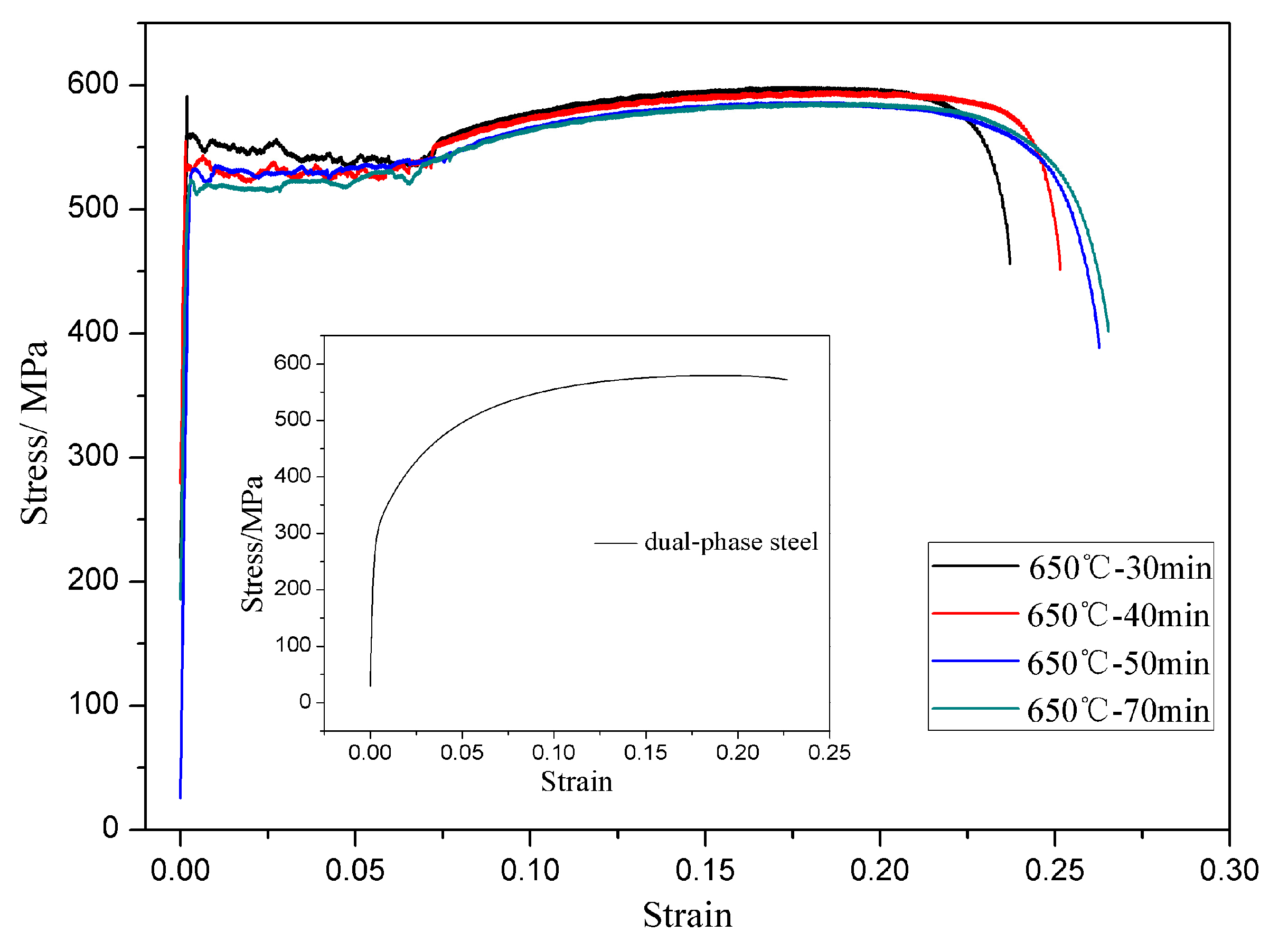
3.2. Mechanical Properties of the Annealed Microstructures
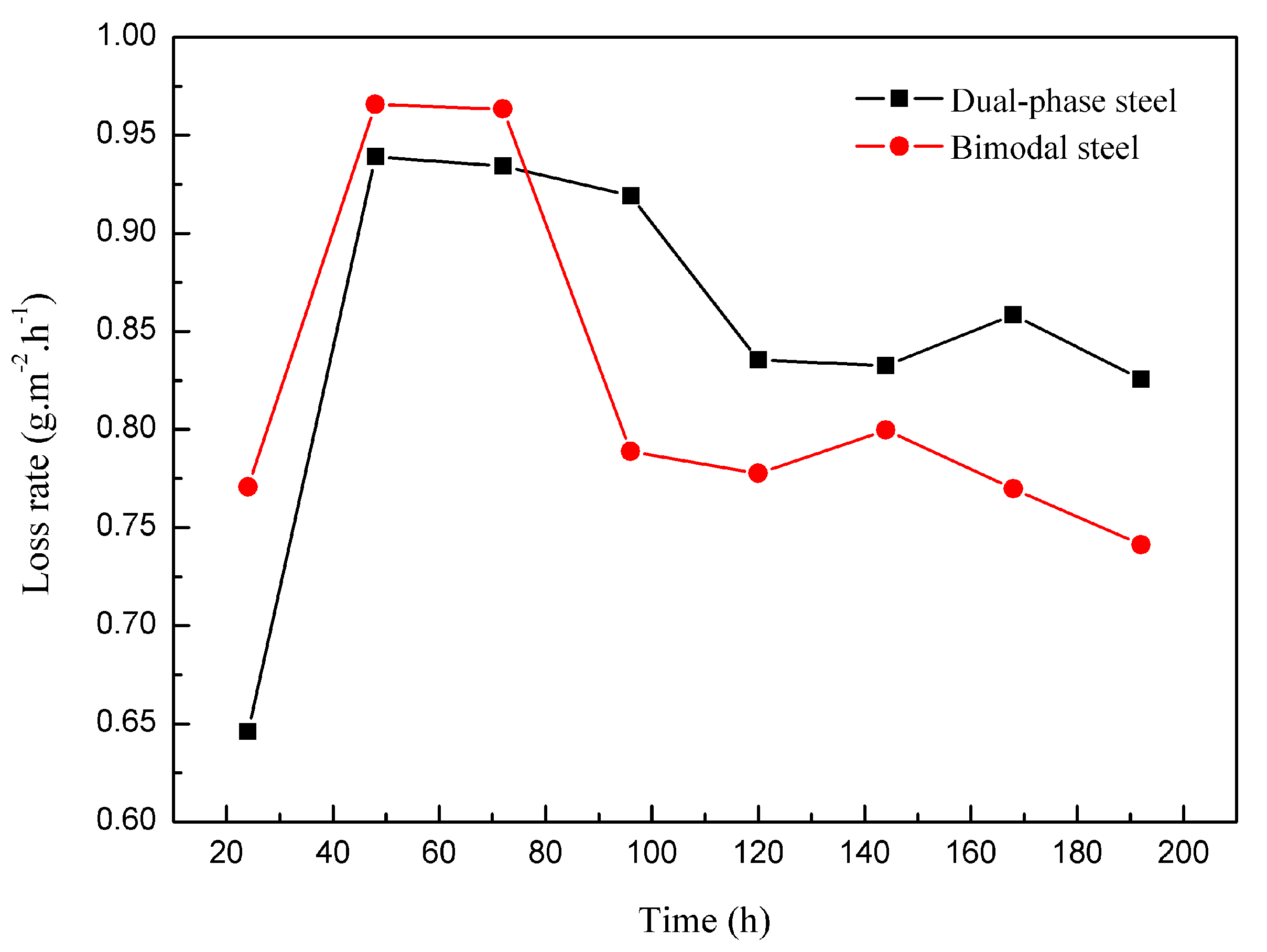
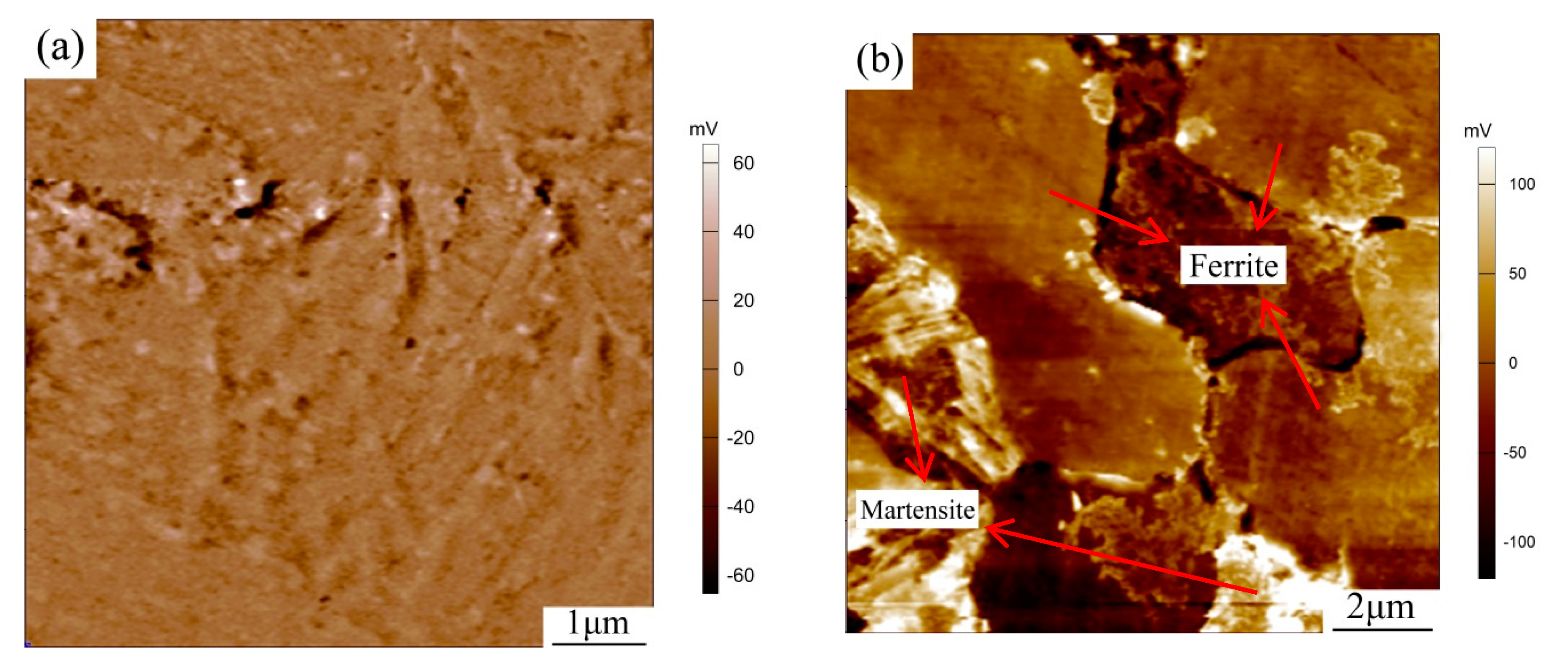
