Production of Clean Steel Using the Nitrogen Elevating and Reducing Method
Abstract
:1. Introduction
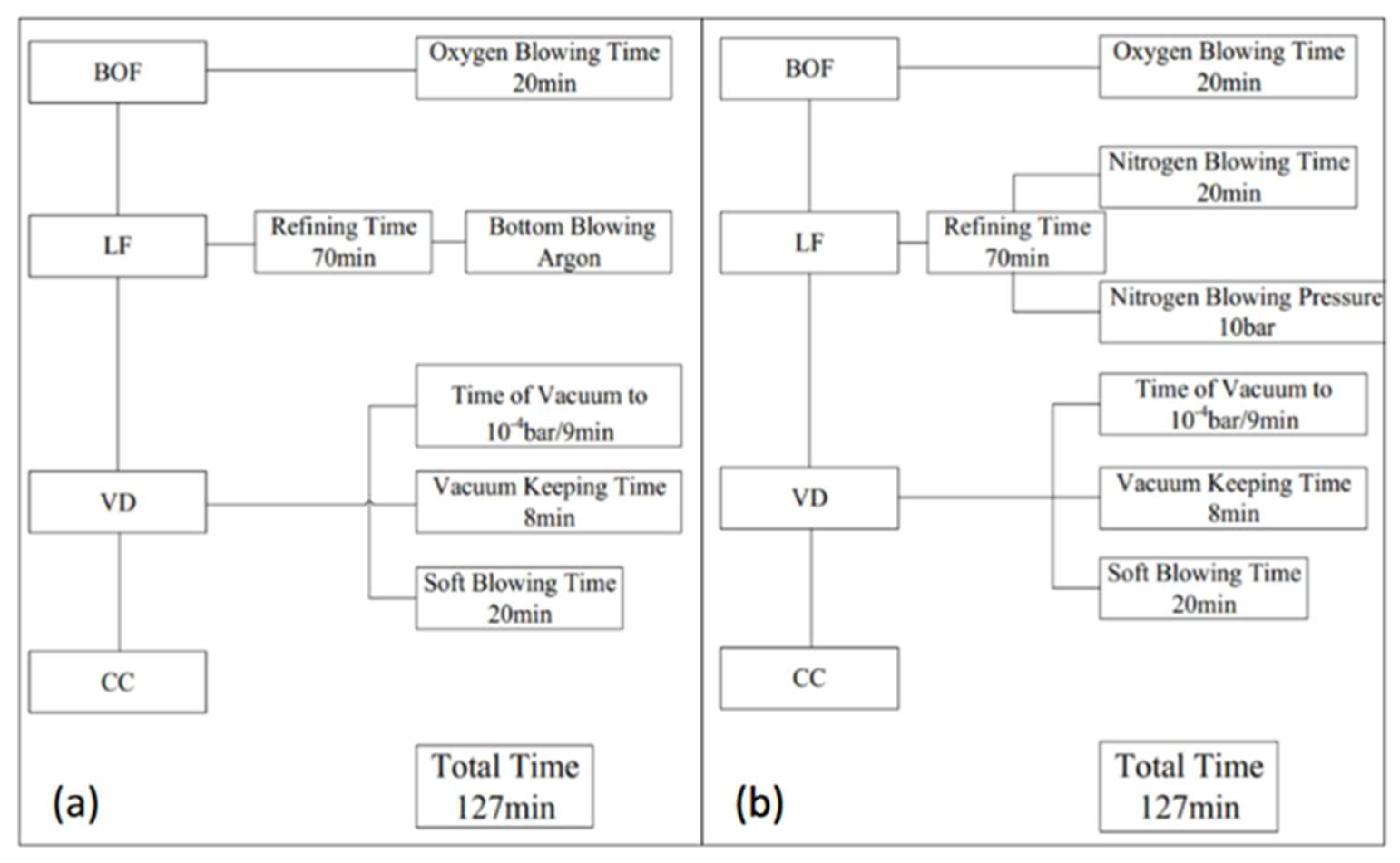
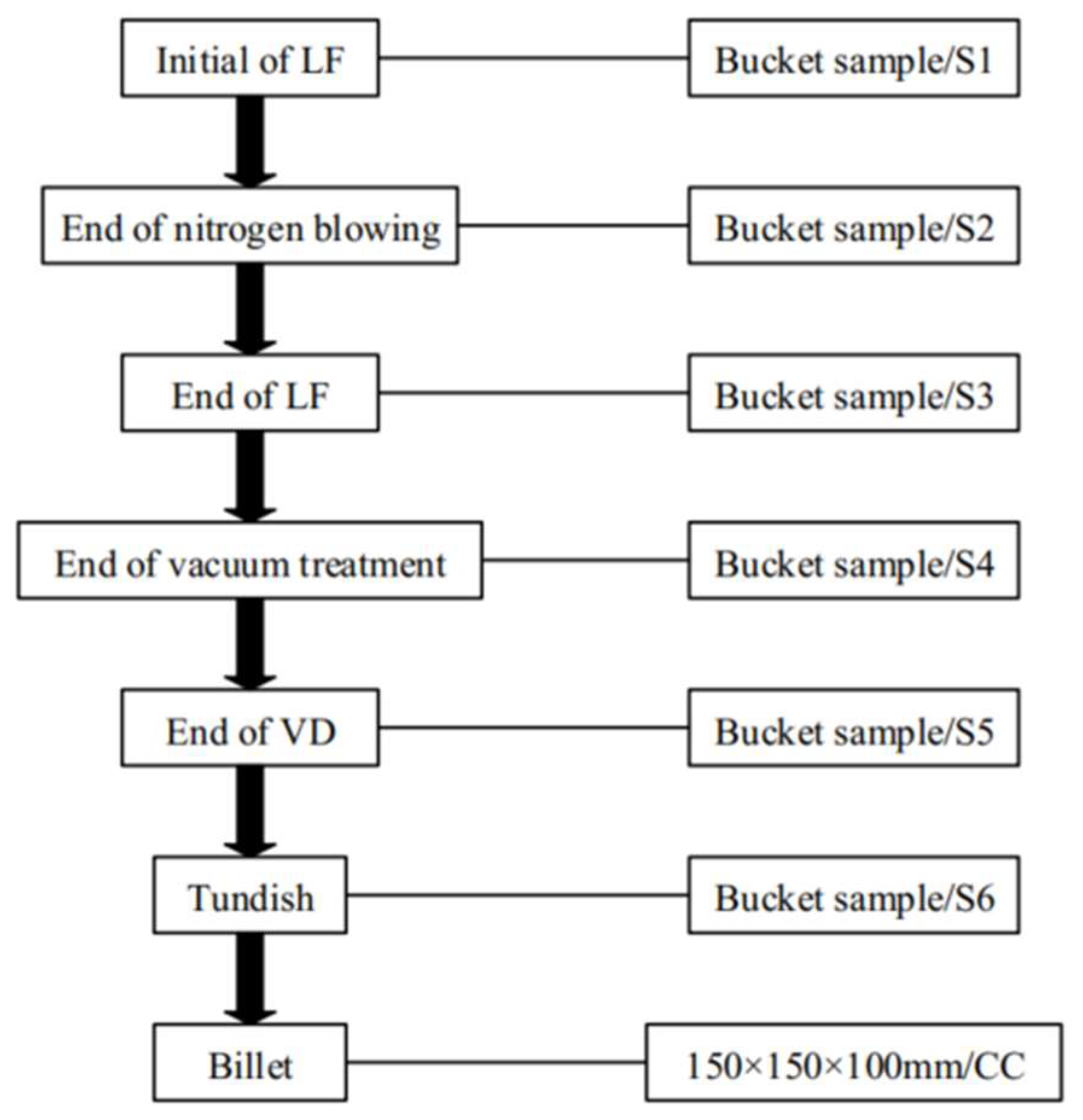
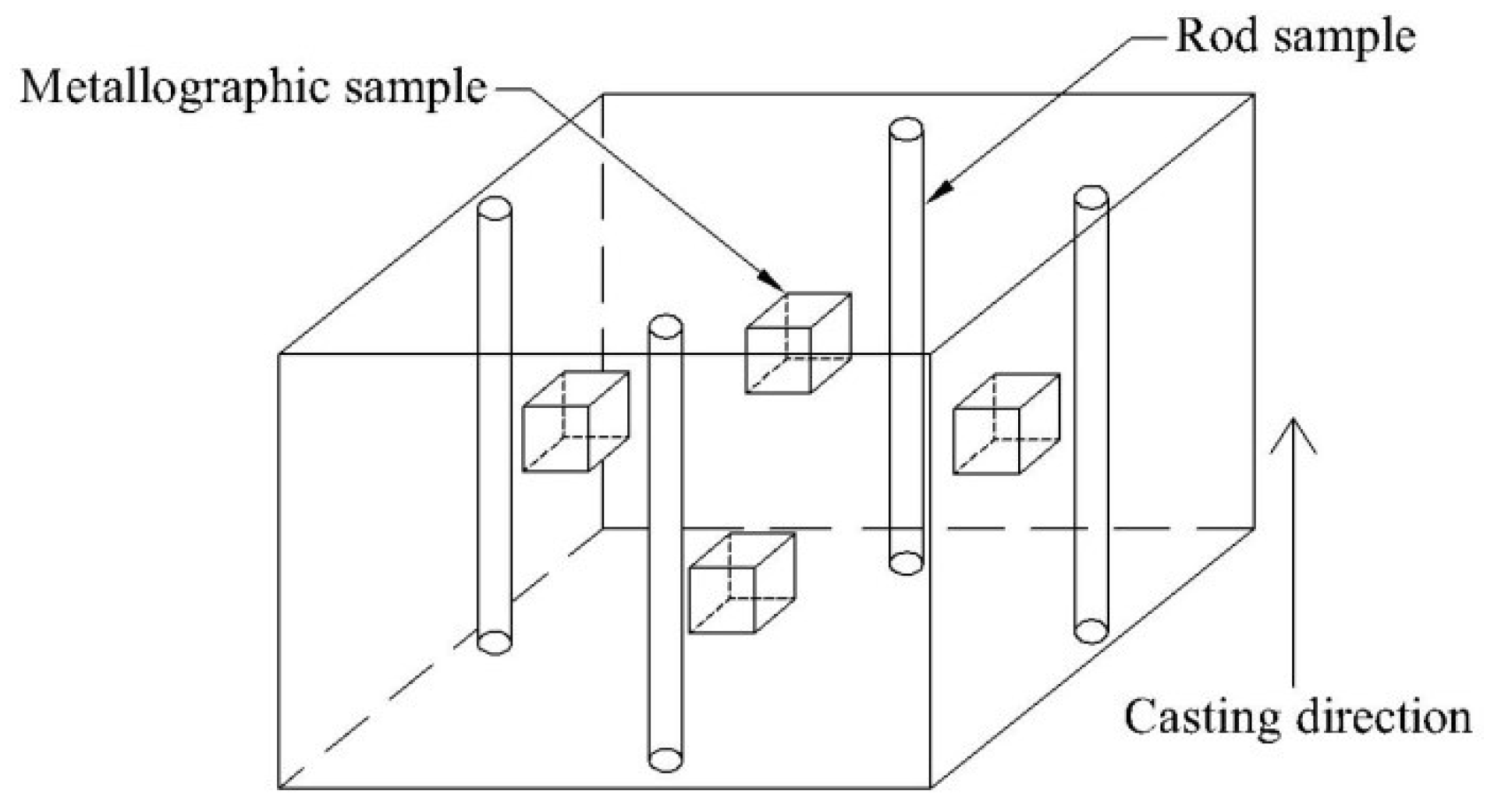
2. Industrial Experiments
3. Results
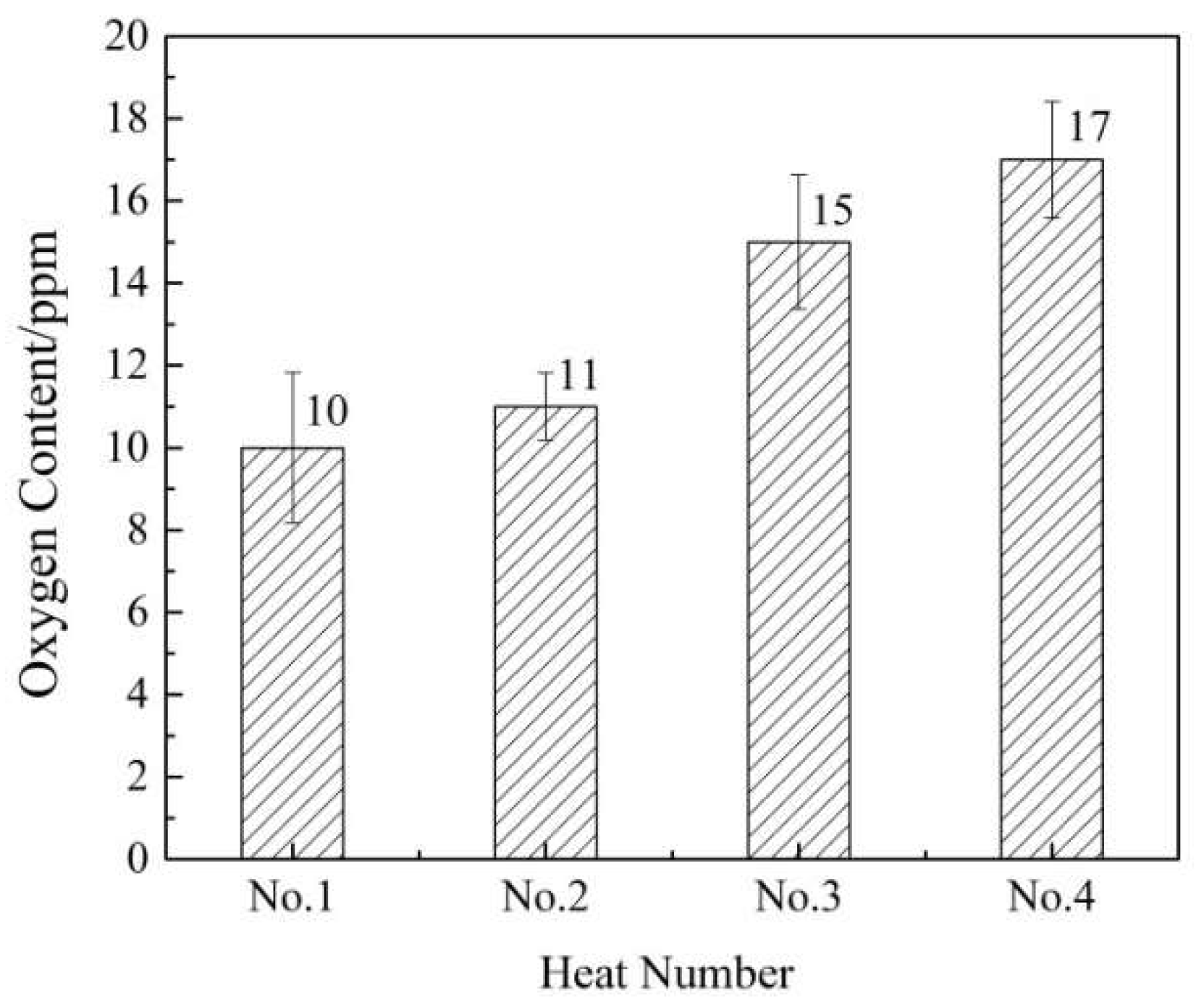
3.1. Changes of Nitrogen/Oxygen Content in Steel
