Extraction of Iron and Manganese from Pyrolusite Absorption Residue by Ammonium Sulphate Roasting–Leaching Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods and Procedure
2.3. Product Analysis
3. Results and Discussion
3.1. Reaction Mechanisms
3.1.1. Reaction between PAR and Ammonium Sulphate
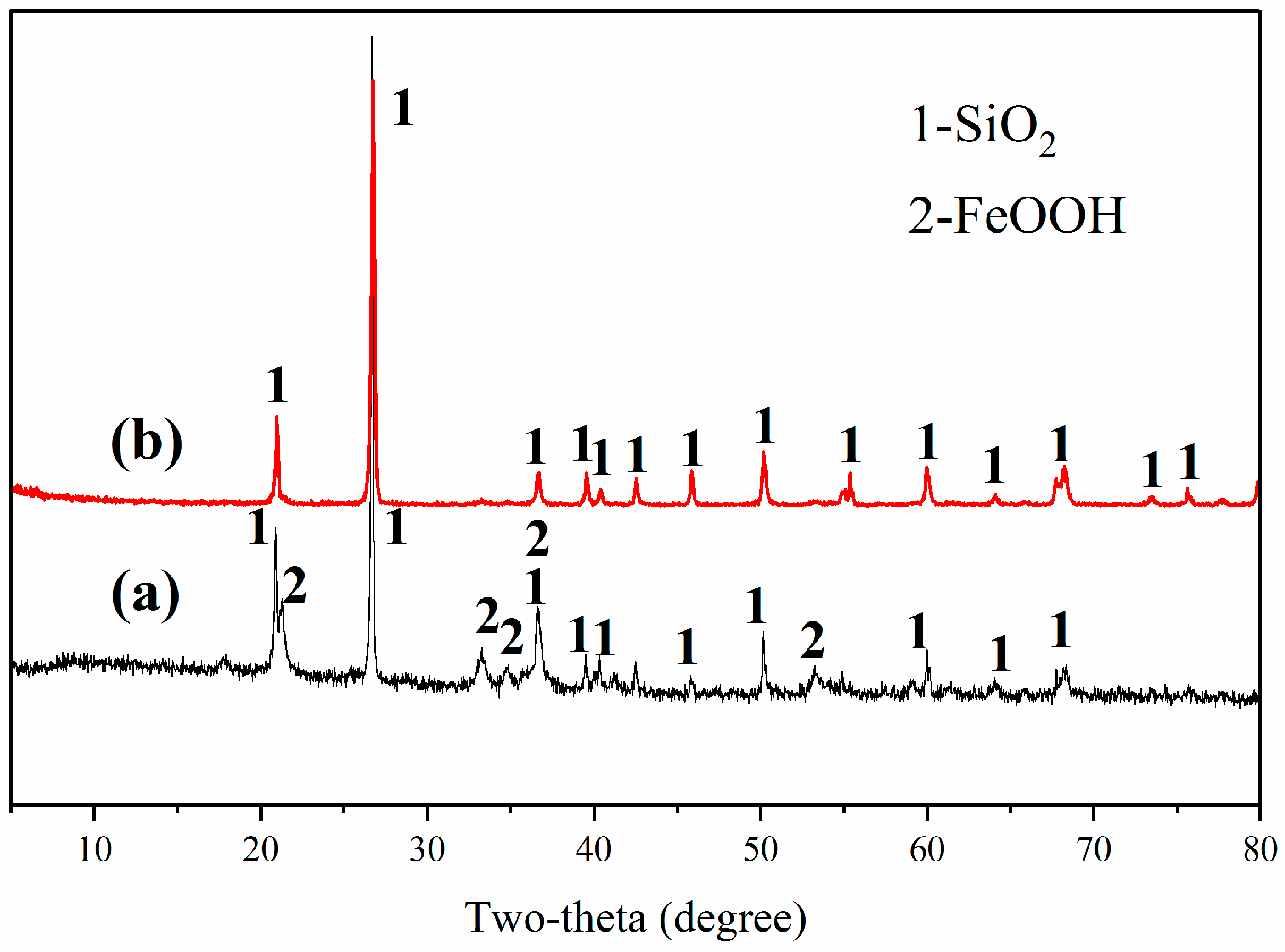
