1. Introduction
Automotive [
1] and aerospace [
2], as well as biomechanics [
3,
4], are just a few examples of the main research and industry branches aimed at advanced lightweight materials, the everlasting research and development of which has resulted in the design of new innovative Mg-based alloys and their production technologies [
5]. The mechanical and physical properties are not the greatest advantages of pure Mg; however, they can both be improved by structural unit refinement and the addition of alloying elements [
6].
Refinement of the structural units can be provided via plastic deformation [
7] (advantageously via severe plastic deformation (SPD) methods [
8] such as equal channel angular pressing [
9,
10], high pressure torsion [
11], and accumulative roll bonding [
12]), processing via methods of powder metallurgy (PM) [
13], by combinations of PM and SPD [
14], or via additions of structure refining elements. Since Mg-based alloys in industry are usually used for die-casting [
15], the effects of the alloying elements are of the utmost importance.
The alloying elements can positively affect the mechanical properties of Mg-based alloys in many ways: for example, by grain refinement, solid solution strengthening, and precipitation hardening [
16,
17]. Aluminium, a common alloying element, provides strengthening via solid solution and precipitation of secondary phases, especially Mg
17Al
12 [
18,
19]. Additions of zinc lead to the formation of Mg–Zn Guinier–Preston zones and precipitation of MgZn
x strengthening phases; in Mg–Al-based alloys, zinc also contributes to the refinement of the Mg
17Al
12 phase [
18]. Alloying by rare earth (RE) metals generally induces precipitation and grain refinement [
20]. Additions of RE can also increase plasticity and thus ensure better formability even during severe plastic deformation treatment at room temperature. For example, additions of La or Y result in the strengthening of Mg-based alloys to more than 400 MPa after processing via extrusion at elevated temperatures [
21,
22]. The strength of Mg–RE alloys can advantageously be further increased by the addition of Zn, which contributes to the formation of various Mg–RE–Zn long-period stacking ordered (LPSO) phases. These have been reported to feature favourable mechanical properties [
23]. Therefore, Mg–RE–Zn alloys are very good candidates for lightweight construction applications. However, similar to grains and precipitates, large and coarse LPSO phases do not contribute to improvements in the mechanical properties as much as fine phases do [
24]. Zirconium has a strong refining effect [
18] and its addition to Mg-based alloys containing RE usually result in quite notable improvements in the mechanical properties [
25]. The effect of Zr on grain refinement is the most favourable when its content is below 17 wt. % [
26]. Conjunctive additions of RE, Zn and Zr also result in the formation of (meta)stable precipitates and RE-rich phases after heat treatments [
27,
28]. Precipitation is quite an issue for Mg-based alloys since this phenomenon can significantly influence mechanical as well as physical properties. Precipitation can be controlled by various means, such as the chemical composition [
29], imposed strain [
30,
31], and the selected heat treatment regime [
32].
This work deals with the investigation of a recently designed Mg–10Dy–3Al–1Zn–0.2Zr alloy. The alloy is based on the AZ31 alloy featuring improved mechanical properties and corrosion resistance when compared to pure Mg [
18]. The AZ alloys are among the most advantageous alternatives to steel in many structural and industrial applications [
33,
34], but they can also be used in biomedicine [
35]. Dy has a favourable effect on strengthening and structure stabilisation, similar to other RE metals, and is also considered to be biocompatible [
29,
36]. Additions of Dy to Mg-based alloys significantly improve corrosion resistance in various solutions [
18]. The amount of added Dy was selected based on the literature research—Mg-10Dy alloys are reported to have very favourable mechanical properties [
37], as well as corrosion resistance [
36] (higher amounts of Dy significantly decrease plasticity as well as increase the corrosion rate). As already mentioned, Zr has a strong refining effect and also exhibits excellent biocompatibility [
38]. Therefore, despite the fact that the alloy was designed to be applicable for construction purposes, it can also be considered for biomedical purposes due to the favourable chemical composition [
36,
39,
40].