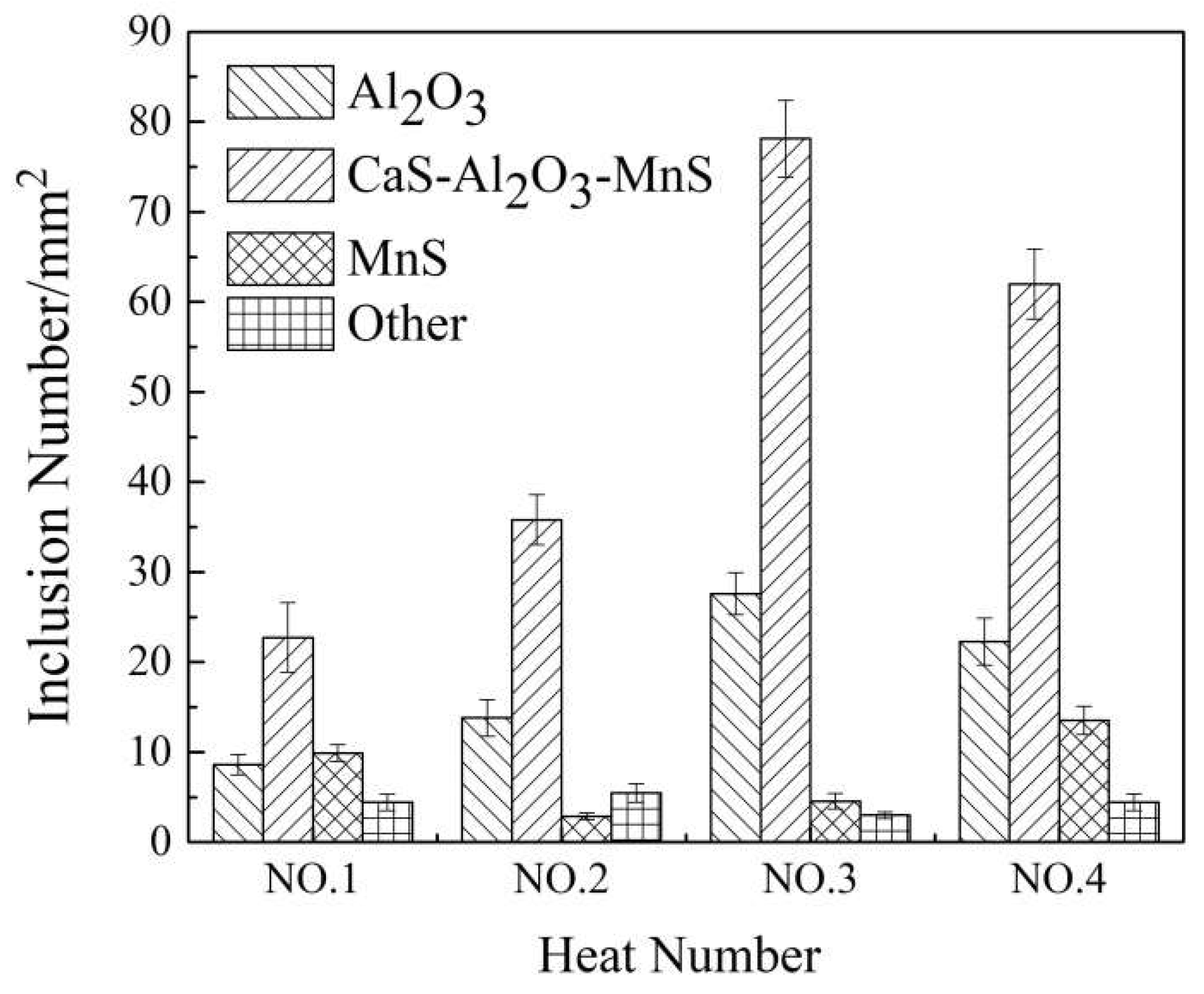
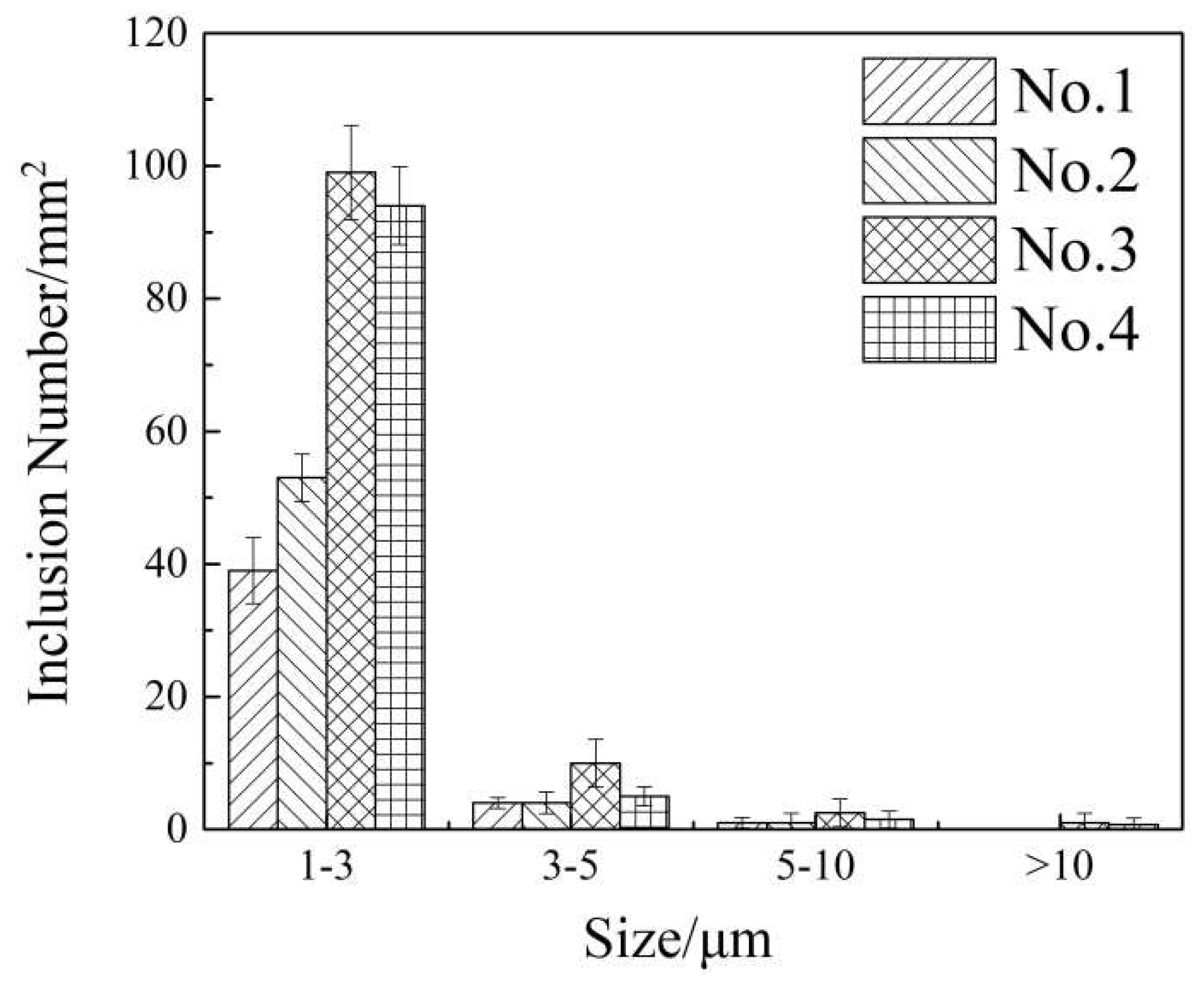
3.2. The Type and Morphology of Inclusions in Billets
3.3. Effect of Inclusion Removal
4. Discussion
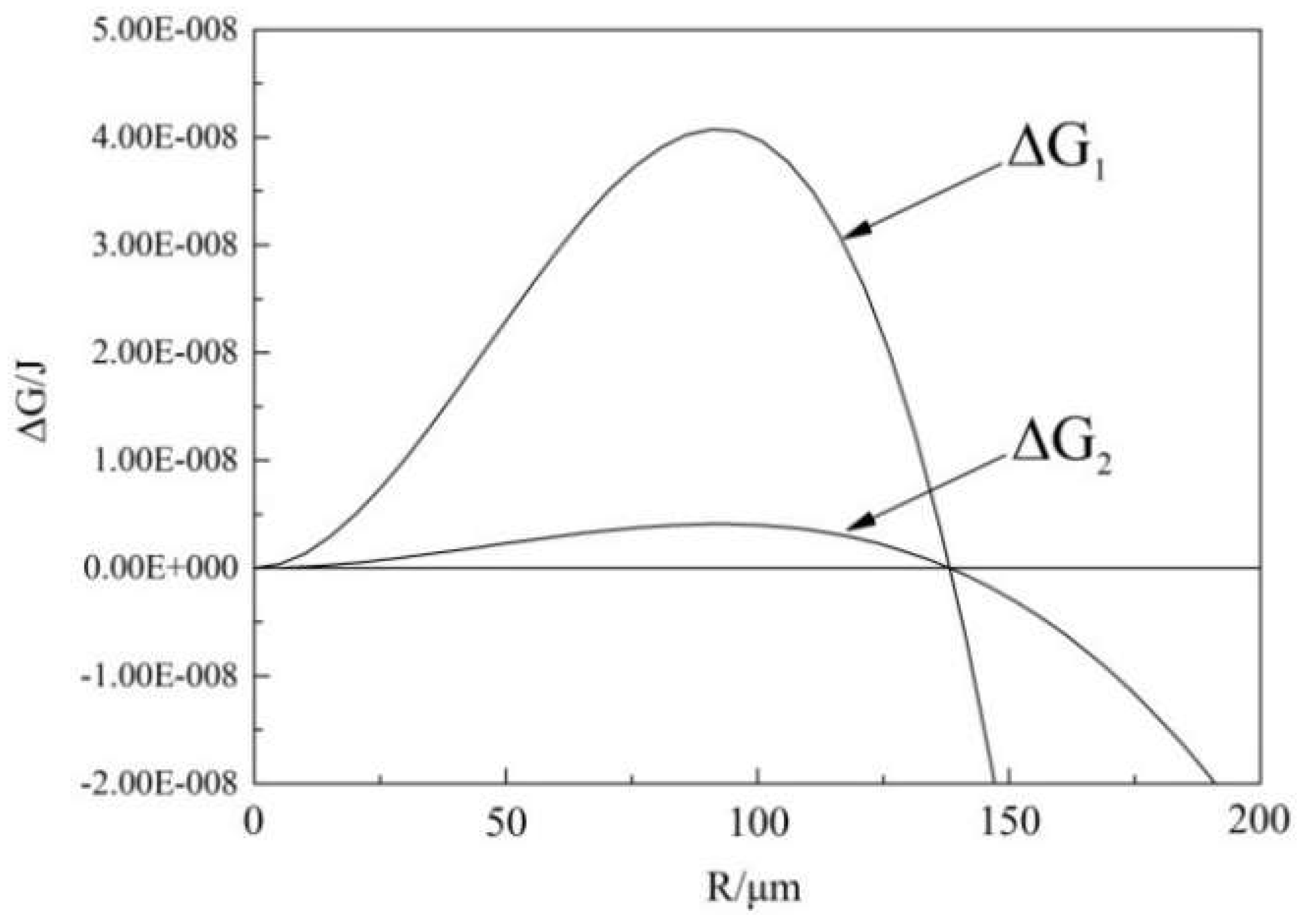
4.1. The Effect of Nitrogen Content on Depth Range of Bubble Nucleation
4.2. Effect of Nitrogen Content on Bubble Nucleation Rate