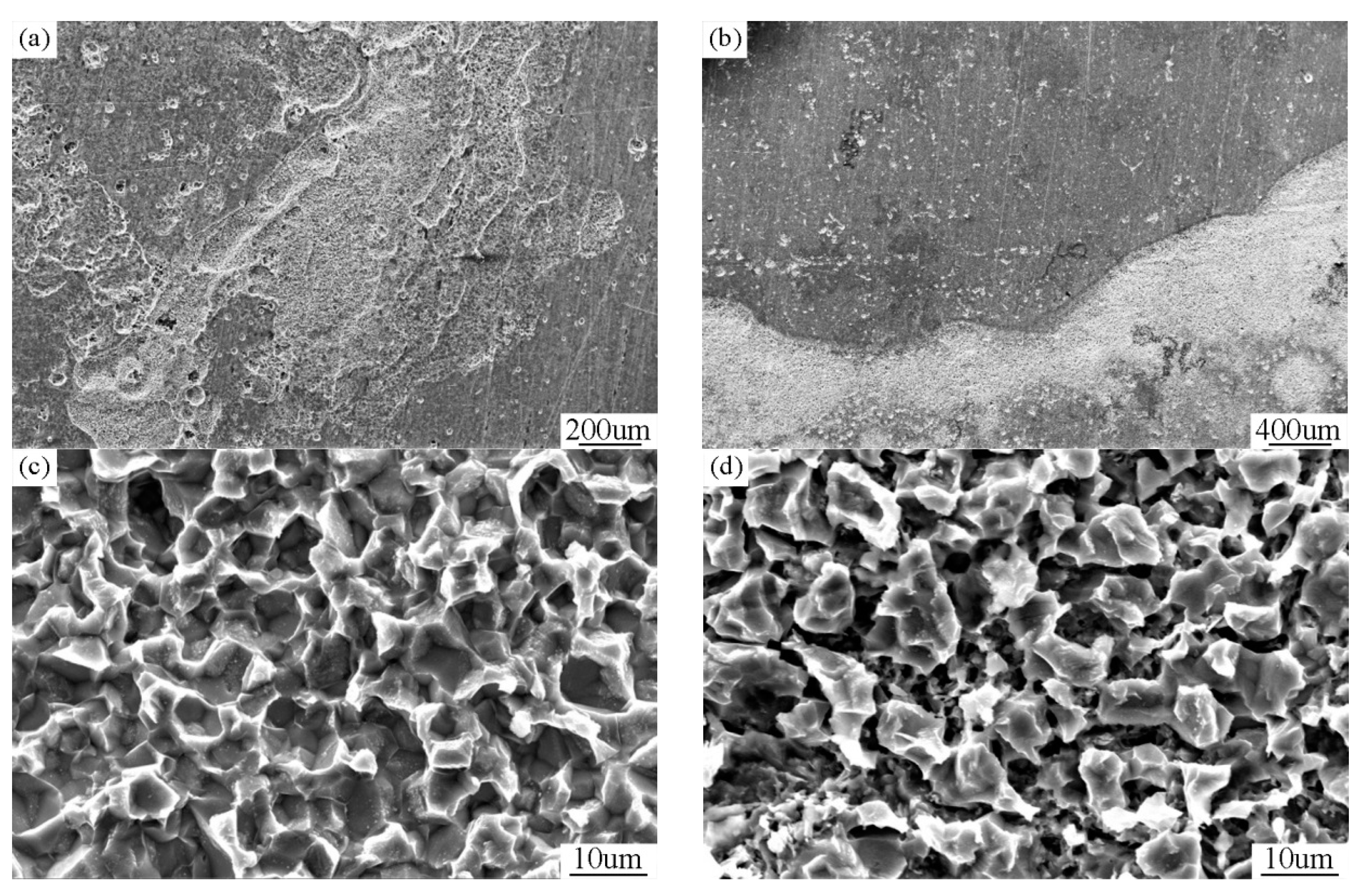
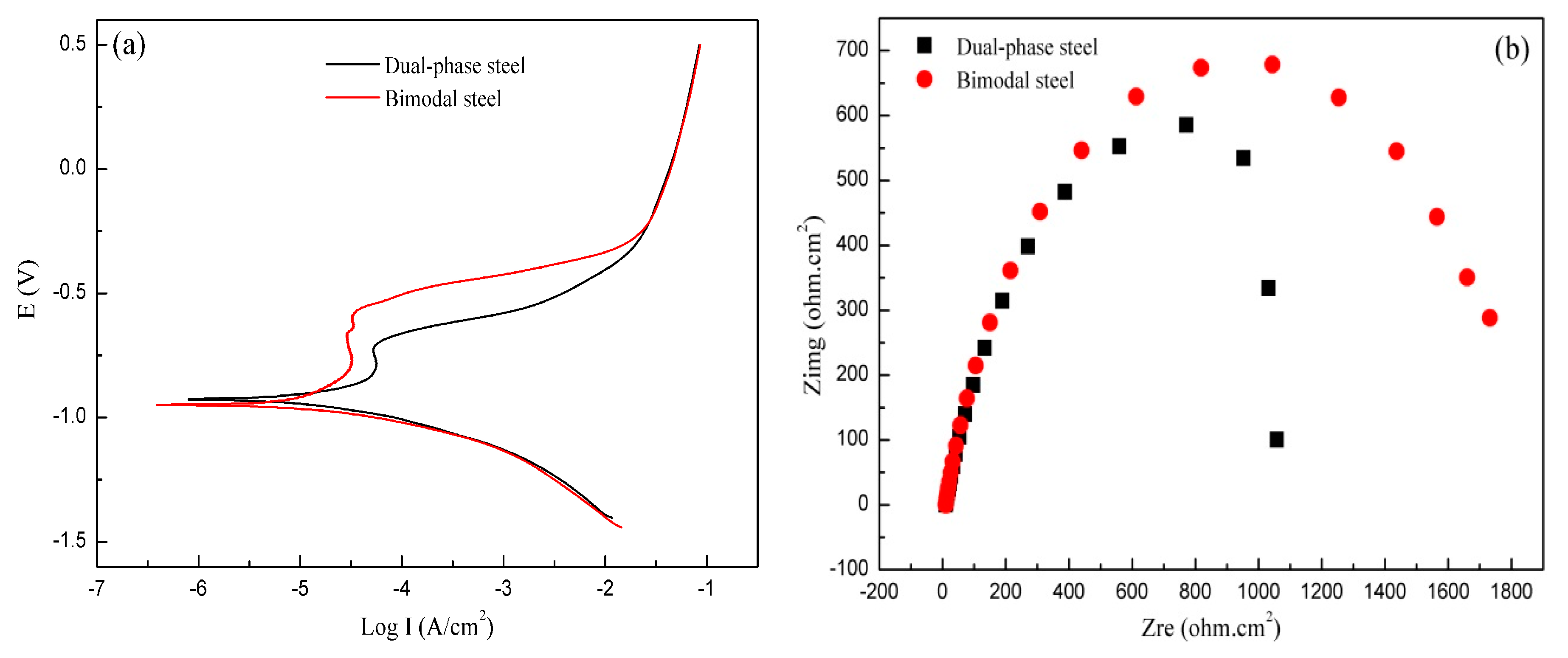
3.3. Corrosion Behavior of Bimodal Ferrite Steel and Dual-Phase Steel
4. Conclusions
- With the annealing time increasing, deformed ferrite and deformed martensite gradually recrystallized. When the samples were annealed at 650 °C for 40 min, a bimodal microstructure was obtained and the fine grain region and coarse grain region were distributed in bands. The peak value of the fine grain region was 0.91 μm, the peak value of coarse grain region was 1.9 μm.