3.1.2. Characterization of Roasting Product
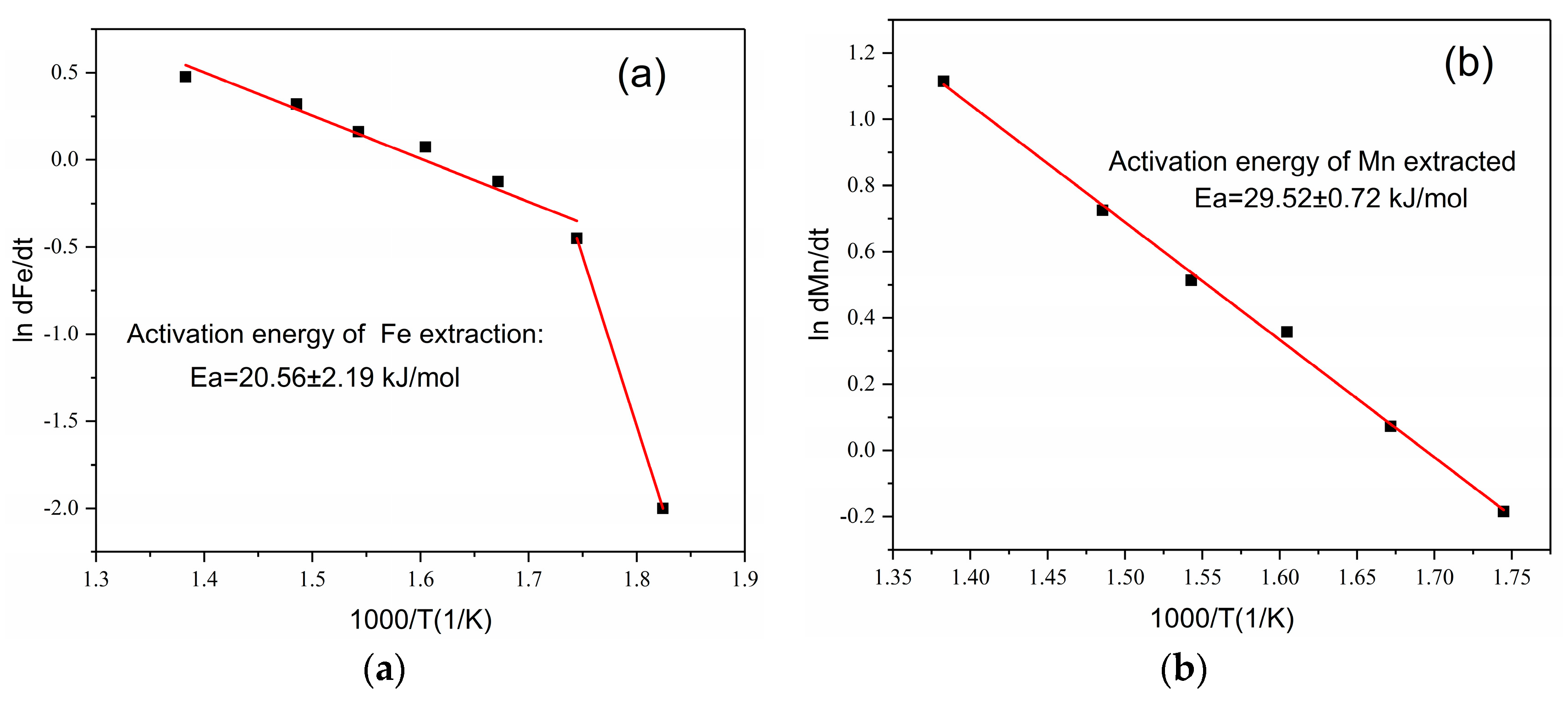
3.2. Roasting Kinetics
3.3. Effect of Conditions Applied for Roasting and Acid Leaching
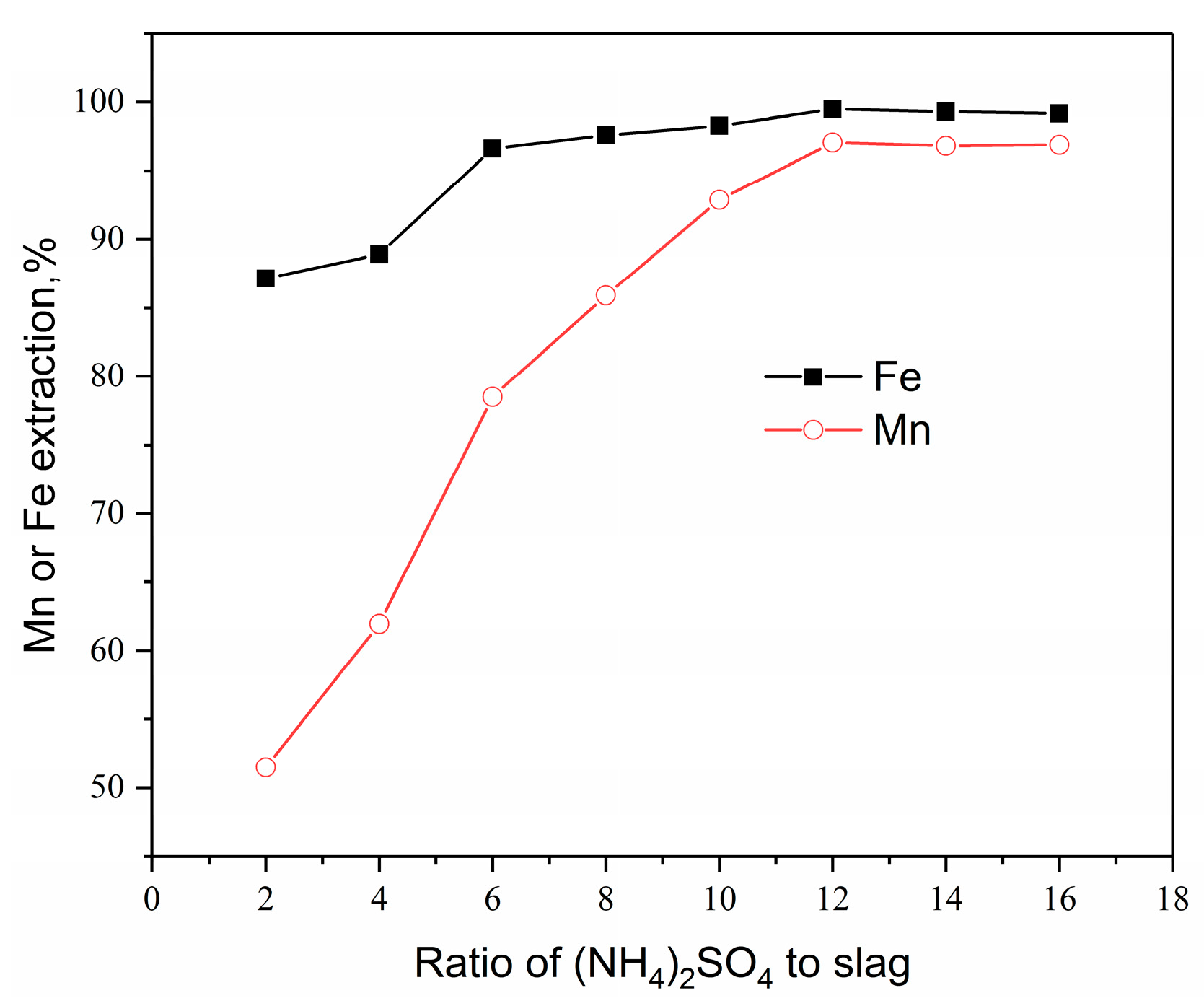
3.3.1. Effect of Ammonium Sulphate: PAR Mass Ratio
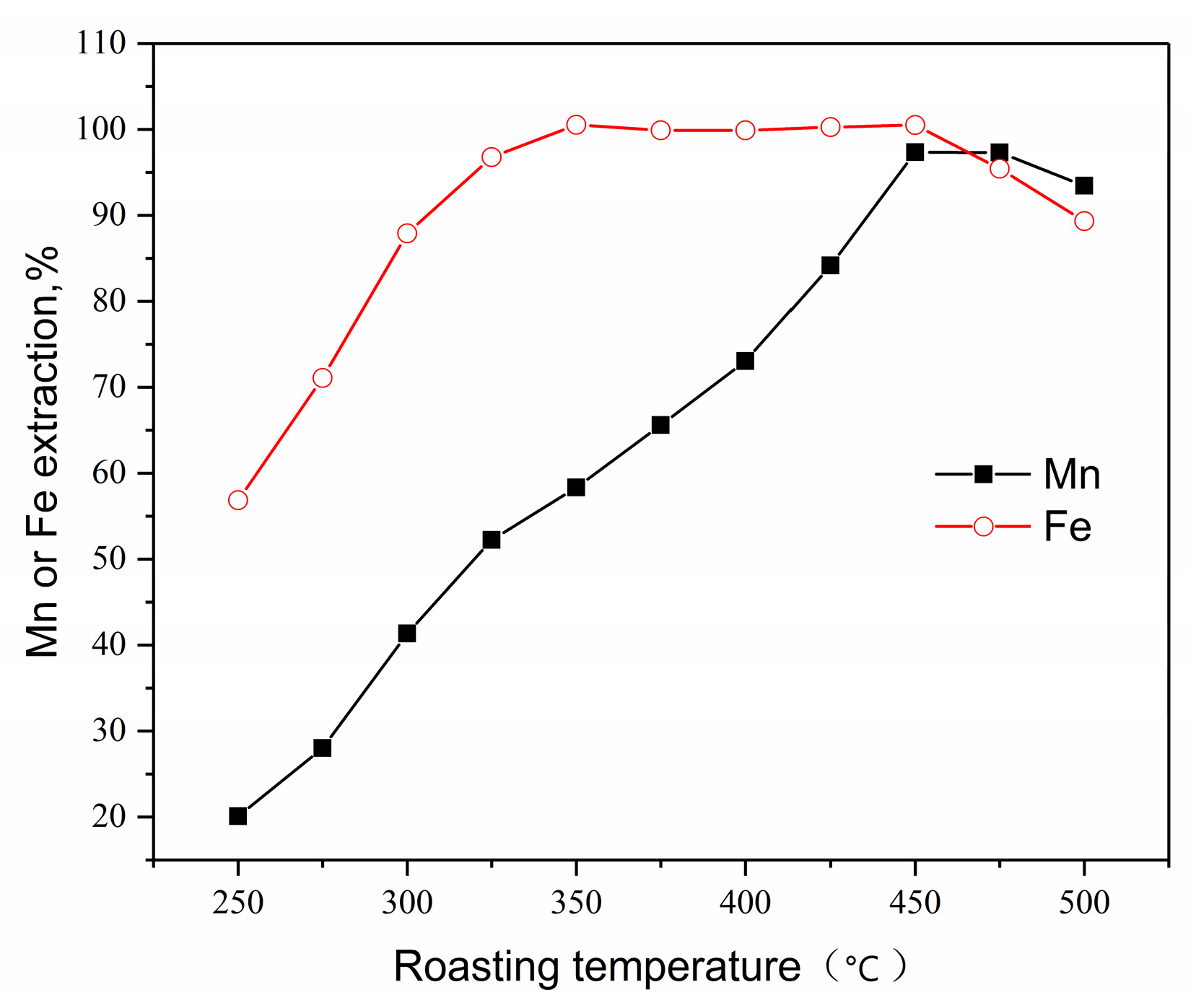
