Chemical Degradation of a Mixture of tri-n-Octylamine and 1-Tridecanol in the Presence of Chromium(VI) in Acidic Sulfate Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods and Equipments
3. Results
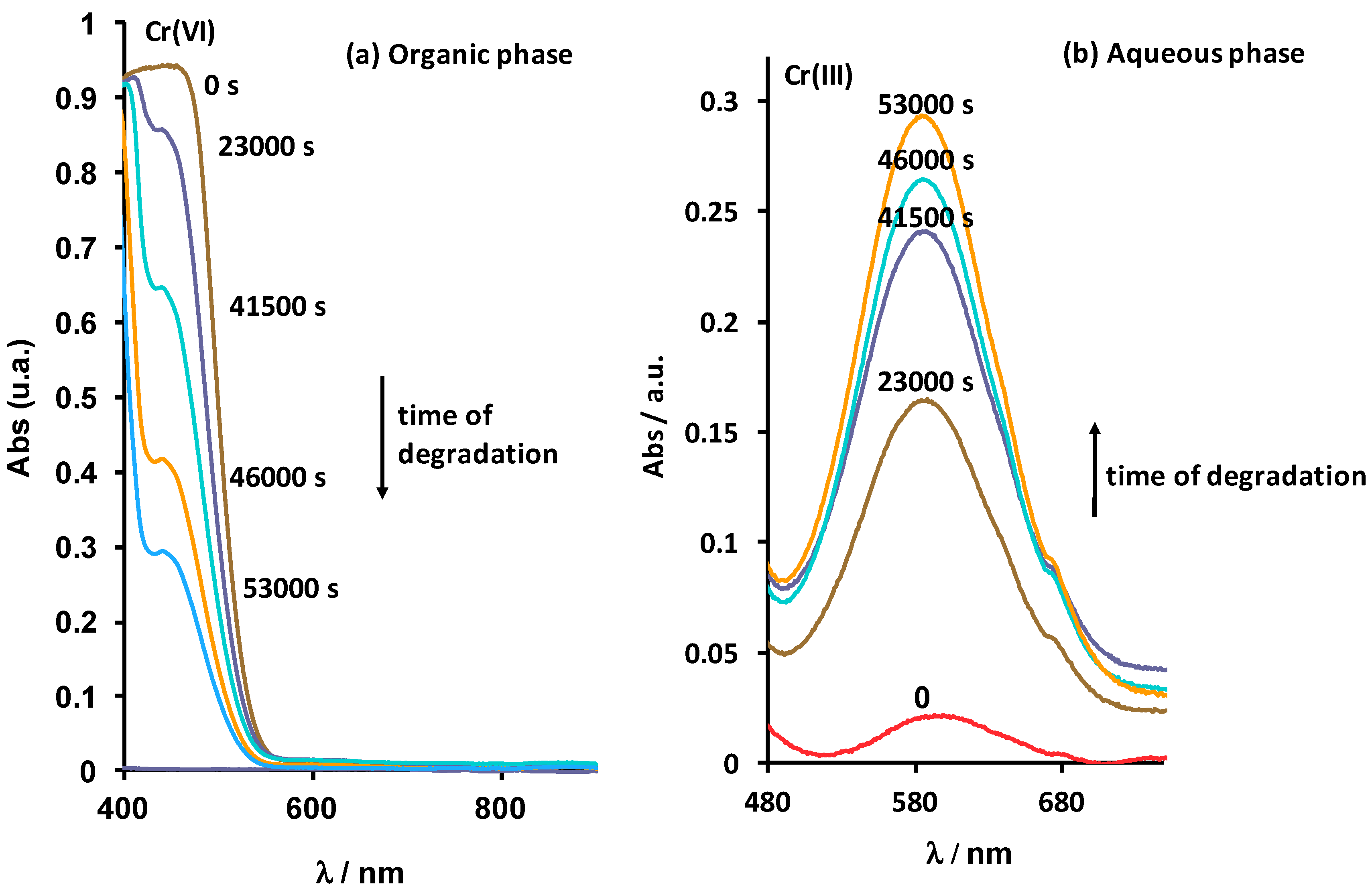
3.1. Identification of Degradation Products
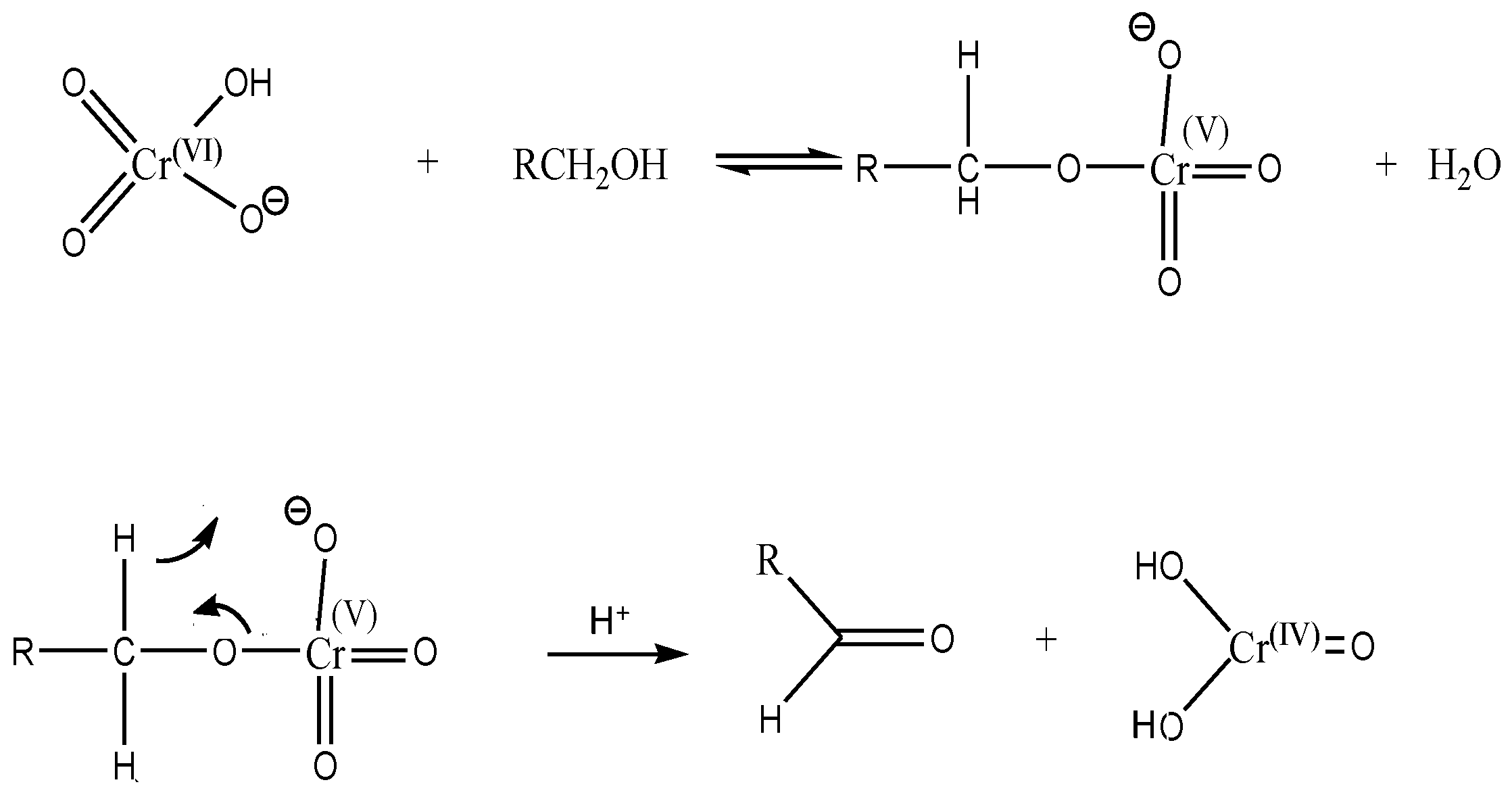
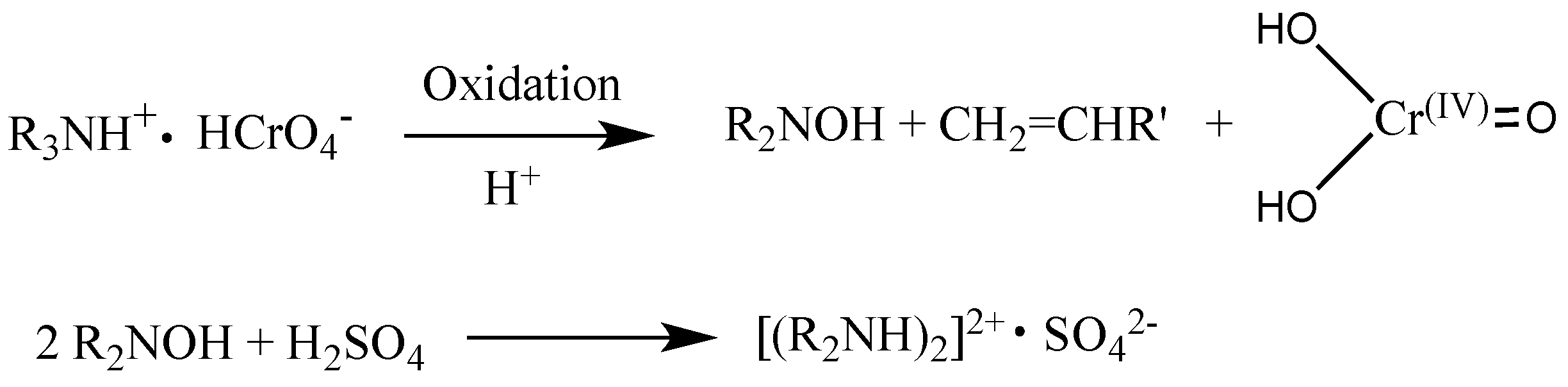
3.2. Degradation Mechanisms
3.3. Kinetics of Degradation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chagnes, A. Fundamentals in Electrochemistry and Hydrometallurgy. In Lithium Process Chemistry: Resources, Extractions, Batteries and Recycling, 1st ed.; Chagnes, A., Swiatowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 41–80. ISBN 978-0-12-801417-2. [Google Scholar]
- Chagnes, A.; Cote, G.; Courtaud, B.; Syna, N.P.; Thiry, J. Influence of the chemical degradation of trioctylamine dissolved in n-dodecane modified with tridecanol on uranium extraction process in a plant located in Niger. In Proceedings of the 3rd Conference on Uranium, 40th Annual Hydrometallurgy Meeting, Saskatoon, SK, Canada, 15–18 August 2010. [Google Scholar]
- Rydberg, J. Solvent Extraction: Principles and Practices, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; p. 750. [Google Scholar]