Whereas our previous study was focused on the influence of SPD processing on the structure of the alloy [
41], the herein presented study was designed with the aim to compare the effects of different heat treatments on the precipitated phases, their sizes and morphologies. The fact that ageing treatments conducted at temperatures between 200 °C and 350 °C support development of the strengthening phases in Mg-based alloys is known and has been reported by others (e.g., [
42,
43]). However, too high temperatures and times can lead to over-ageing and coarsening of the precipitates. For example, Apps et al. [
44] reported the effect of increasing the ageing time period on coarsening of Mg–RE precipitates, and Rokhlin [
20] reported decreasing mechanical properties of Mg–Dy-based alloys to be related to the effect of increasing ageing temperature. This study is a report on the structure and substructure of the alloy subjected to different heat treatments; the selected temperatures were higher and the times were shorter than for conventional ageing treatments and the effects are discussed with conclusions drawn from the available literature. The first annealing temperature applied was 520 °C. This temperature is most commonly used for solution treatments of Mg-based alloys with additions of RE metals according to the available literature [
13,
26,
28,
29,
30,
31,
32]. At 520 °C, the two time periods of 8 and 16 h were applied, and also for two different temperatures of 480 °C and 560 °C. The temperature of 480 °C was selected as the lower temperature limit since no significant structure rearrangement has been found in Mg–RE-based alloys when annealed below 480 °C for relatively short times [
45]; the value of 560 °C was selected based on the maximum solubility of Dy in Mg being at 561 °C [
20]. The 8-h treatment was selected based on the research of Mg–Dy-based alloys performed by others (e.g., Mg–Dy–Gd–Zr [
39], Mg–Dy–Zn [
46,
47], etc.); the time of 16 h (double the first time period) was selected to observe possible structural changes after exposure to the identical temperature for a longer time. The sizes of the precipitated phases and their effects on the mechanical properties were also evaluated. However, deeper studies addressing the precipitation kinetics and diffusion parameters are planned for a subsequent publication.