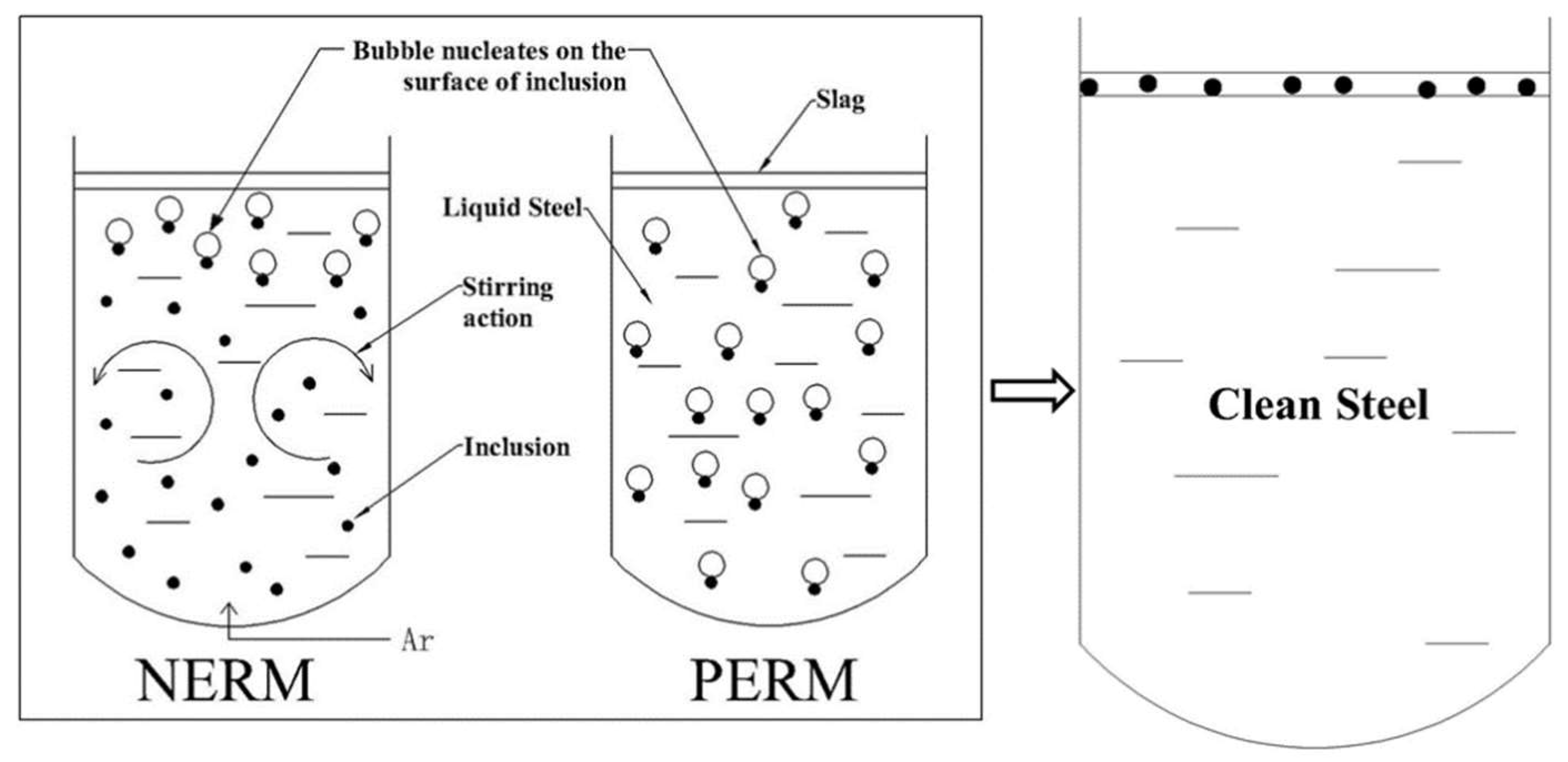
4.3. The Mechanism of the Pressure Elevating and Reducing Method (PERM) and NERM
5. Conclusions
- (1)
- Using NERM can remove the inclusions in steel effectively in actual industrial production. Compared with conventional method, the amount of inclusions was reduced by 52.9% by NERM. In addition, the number of inclusions