- Munyungano, B.; Feather, A.; Virnig, M. Degradation problems with the solvent extraction organic at Rössing uranium. In Proceedings of the International Solvent Extraction Conference (ISEC 2008)—Solvent Extraction: Fundamentals to Industrial Applications, Tucson, AZ, USA, 15–19 September 2008; Moyer, B., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2008; Volume 1, pp. 269–274. [Google Scholar]
- Feather, A.; Virnig, M.; Bender, J.; Crane, P. Degradation problems with uranium solvent extraction organic. In Proceedings of the ALTA International Uranium Conference, Melbourne, QC, Canada, 23–27 May 2009; ALTA Metallurgical Services: Perth, Australia, 2009. [Google Scholar]
- Chagnes, A.; Fossé, C.; Courtaud, B.; Thiry, J.; Cote, G. Chemical degradation of tri-n-octylamine and 1-tridecanol dissolved in n-dodecane in solvent extraction processes involving aqueous acidic sulfate solutions containing vanadium(V). Hydrometallurgy 2011, 105, 328–333. [Google Scholar] [CrossRef]
- Chagnes, A.; Rager, M.-N.; Courtaud, B.; Thiry, J.; Cote, G. Speciation of vanadium (V) extracted from acidic sulfate media by tri-n-octylamine in n-dodecane modified with 1-tridecanol. Hydrometallurgy 2010, 104, 20–24. [Google Scholar] [CrossRef]
- Collet, S.; Chagnes, A.; Courtaud, B.; Thiry, J.; Cote, G. Solvent Extraction of Uranium from Acidic Sulfate Media by Alamine® 336: Computer Simulation and Optimization of the Flowsheets. J. Chem. Technol. Biotechnol. 2009, 84, 1331–1337. [Google Scholar] [CrossRef]