4. Discussion
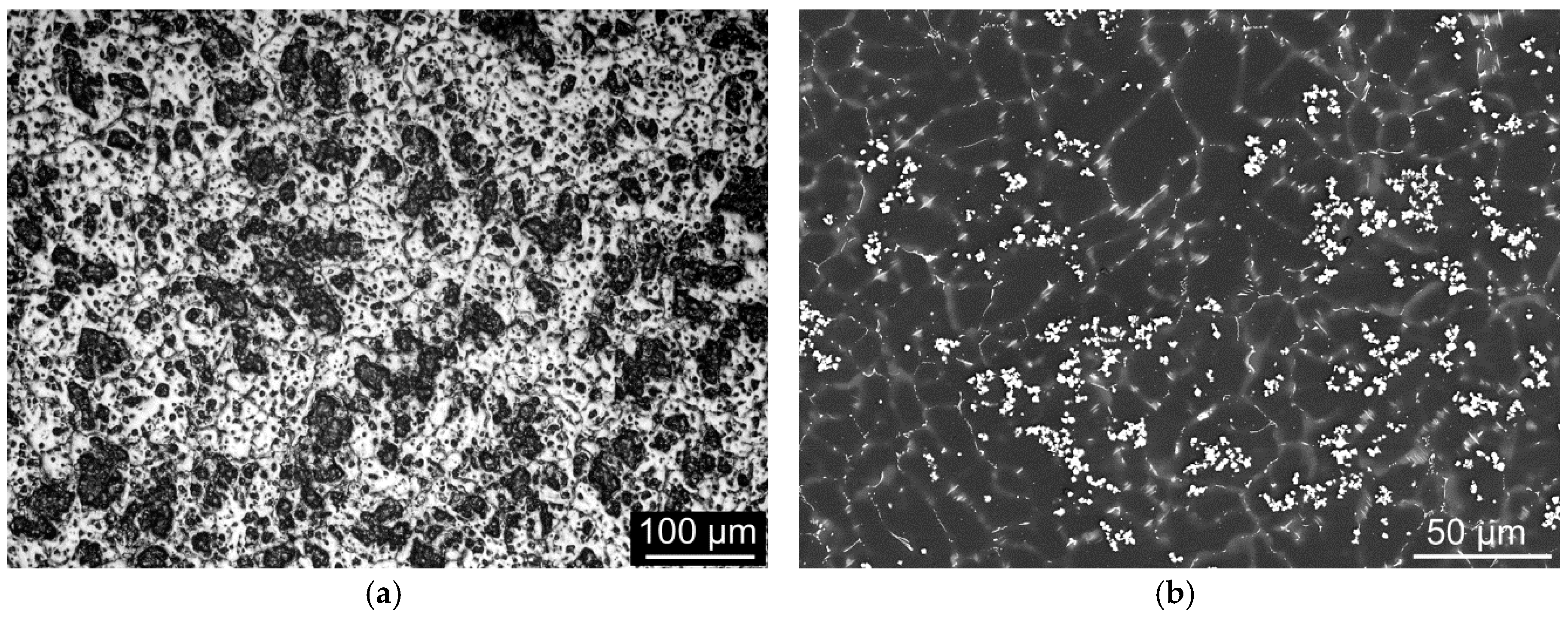
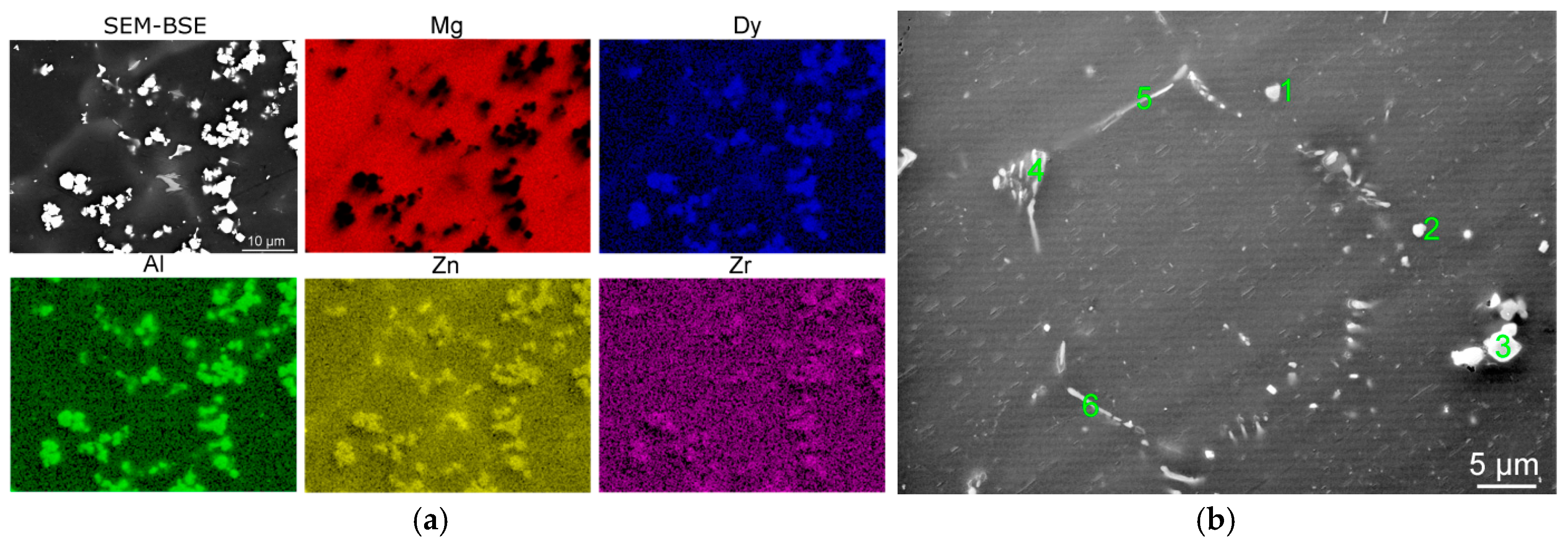
All of the annealing regimes caused homogenisation of the structure since the as-cast state obviously exhibited significant segregations on the grain boundaries and polygon-shaped precipitates (
Figure 1b). The segregations were identified as β-phase of the continuous type, while the polygon-shaped precipitates were rich in Dy and exhibited a mixture of various chemical compositions.
Heat treatment at 480 °C caused partial homogenisation of the structure. Although the temperature was too low for dissolution of the polygon-shaped Dy-rich precipitates (highly stable due to relatively high Dy melting point), the phase segregations along the grain boundaries dissolved the more the longer was the annealing time. The β-phase melting point was reported to be 437 °C [
48]. Therefore, at 480 °C, the grain boundary segregations dissolved to the solid solution and re-precipitated in the form of thin elongated β-phase particles, which can for this material be characterised as Mg
17(AlZnDy)
12. This significant change is also advantageous for mechanical properties since grain boundary segregations of brittle Mg
17(Al)
12-type phases have repeatedly been reported to deteriorate strength, as well as the plasticity of Mg-based alloys [
19,
48,
49]. Dy-rich phases usually precipitate from the SSS during ageing—when heat treated at temperatures higher than 300 °C for long time periods (sometimes in hundreds of hours) [
32,
44]. Nevertheless, increasing the annealing temperature to 480 °C together with decreasing the annealing time to 16 h had similar effects on the precipitation. Therefore, this particular heat treatment not only induced re-formation of strengthening precipitates, but also influenced the β-phase distribution and caused homogenisation of the structure (comparing to conventional ageing treatments at lower temperatures and longer time periods, which usually only have the first mentioned effect). The compositions of the Dy-rich precipitates corresponded to the Mg
3Dy phase, which had been reported to develop in Mg–Dy alloys subjected to annealing temperatures lower than 520 °C [
20]. However, the high amount of Al substituting Mg in these precipitates imparts their more precise determination to be (AlMg)
3Dy.