3.3.2. Effect of Roasting Temperature
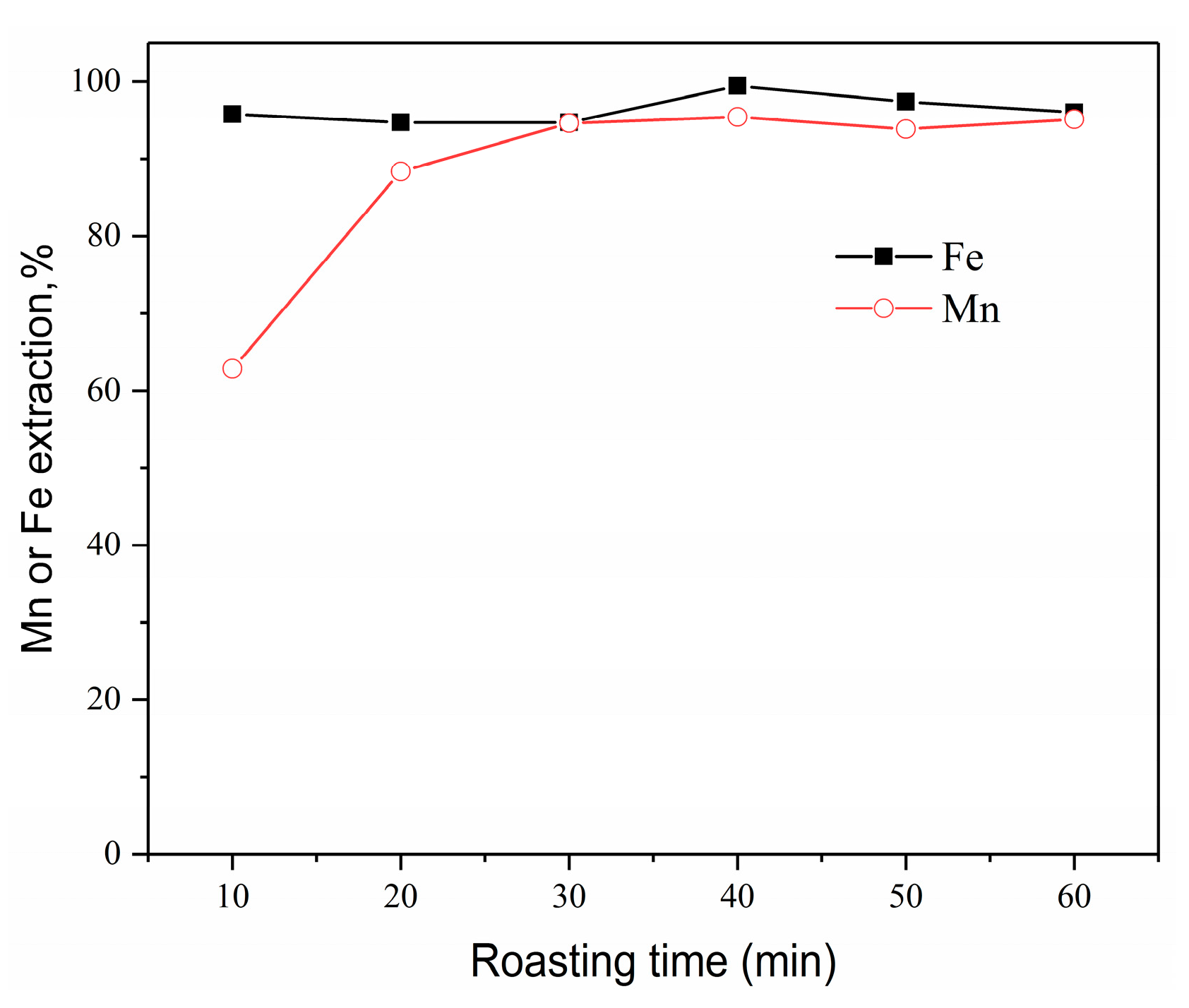
3.3.3. Effect of Roasting Time
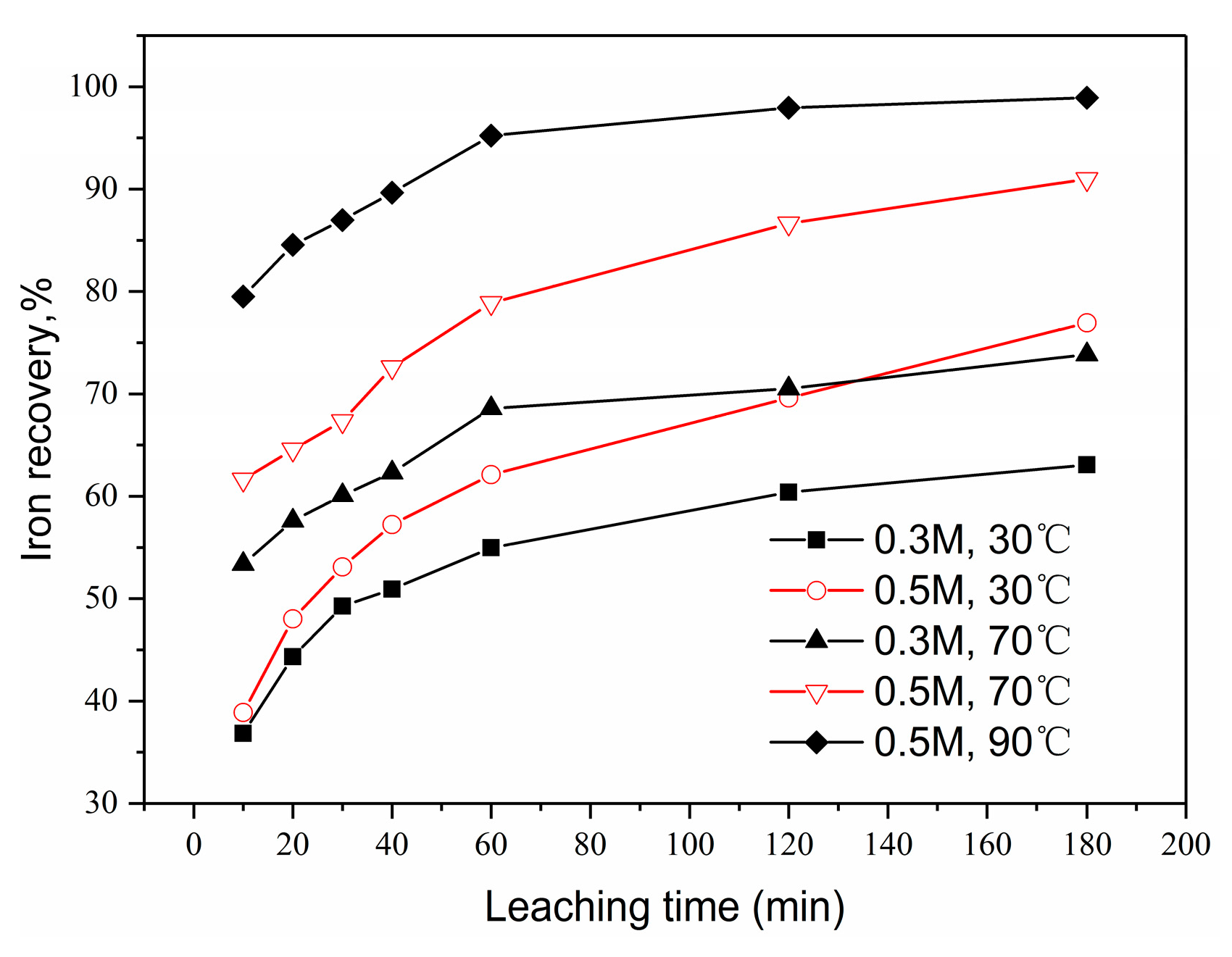
3.3.4. Effect of Leaching Temperature and Acidity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gui, Y.L.; Yan, Q.C.; Yan, Y.K.; Song, C.Y. Chemical Reaction Mechanism of Sintering Flue Gas Desulphurization with Pyrolusite Slurry. In Applications of Engineering Materials, Pts 1–4. Advanced Materials Research; Bu, J.L., Wang, P.C., Ai, L., Sang, X.M., Li, Y.G., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Swizerland, 2011; Volume 287–290, pp. 2937–2940. [Google Scholar]
- Sun, W.-Y.; Ding, S.-L.; Zeng, S.-S.; Su, S.-J.; Jiang, W.-J. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone. J. Hazard. Mater. 2011, 192, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-Y.; Wang, Q.-Y.; Ding, S.-L.; Su, S.-J. Simultaneous absorption of SO2 and NOx with pyrolusite slurry combined with gas-phase oxidation of NO using ozone: Effect of molar ratio of O2/(SO2 + 0.5NOx) in flue gas. Chem. Eng. J. 2013, 228, 700–707. [Google Scholar] [CrossRef]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- An Introduction of Pyrolusite Ore. Available online: https://en.wikipedia.org/wiki/Pyrolusite (accessed on 3 November 2017).