- Best, P.; Littler, J.S.; Waters, W.A. The mechanism of oxidation of cyclohexanone under acid conditions. Part 1. Two-electron oxidants. J. Chem. Soc. 1962, 822. [Google Scholar] [CrossRef]
- Hartford, W.H.; Darrin, M. The chemistry of chromyl compounds. Chem. Rev. 1958, 58, 1–61. [Google Scholar] [CrossRef]
- Rocek, J.; Riehl, A. Mechanism of the chromic acid oxidation of ketones. J. Am. Chem. Soc. 1967, 89, 6691–6695. [Google Scholar] [CrossRef]
- Stewart, R. Oxidation Mechanisms—Application to Organic Chemistry; Benjamin, W.A., Ed.; Wiley: New York, NY, USA, 1964; pp. 33–57. [Google Scholar]
- Wagner, R.B.; Moore, J.A. The rearrangement of α,α’-dibromoketones. J. Am. Chem. Soc. 1950, 72, 974–977. [Google Scholar] [CrossRef]
- Puigdomenech, I. Medusa Software; KTH University: Stockholm, Sweden, 2000. [Google Scholar]
- Da Silva, M.F.C.G.; Da Silva, J.A.L.; Da Silva, J.J.R.F.; Pombeiro, A.J.L.; Amatore, C.; Verpeaux, J.-N. Evidence for a Michaelis-Menten type mechanism in the electrocatalytic oxidation of mercaptopropionic acid by an amavadine model. J. Am. Chem. Soc. 1996, 118, 7568–7573. [Google Scholar] [CrossRef]
- Katz, S.A.; Salem, H. The Biological Environmental Chemistry of Chromium; VCH Publishers Inc.: New York, NY, USA, 1994; p. 65. [Google Scholar]
- O’Brien, P.; Kotenkamp, A. The chemistry underlying chromate toxicity. Transit. Met. Chem. 1995, 20, 636–642. [Google Scholar] [CrossRef]