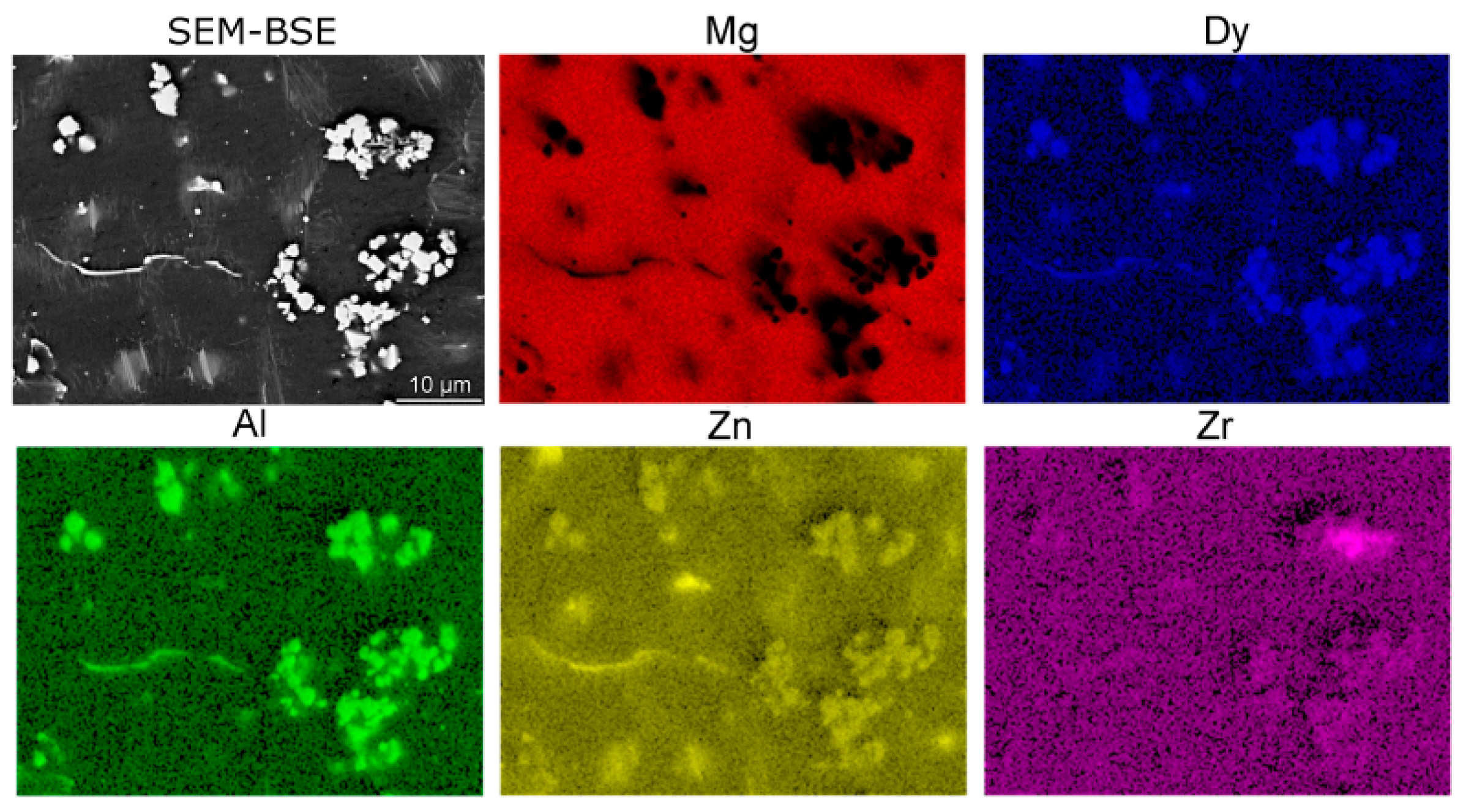
The samples treated at 520 °C did not exhibit any notable grain boundary segregation. When compared to 480 °C, the increased temperature caused the alloying elements to dissolve to the SS more intensively, which resulted in the formation of more homogenously distributed fine precipitates, as well as higher amounts of Zn and Dy dissolved in the SS. The dissolubility of Dy in Mg at 520 °C is 22 wt. % [
39]. However, rapid solidification introduced by quenching resulted in preserving the non-equilibrium conditions and the formation of phases and compositions slightly different from the assumptions made based on the phase diagrams [
50]. Dy was primarily present in the polygon-shaped precipitates with compositions very close to Mg
24(Dy)
5—i.e., Mg
24(DyZnAl)
5. According to Rokhlin [
20], the ideal temperature for the formation of Mg
24(DyZn)
5 precipitates is 561 °C. However, some of the particles were identified as a combination of phases, such as Mg
24(DyZnAl)
5 plus Mg
3Dy. Such combined precipitates occurred in locations with higher concentrations of Dy, which were not able to sufficiently dissolve and homogenise through diffusion due to the relatively