- Bimodal structured ultrafine-grained ferrite steel had better comprehensive mechanical properties than dual-phase steel. This is mainly because the hard fine grains were embedded in the soft coarse grains to form a lamellar interphase structure. The fine-grained strengthening, back-stress strengthening, and precipitation strengthening produced by the lamellar interphase structure contributed to the excellent match of strength and ductility. Meanwhile, complete ferrite with ultrafine grains was conducive to the appearance of the yield plateau.
- The neutral salt spray, immersion, SKPFM, and the electrochemistry tests showed that the corrosion resistance of bimodal ferrite steel was better than that of dual-phase steel. The main reason was there were several defects in the martensite substrate and the potential difference between martensite and ferrite was large, which accelerated the corrosion process and was not conducive to the formation of a compact rust layer. On the contrary, the substrate of bimodal ferrite microstructure contained fewer defects and there was no obvious potential difference, which was detrimental to the occurrence and development of corrosion and conducive to forming a protective rust layer. Therefore, the bimodal structured ultrafine-grained ferrite steel possessed excellent corrosion resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, S.; Singh, A.K.; Mula, S.; Chanda, P.; Mahashabde, V.V.; Roy, T.K. Mechanical properties, formability and corrosion resistance of thermomechanically controlled processed Ti-Nb stabilized IF steel. Mater. Sci. Eng. A 2017, 684, 22–36. [Google Scholar] [CrossRef]
- Qu, S.; Pang, X.; Wang, Y.; Gao, K. Corrosion behavior of each phase in low carbon microalloyed ferrite-bainite dual-phase steel: Experiments and modeling. Corros. Sci. 2013, 75, 67–77. [Google Scholar] [CrossRef]
- Wu, H.; Liang, J.; Tang, D.; Liu, X.; Zhang, P.; Yue, Y. Influence of Inclusion on Corrosion Behavior of E36 Grade Low-alloy Steel in Cargo Oil Tank Bottom Plate Environment. J. Iron Steel Res. Int. 2014, 21, 1016–1021. [Google Scholar] [CrossRef]
- Al-Duheisat, S.A.; El-Amoush, A.S. Effect of deformation conditions on the corrosion behavior of the low alloy structural steel girders. Mater. Des. 2016, 89, 342–347. [Google Scholar] [CrossRef]
- Osório, W.R.; Peixoto, L.C.; Garcia, A. Electrochemical corrosion behaviour of a Ti-IF steel and a SAE 1020 steel in a 0.5 M NaCl solution. Mater. Corros. 2010, 61, 407–411. [Google Scholar] [CrossRef]