- Watanabe, W.; Westheimer, F.H. The kinetics of the chromic acid oxidation of isopropyl alcohol: The induced oxidation of manganous ion. J. Chem. Phys. 1949, 17, 61–70. [Google Scholar] [CrossRef]
- Rocek, J.; Radkowsky, A.E. Mechanism of the chromic acid oxidation of cyclobutanol. J. Am. Chem. Soc. 1973, 95, 7123–7132. [Google Scholar] [CrossRef]
- Sreelatha, G.; Rao, M.P. Kinetics and mechanism of oxidation of allyl, crotyl and cinnamyl alcohol by chromium (V). Transit. Met. Chem. 1990, 15, 31–33. [Google Scholar] [CrossRef]
- Patel, S.; Mishra, B.K. Oxidation of Alcohol by Lipopathic Cr(VI): A Mechanistic Study. J. Org. Chem. 2006, 71, 6759–6766. [Google Scholar] [CrossRef] [PubMed]
- Murashashi, S.I. Synthetic Aspects of Metal-Catalyzed Oxidations of Amines and Related Reactions. Angew. Chem. Int. Ed. 1995, 34, 2443–2465. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal-Catalysed Oxidations of Organic Compounds: Mechanistic Principles and Synthetics Methodology Including Biomedical Processes; Academic Press: New York, NY, USA, 1981; Chapter 13; pp. 387–397. [Google Scholar]