low heat treatment temperature and time (found especially in the sample annealed for 8 h). Very fine elongated Dy-rich precipitates, the characterisation of which needed to be performed via TEM, were also found (e.g., location 3 in
Figure 4b). These precipitates, identified as the 18R-type LPSO phase, were especially found in the sample annealed for 16 h. The LPSO phases have been reported to develop in Mg–RE (Mg–Zn–RE) alloys subjected to heat treatments at higher temperatures and/or longer times, which provide the alloying elements with sufficient time and energy to perform full diffusion to stacking planes and form these phases [
45,
51,
52,
53]. Although LPSO phases were also observed in the samples annealed at 520 °C (e.g.,
Table 2), the samples heat treated at 560 °C exhibited their more notable formation (
Figure 9c).
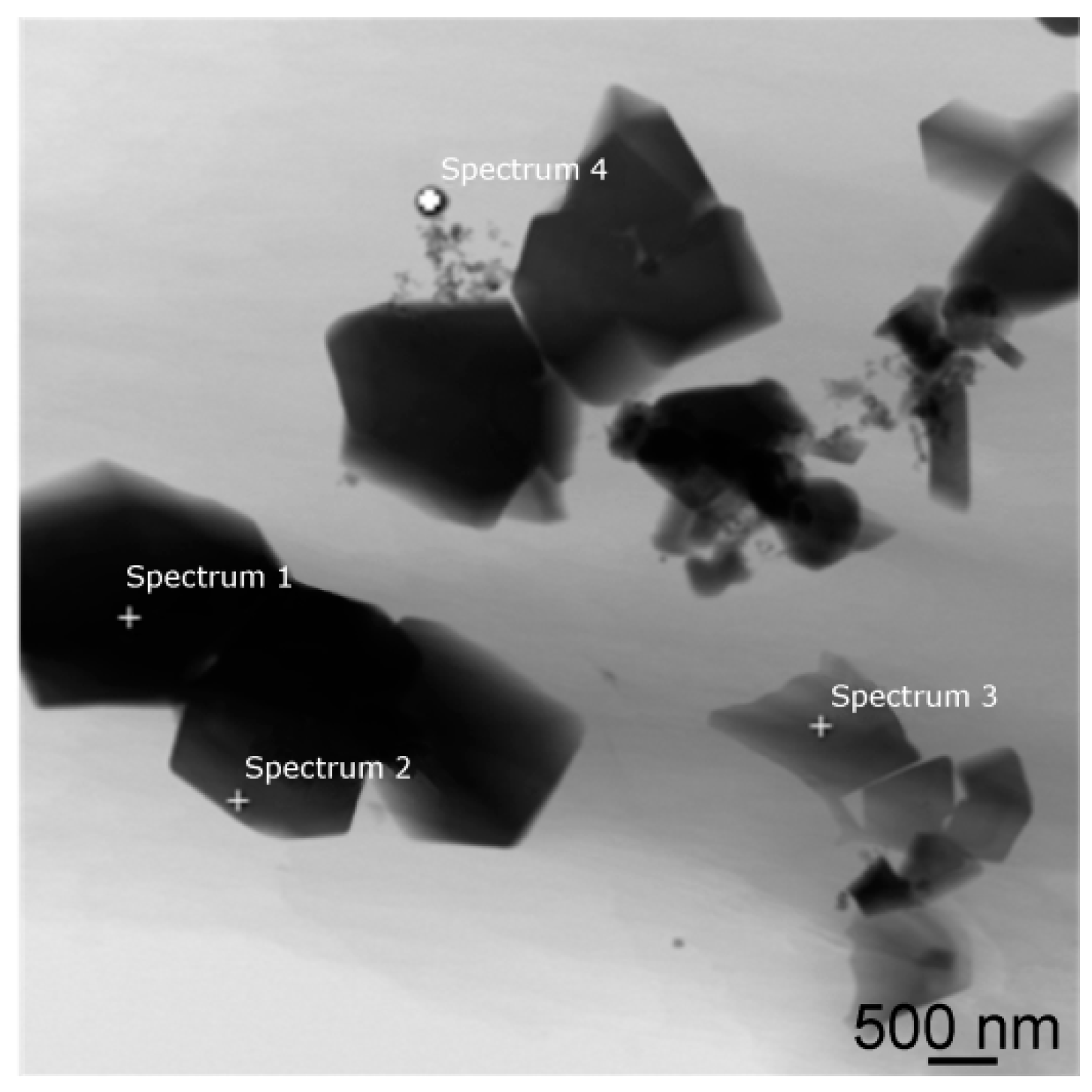
The structures annealed at 560 °C featured quite a significant presence of very fine polygon-shaped precipitates and thin elongated precipitates, identified as Mg
24Dy
5—more precisely Mg
24(DyZn)
5—phase (containing ~60 wt. % of Dy) and LPSO phases, respectively. Based on the works published by others [
20,
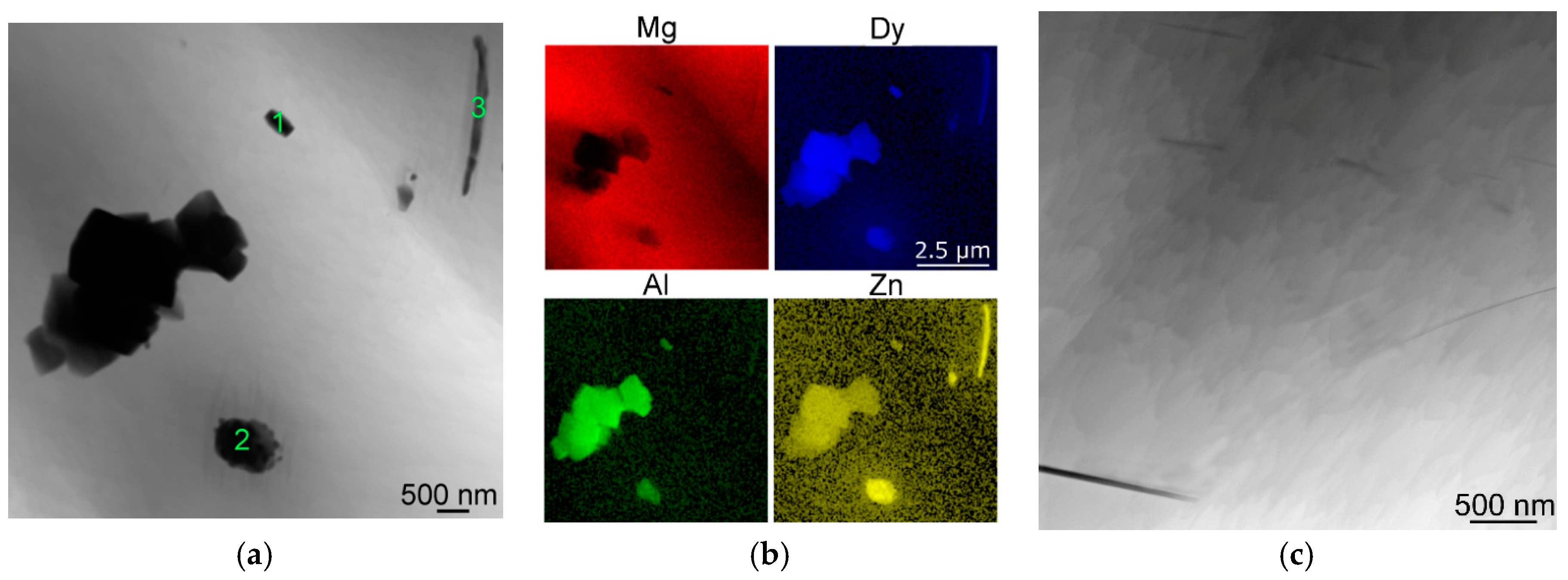
37], the maximum solubility of Dy in Mg is about 25 wt. % at 561 °C and decreases with decreasing temperature. Therefore, rapid cooling (quenching) from 560 °C resulted in preserving the highest amount of Dy in the matrix of all the investigated annealed samples (approx. 4.5 wt. %). However, the majority of this element was again found in the secondary phases. The 560 °C/16-h sample exhibited significant re-precipitation of the β-phase in the form of coarse plate-like structures (not observed after 8 h annealing), as well as the presence of Dy-rich precipitates and LPSO phases, differing in their morphology although having similar chemical composition—e.g., the morphology of the particles depicted in locations 2 and 3 in
Figure 9a pointed to their different nature. The more or less equiaxed-shape precipitate 2 had a cubic lattice corresponding to (Mg,Zn)
x Dy phase [
45]. On the other hand, the morphology and composition of the thin elongated precipitate 3 revealed the precipitate to be one of the hexagonal LPSO phases of the 18R type. These phases have been reported to significantly increase mechanical properties by creating obstacles for non-basal slip deformation mechanisms [
49].