- Sun, W.-Y.; Wang, Q.-Y.; Ding, S.-L.; Su, S.-J.; Jiang, W.-J.; Zhu, E.-G. Reaction mechanism of NOx removal from flue gas with pyrolusite slurry. Sep. Purif. Technol. 2013, 118, 576–582. [Google Scholar] [CrossRef]
- Sun, W.-Y.; Shen, W.; Wang, Q.Y.; Ding, S.L.; Su, S.-J. Adorption of chromium from aqueous solution onto aluminum pillared pyrolusite leaching residue. Fresen. Environ. Bull. 2013, 22, 2645–2650. [Google Scholar]
- Liao, B.; Shen, W.; Sun, W.; Wu, B.; Su, S.; Ding, S. Adsorption of methylene blue from aqueous solution onto pyrolusite leaching residue and degradation in H2O2/H2SO4 system. Fresen. Environ. Bull. 2014, 23, 1618–1625. [Google Scholar]
- Shen, W.; Liao, B.; Sun, W.; Su, S.; Ding, S. Adsorption of Congo red from aqueous solution onto pyrolusite reductive leaching residue. Desalination Water Treat. 2014, 52, 3564–3571. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Lang, T.; Xu, D.D.; Yi, M.Y.; Sun, W.Y.; Shen, W.; Su, S.J. Preparation of sulphoaluminate cement clinkers from pyrolusite flue desulfurization slag. Environ. Eng. 2014, 32, 108–112. [Google Scholar]
- Zhang, Y.; Du, M.; Liu, B.; Su, Z.; Li, G.; Jiang, T. Separation and recovery of iron and manganese from high-iron manganese oxide ores by reduction roasting and magnetic separation technique. Sep. Sci. Technol. 2017, 52, 1321–1332. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Xue, X.-X.; Yuan, S. Separation and Recovery of Iron and Rare Earth from Bayan Obo Tailings by Magnetizing Roasting and (NH4)(2)SO4 Activation Roasting. Metals 2017, 7, 195. [Google Scholar] [CrossRef]
- Zhu, D.-Q.; Chun, T.-J.; Pan, J.; He, Z. Recovery of Iron from High-Iron Red Mud by Reduction Roasting with Adding Sodium Salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, B.; Tang, Y.; Wan, P.; Chen, Y.; Lv, Z. Recycling of iron from red mud by magnetic separation after co-roasting with pyrite. Thermochim. Acta 2014, 588, 11–15. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Shi, Z.-X.; Chen, Y.-M.; Niu, Y.-J.; Wang, Y.-X.; Wan, P.-Y. Red-mud treatment using oxalic acid by UV irradiation assistance. Trans. Nonferr. Met. Soc. 2012, 22, 456–460. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Recovery of iron from red mud by selective leach with oxalic acid. Hydrometallurgy 2015, 157, 239–245. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Li, G.; Jiang, T. Manganese extraction by sulfur-based reduction roasting-acid leaching from low-grade manganese oxide ores. Hydrometallurgy 2013, 133, 126–132. [Google Scholar] [CrossRef]
- You, Z.; Li, G.; Zhang, Y.; Peng, Z.; Jiang, T. Extraction of manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching. Hydrometallurgy 2015, 156, 225–231. [Google Scholar] [CrossRef]
- Freitas, L.R.; Amaral, J.C.; Mendonca, C.F. Sulphation of Carajas manganese ore with gaseous SO2. Trans. Inst. Min. Metall. Sect. C 1993, 102, 130–131. [Google Scholar]
- Vu, H.; Jandová, J.; Lisá, K.; Vranka, F. Leaching of manganese deep ocean nodules in FeSO4–H2SO4–H2O solutions. Hydrometallurgy 2005, 77, 147–153. [Google Scholar] [CrossRef]
- Yi, A.-F.; Wu, M.-N.; Liu, P.-W.; Feng, Y.-L.; Li, H.-R. Reductive leaching of low-grade manganese ore with pre-processed cornstalk. Int. J. Miner. Metall. Mater. 2015, 22, 1245–1251. [Google Scholar] [CrossRef]