- Velusamy, S.; Punniyamurthy, T. Novel Vanadium-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones under Atmospheric Oxygen. Org. Lett. 2004, 6, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.R.; Garratt, D.G.; De Cesare, J.M.; Olivier, A.J. Solvent Degradation and High Acid Stripping in the Rabbit Lake Uranium Mill. In Proceedings of the SME Annual Meeting, Society of Mining Engineers, Phoenix, AZ, USA, 25–28 January 1988; pp. 1–18. [Google Scholar]
- Yaou-Huei, H.; Chen, C.-Y.; J-Kuo, J.-F. Chromium(VI) complexation with triisooctylamine in organic solvents. Bull. Chem. Soc. Jpn. 1991, 64, 3059–3062. [Google Scholar]


| Products | Retention Time/min |
|---|---|
| n-dodecane | 6.20 |
| 1-tridecanal | 9.10 |
| 1-tridecanol | 9.60 |
| Unidentified compound | 10.40 |
| n-dodecane tridecanoate | 11.30 |
| tri-n-Octylamine | 20.00 |
| di-n-Octylamine | 20.60 |
| N,N,N-octen-1-yl-dioctylamine | 29.50 |
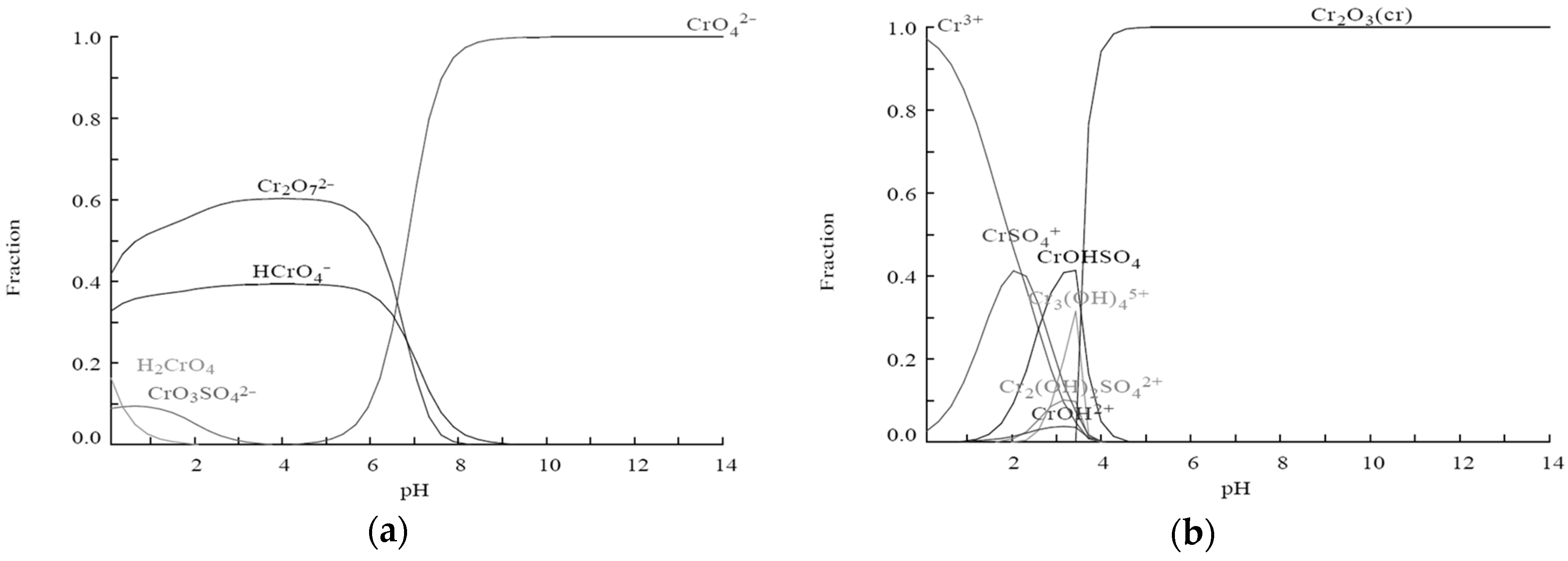
| Equilibria | Thermodynamic Constants at 25 °C |
|---|---|
| CrO42− + H+ = HCrO4− | 3.5 × 106 |
| CrO42− + 2H+ = H2CrO4 | 2.2 × 106 |
| 2CrO42− + 2H+ = Cr2O72− + H2O | 4.9 × 1014 |
| CrO42− + 2H+ + SO42− = CrO3SO42− + H2O | 9.9 × 108 |
| Cr3+ + SO42− = CrSO4+ | 2.2 × 101 |
| Cr3+ + SO42− + H2O = H+ + CrOHSO4 | 4.5 × 10−2 |
| 3Cr3+ + 4H2O = 4H+ + Cr3(OH)45+ | 7.1 × 10−9 |
| 2Cr3+ + 2H2O + SO42− = 2H+ + Cr2(OH)2SO42+ | 8.3 × 10−4 |
| Cr3+ + H2O = CrOH2+ + H+ | 2.7 × 10−4 |
| [H2SO4] mol·kg−1 | [Cr(VI)] mol·kg−1 | [1-Tridecanol] wt % | [tri-n-Octylamine] mol·kg−1 | kobs s−1 |
|---|---|---|---|---|