- Zeng, Y.; Jiang, B.; Li, R.; Yin, H.; Al-Ezzi, S. Grain Refinement Mechanism of the As-Cast and As-Extruded Mg–14Li Alloys with Al or Sn Addition. Metals 2017, 7, 172. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Liu, F.; Wen, Y.; Zhang, L.; Dou, Y. ODS ferritic steel engineered with bimodal grain size for high strength and ductility. Mater. Lett. 2011, 65, 1672–1674. [Google Scholar]
- Odnobokova, M.; Belyakov, A.; Kaibyshev, R. Development of nanocrystalline 304L stainless steel by large strain cold working. Metals 2015, 5, 656–668. [Google Scholar] [CrossRef]
- Zhao, M.; Yin, F.; Hanamura, T.; Nagai, K.; Atrens, A. Relationship between yield strength and grain size for a bimodal structural ultrafine-grained ferrite/cementite steel. Scr. Mater. 2007, 57, 857–860. [Google Scholar] [CrossRef]
- Yuan, W.; Panigrahi, S.K.; Su, J.Q.; Mishra, R.S. Influence of grain size and texture on Hall–Petch relationship for a magnesium alloy. Scr. Mater. 2011, 65, 994–997. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Zhu, L.; Liu, Y.; Lei, X.; Wang, G.; Gao, H. Evading the strength-ductility trade-off dilemma in steel through gradient hierarchical nanotwins. Nat. Commun. 2014, 5, 3580. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuan, F.; Yang, M.; Jiang, P.; Zhang, C.; Chen, L.; Ma, E. Nanodomained nickel unite nanocrystal strength with coarse-grain ductility. Sci. Rep. 2015, 5, 11728. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kakihata, K.; Kaneko, Y.; Hashimoto, S.; Vinogradov, A. Nanostructurization assisted by twinning during equal channel angular pressing of metastable 316L stainless steel. J. Mater. Sci. 2011, 46, 4276–4283. [Google Scholar] [CrossRef]
- Wu, H.; Niu, G.; Wu, F.; Tang, D. Reverse-transformation austenite structure control with micro/nanometer size. Int. J. Miner. Metall. Mater. 2017, 24, 530–537. [Google Scholar] [CrossRef]
- Karmakar, A.; Karani, A.; Patra, S.; Chakrabarti, D. Development of bimodal ferrite-grain structures in low-carbon steel using rapid intercritical annealing. Metall. Mater. Trans. A 2013, 44, 2041–2052. [Google Scholar] [CrossRef]
- Wu, X.; Yang, M.; Yuan, F.; Wu, G.; Wei, Y.; Huang, X.; Zhu, Y. Heterogeneous lamella structure unites ultrafine-grain strength with coarse-grain ductility. Proc. Natl. Acad. Sci. USA 2015, 112, 14501–14505. [Google Scholar] [CrossRef] [PubMed]