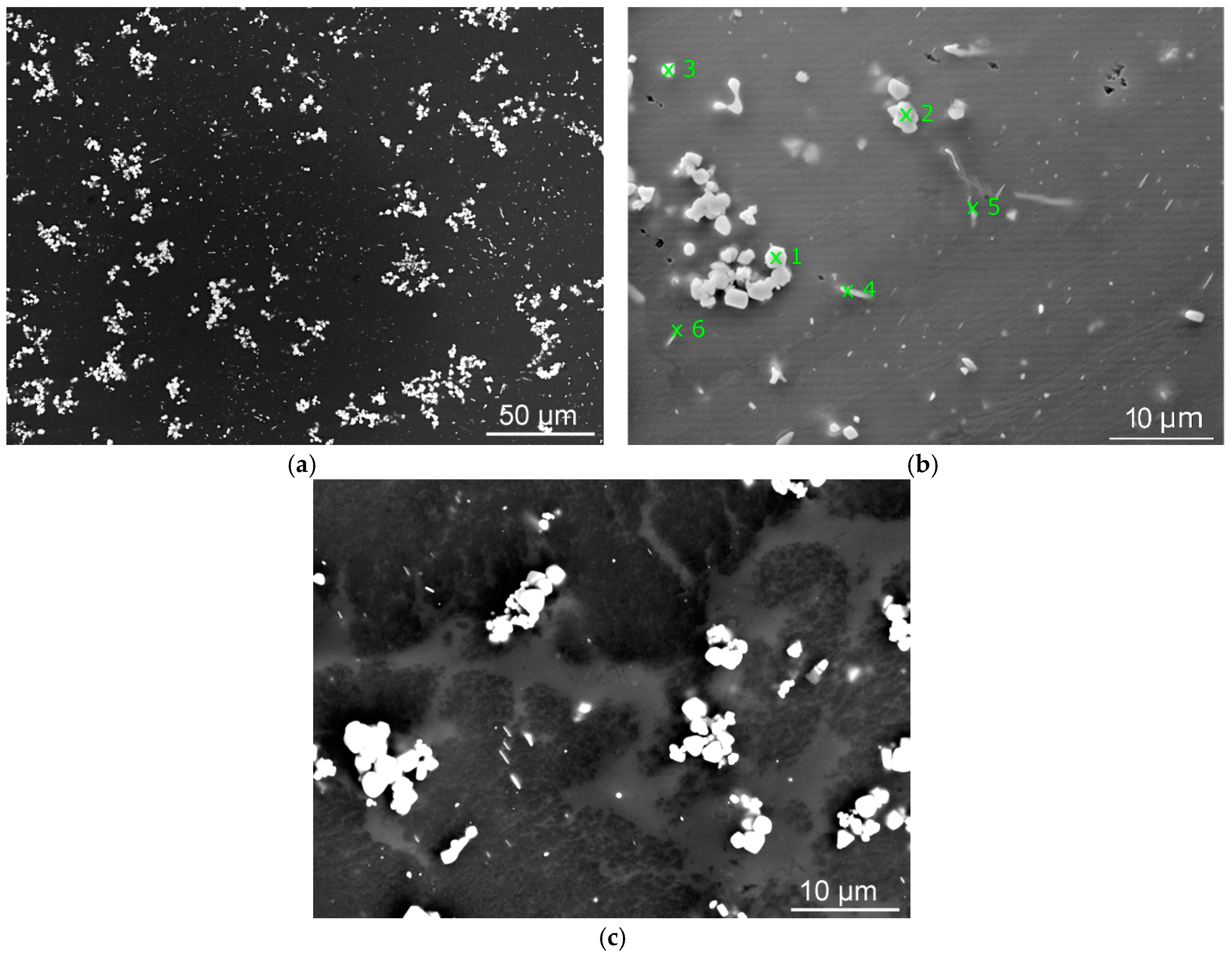
The average volume of the fine precipitates in the 480 °C/8-h sample was 4%. This corresponds to the fact that most of the alloying elements were still conserved in the grain boundary segregations formed during casting and did not have sufficient energy to re-precipitate. On the other hand, the longer annealing time of 16 h caused the volume of precipitates to increase to 5.8%. This was mostly due to the better dissolution of the continuous β-phase. The average precipitate size for this annealing temperature was the highest of all those investigated due to the insufficient dissolution and aggravated diffusion. The precipitates were mostly fragments of those that had formed during casting and subsequent quenching. The activation energy was insufficient for the diffusion to proceed effectively.