| 0.1 | 0.02 | 5 | 0.2 | 9.7 × 10−6 (0.989) |
| 0.1 | 0.04 | 5 | 0.2 | 9.5 × 10−6 (0.977) |
| 0.1 | 0.05 | 5 | 0.2 | 9.6 × 10−6 (0.972) |
| 0.1 | 1 | 5 | 0.2 | 2.0 × 10−5 (0.985) |
| 1 | 0.02 | 5 | 0.2 | 1.1 × 10−3 (0.995) |
| 1 | 0.04 | 5 | 0.2 | 1.1 × 10−3 (0.993) |
| 1 | 0.05 | 5 | 0.2 | 1.0 × 10−3 (0.994) |
| 1 | 1 | 5 | 0.2 | 1.0 × 10−3 (0.978) |
| 0.1 | 0.02 | 4 | 0.2 | 9.6 × 10−6 (0.975) |
| 0.1 | 0.04 | 5 | 0.2 | 9.6 × 10−6 (0.972) |
| 0.1 | 0.05 | 6 | 0.2 | 9.6 × 10−6 (0.965) |
| 0.1 | 1 | 7 | 0.2 | 9.6 × 10−6 (0.961) |
| 0.1 | 0.05 | 5 | 0.2 | 9.6 × 10−6 (0.972) |
| 0.1 | 0.05 | 5 | 0.4 | 2.5 × 10−6 (0.992) |
| 0.1 | 0.05 | 5 | 0.5 | 1.8 × 10−6 (0.993) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagnes, A.; Cote, G. Chemical Degradation of a Mixture of tri-n-Octylamine and 1-Tridecanol in the Presence of Chromium(VI) in Acidic Sulfate Media. Metals 2018, 8, 57. https://doi.org/10.3390/met8010057
Chagnes A, Cote G. Chemical Degradation of a Mixture of tri-n-Octylamine and 1-Tridecanol in the Presence of Chromium(VI) in Acidic Sulfate Media. Metals. 2018; 8(1):57. https://doi.org/10.3390/met8010057
Chicago/Turabian StyleChagnes, Alexandre, and Gérard Cote. 2018. "Chemical Degradation of a Mixture of tri-n-Octylamine and 1-Tridecanol in the Presence of Chromium(VI) in Acidic Sulfate Media" Metals 8, no. 1: 57. https://doi.org/10.3390/met8010057