- Vajpai, S.K.; Ota, M.; Zhang, Z.; Ameyama, K. Three-dimensionally gradient harmonic structure design: An integrated approach for high performance structural materials. Mater. Res. Lett. 2016, 4, 191–197. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of annealing temperature on the pitting corrosion resistance of super duplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Örnek, C.; Engelberg, D.L. SKPFM measured Volta potential correlated with strain localisation in microstructure to understand corrosion susceptibility of cold-rolled grade 2205 duplex stainless steel. Corros. Sci. 2015, 99, 164–171. [Google Scholar] [CrossRef]
- Nakhaie, D.; Davoodi, A.; Imani, A. The role of constituent phases on corrosion initiation of NiAl bronze in acidic media studied by SEM-EDS, AFM and SKPFM. Corros. Sci. 2014, 80, 104–110. [Google Scholar] [CrossRef]
- Kisko, A.; Hamada, A.S.; Talonen, J.; Porter, D.; Karjalainen, L.P. Effects of reversion and recrystallization on microstructure and mechanical properties of Nb-alloyed low-Ni high-Mn austenitic stainless steels. Mater. Sci. Eng. A 2016, 657, 359–370. [Google Scholar] [CrossRef]
- Wu, H.; Niu, G.; Cao, J.; Yang, M. Annealing of strain-induced martensite to obtain micro/nanometre grains in austenitic stainless. Mater. Sci. Technol. 2017, 33, 480–486. [Google Scholar] [CrossRef]
- Meng, Y.; Sugiyama, S.; Yanagimoto, J. Microstructural evolution during RAP process and deformation behavior of semi-solid SKD61 tool steel. J. Mater. Process. Technol. 2012, 212, 1731–1741. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; He, J.; Nieh, T.; Lu, Z. Grain growth and the Hall–Petch relationship in a high-entropy FeCrNiCoMn alloy. Scr. Mater. 2013, 68, 526–529. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Zaefferer, S.; Raabe, D. The effect of grain size and grain orientation on deformation twinning in a Fe–22 wt. % Mn–0.6 wt. % C TWIP steel. Mater. Sci. Eng. A 2010, 527, 3552–3560. [Google Scholar] [CrossRef]
- Thijs, L.; Sistiaga, M.L.M.; Wauthle, R.; Xie, Q.; Kruth, J.P.; Van Humbeeck, J. Strong morphological and crystallographic texture and resulting yield strength anisotropy in selective laser melted tantalum. Acta Mater. 2013, 61, 4657–4668. [Google Scholar] [CrossRef]
- Sinclair, C.; Saada, G.; Embury, J. Role of internal stresses in co-deformed two-phase materials. Philos. Mag. 2006, 86, 4081–4098. [Google Scholar] [CrossRef]
- Mughrabi, H. Dislocation wall and cell structures and long-range internal stresses in deformed metal crystals. Acta Metall. 1983, 31, 1367–1379. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of high strength hot-rolled sheet steel consisting of ferrite and nanometer-sized carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Kapoor, M.; Isheim, D.; Ghosh, G.; Vaynman, S.; Fine, M.E.; Chung, Y.W. Aging characteristics and mechanical properties of 1600 MPa body-centered cubic Cu and B2-NiAl precipitation-strengthened ferritic steel. Acta Mater. 2014, 73, 56–74. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.B.; Ray, K.K. Influence of bainite/martensite-content on the tensile properties of low carbon dual-phase steels. Mater. Sci. Eng. A 2008, 474, 270–282. [Google Scholar] [CrossRef]