- Rolf, R.F. Process for the Selective Recovery of Manganese and Iron. U.S. Patent 3471285DA, 7 October 1969. [Google Scholar]
- Li, J.; Chen, Z.; Shen, B.; Xu, Z.; Zhang, Y. The extraction of valuable metals and phase transformation and formation mechanism in roasting-water leaching process of laterite with ammonium sulfate. J. Clean. Prod. 2016, 140, 1148–1155. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Peng, B.; Min, X.; Hu, M.; Peng, N.; Yuang, Y.; Lei, J. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate. Hydrometallurgy 2015, 158, 42–48. [Google Scholar] [CrossRef]
- Mohamed, S.; Merwe, E.M.V.D.; Altermann, W.; Doucet, F.J. Process development for elemental recovery from PGM tailings by thermochemical treatment: Preliminary major element extraction studies using ammonium sulphate as extracting agent. Waste Manag. 2016, 50, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Doucet, F.J.; Mohamed, S.; Neyt, N.; Castleman, B.A.; van der Merwe, E.M. Thermochemical processing of a South African ultrafine coal fly ash using ammonium sulphate as extracting agent for aluminium extraction. Hydrometallurgy 2016, 166, 174–184. [Google Scholar] [CrossRef]
- Lee, C.T.; Sohn, H.Y. Recovery of synthetic rutile and iron oxide from ilmenite ore by sulfation with ammonium sulfate. Ind. Eng. Chem. Res. 1989, 28, 1802–1808. [Google Scholar] [CrossRef]
- Xin, H.X.; Wu, Y.; Liu, S.M.; Zhai, Y.C. Recovery of aluminum and iron from high-iron bauxite by roasting method using ammonium sulfate. Conserv. Util. Miner. Resour. 2013, 37, 37–40. [Google Scholar] [CrossRef]
- Dixo, P. Formation of sulphamic acid during the thermal decomposition of ammonium sulphate. Nature 1944, 154, 706. [Google Scholar] [CrossRef]
- Kiyoura, R.; Urano, K. Mechanism, Kinetics, and Equilibrium of Thermal Decomposition of Ammonium Sulfate. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 489–494. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Li, G.-G.; Bi, C.-M.; Wang, B.; Li, R.-G. Research on the preparation of cementitious material using slag, desulfuration residue and cinder. J. Wuhan Univ. Technol. 2010, 32, 6–10. [Google Scholar] [CrossRef]
- Liu, X.-W.; Feng, Y.-L.; Li, H.-R.; Yang, Z.-C.; Cai, Z.-L. Recovery of valuable metals from a low-grade nickel ore using an ammonium sulfate roasting-leaching process. Int. J. Miner. Metall. Mater. 2012, 19, 377–383. [Google Scholar] [CrossRef]


| Elements | Fe | Mn | Al | Ca | Ba | Zn | Ni | Cu |
|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 30.1 | 4.03 | 1.75 | 0.761 | 0.491 | 0.044 | 0.029 | 0.021 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Qu, B.; Su, S.-J.; Ding, S.-L.; Sun, W.-Y. Extraction of Iron and Manganese from Pyrolusite Absorption Residue by Ammonium Sulphate Roasting–Leaching Process. Metals 2018, 8, 38. https://doi.org/10.3390/met8010038
Deng L, Qu B, Su S-J, Ding S-L, Sun W-Y. Extraction of Iron and Manganese from Pyrolusite Absorption Residue by Ammonium Sulphate Roasting–Leaching Process. Metals. 2018; 8(1):38. https://doi.org/10.3390/met8010038
Chicago/Turabian StyleDeng, Lin, Bing Qu, Shi-Jun Su, Sang-Lan Ding, and Wei-Yi Sun. 2018. "Extraction of Iron and Manganese from Pyrolusite Absorption Residue by Ammonium Sulphate Roasting–Leaching Process" Metals 8, no. 1: 38. https://doi.org/10.3390/met8010038