The samples that were annealed at 520 °C exhibited an increase in the presence of precipitates; however, their average size decreased slightly. The higher annealing temperature provided sufficient energy for the β-phase to dissolve and supported the diffusion process. The polygon-shaped precipitates re-precipitated as well and their chemical composition changed slightly when compared to the 480 °C samples. Nevertheless, the temperature and time were still not sufficient for the elements to dissolve into the SS to a greater extent and subsequent quenching caused precipitation of various phases from the SSS. On the other hand, most of the coarse precipitates formed during cooling after casting dissolved and subsequently the formation of new fine precipitates from the SSS occurred during quenching. For this reason, the precipitates refined and their average size was smaller than for the 480 °C treated samples.
Samples annealed at 560 °C exhibited a decrease in the presence and size of precipitates; Dy was dissolved in the solid solution in the greatest extent. The volume of precipitates in the 560 °C/8-h sample was slightly higher than in the 560 °C/16-h sample (5.8% compared to 5%); however, the 16 h’ sample exhibited re-precipitation of the continuous β-phase, which was neglected during these analyses. The average precipitate size in both the samples was approximately 1 µm, which was the smallest of all the investigated states. This was caused by the annealing temperature, which provided all the alloying elements with favourable solubility, as well as simplified diffusion. These phenomena finally contributed to the formation of very fine precipitates during subsequent quenching.
As reported by Rokhlin [
20], (micro)hardness measurement is an advantageous method to evaluate structural changes in Mg–RE alloys. For all the samples, the differences between both the measured diagonals were negligible. However, all of the samples exhibited variations along the measured directions. The 480 °C/8-h sample exhibited the largest variation in HV values along the measured diagonals. This phenomenon was caused by the inhomogeneous precipitate distribution and their occurrence in clusters (as already mentioned, the observed precipitates were mostly fragments of the larger particles developed after casting, a significant portion of the alloying elements was still conserved in the β-phase at the original grain boundaries); the large precipitates mostly cracked when loaded by the indenter and caused local brittleness. The average microhardness increased slightly for the sample annealed for 16 h, in which the hard and brittle β-phase dissolved significantly. However, the precipitates were still too large to contribute substantially to the mechanical properties (similar effect as over-ageing [
20]). The 520 °C samples exhibited more uniform HV0.2 distribution due to the more or less homogeneous distribution of finer precipitates. Precipitation of the alloying elements in fine Dy-rich polygon-shaped and the β-phase fine elongated particles increased the microhardness similarly as optimised ageing treatment [
41]. However, the sample that was annealed for 16 h exhibited a slight decrease in HV values. The increased annealing time resulted in the dissolution of a portion of the alloying elements into the SS, which decreased microhardness, but would most probably slightly increase the strength and improve the plastic properties. An increase in the annealing temperature to 560 °C caused a slight decrease in the microhardness for the 8-h sample—due to further dissolution of the alloying elements—and an increase for the 16-h sample. This ensues from the nature of the structures. While the 560 °C/8-h sample exhibited fine and quite homogeneously distributed precipitates, the 16-h sample featured plate-like β-phase precipitates that were very hard but also very brittle [
52].
Based on the results, the 560 °C/8-h heat treatment regime would most probably impart the most advantageous mechanical properties (strength, as well as plasticity) due to the favourable size and homogeneous distribution of precipitates, as well as the presence of fine LPSO phases. A deeper study of the mechanical properties imparted by this heat treatment regime and the precipitation kinetics is planned for a consequent publication.