- Ucak, A.; Tsopelas, P. Constitutive model for cyclic response of structural steels with yield plateau. J. Struct. Eng. 2010, 137, 195–206. [Google Scholar] [CrossRef]
- Cottrell, A.H.; Bilby, B.A. Dislocation theory of yielding and strain ageing of iron. Proc. Phys. Soc. Sect. A 1949, 62, 49. [Google Scholar] [CrossRef]
- Schulson, E.M.; Weihs, T.P.; Viens, D.V.; Baker, I. The effect of grain size on the yield strength of Ni3Al. Acta Metall. 1985, 33, 1587–1591. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.A.; Stolyarov, V.V.; Valiev, R.Z.; Liao, X.Z.; Zhu, Y.T. Corrosion resistance of ultrafine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II.: EIS interpretation of corrosion behavior. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Hoseinpoor, M.; Momeni, M.; Moayed, M.H.; Davoodi, A. EIS assessment of critical pitting temperature of 2205 duplex stainless steel in acidified ferric chloride solution. Corros. Sci. 2014, 80, 197–204. [Google Scholar] [CrossRef]
- Ghaffari, M.; Saeb, M.R.; Ramezanzadeh, B.; Taheri, P. Demonstration of epoxy/carbon steel interfacial delamination behavior: Electrochemical impedance and X-ray spectroscopic analyses. Corros. Sci. 2016, 102, 326–337. [Google Scholar] [CrossRef]
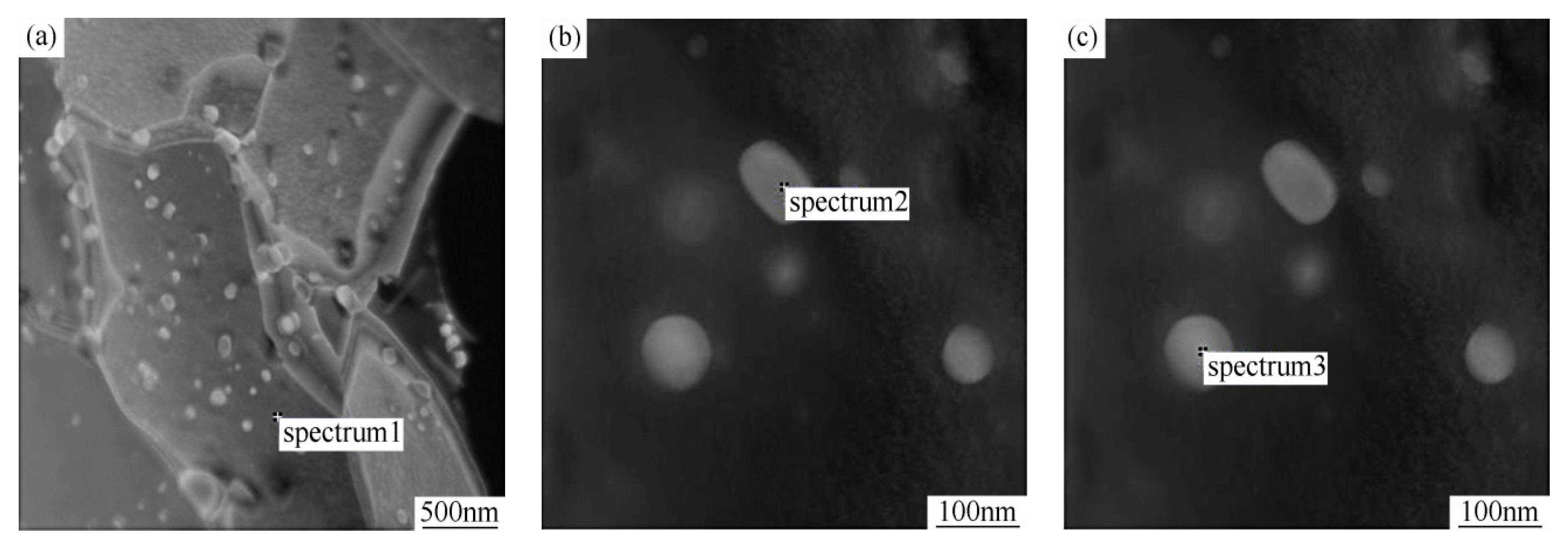
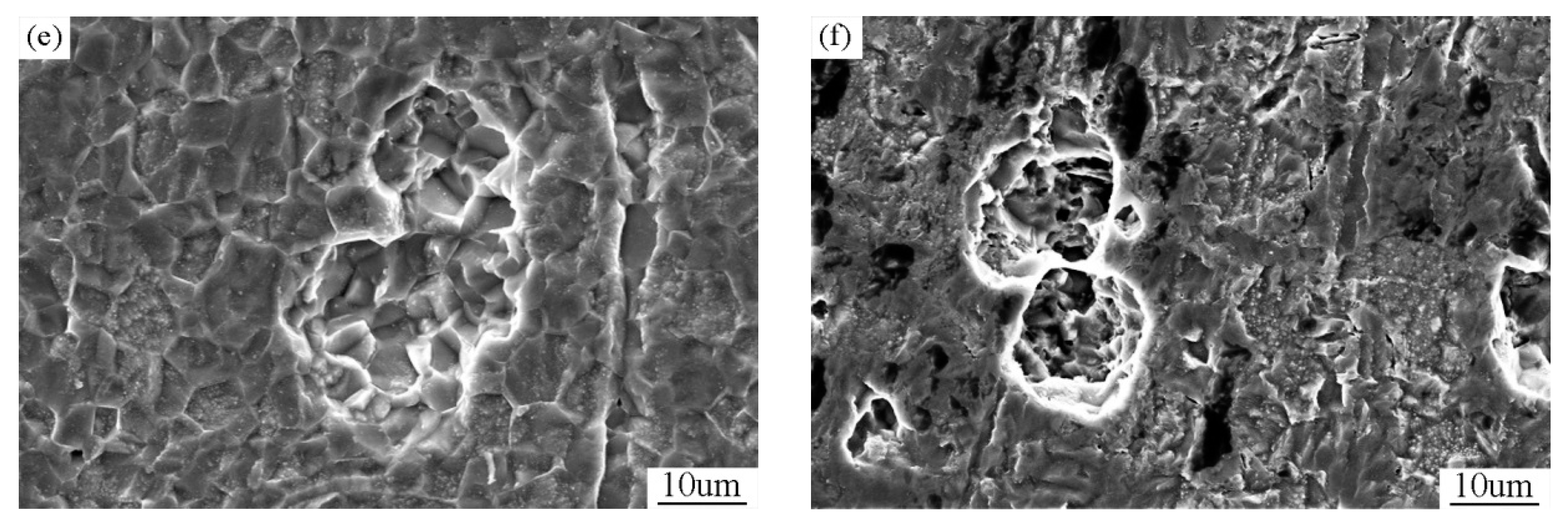
| Alloy Element | Spectrum1 (Matrix) | Spectrum2 (Precipitate) | Spectrum3 (Precipitate) |
|---|---|---|---|
| Si | 1.5 | 1.2 | 0.8 |
| Fe | 97.0 | 81.3 | 77.7 |
| Mn | 1.0 | 9.0 | 12.1 |
| Cr | 0.5 | 6.6 | 7.7 |
| Mo | - | 2.0 | 1.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, G.; Wu, H.; Zhang, D.; Gong, N.; Tang, D. Study on Microstructure and Properties of Bimodal Structured Ultrafine-Grained Ferrite Steel. Metals 2017, 7, 316. https://doi.org/10.3390/met7080316
Niu G, Wu H, Zhang D, Gong N, Tang D. Study on Microstructure and Properties of Bimodal Structured Ultrafine-Grained Ferrite Steel. Metals. 2017; 7(8):316. https://doi.org/10.3390/met7080316
Chicago/Turabian StyleNiu, Gang, Huibin Wu, Da Zhang, Na Gong, and Di Tang. 2017. "Study on Microstructure and Properties of Bimodal Structured Ultrafine-Grained Ferrite Steel" Metals 7, no. 8: 316. https://doi.org/10.3390/met7080316