smaller than 3 μm in billet was reduced significantly, and the inclusions larger than 10 μm were completely removed.
- (2)
- The average oxygen content in billets was 10.5 ppm after the treatment of NERM. The average oxygen content in billets was 16 ppm using conventional method. The average oxygen content of the billets decreased by 34.38%.
- (3)
- The increase of nitrogen content in molten steel reduces the difficulty of bubble nucleation. The number and the density of bubbles increase with the increase of nitrogen content, which can improve the removal effect of inclusions.
- (4)
- Bubbles can only nucleate in the upper part of the molten steel by NERM. However, the argon gas was blown from the bottom of the molten steel in the vacuum process, so that the molten steel in the lower part of the ladle move upwards. The inclusions contained in this part of molten steel also move to the upper part of the molten steel; bubbles can nucleate on the surface of them and float to the top, while bubbles can nucleate within the full depth of molten steel using PERM.
- (5)
- NERM has the advantage that molten steel can be treated under normal or low pressures with the existing processes in a steel plant without retrofitting equipment or increasing the process time. NERM is more applicable.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, L.H.; Huo, J.S. Concrete-filled hollow structural steel columns after exposure to ISO834 fire standard. J. Struct. Eng.-Asce. 2003, 129, 68–78. [Google Scholar] [CrossRef]
- Naser, M.Z.; Kodur, V.K.R. Comparative fire behavior of composite girders under flexural and shear loading. Thin Wall. Struct. 2017, 116, 82–90. [Google Scholar] [CrossRef]
- Naser, M.Z.; Chehab, A.I. Materials and design concepts for space-resilient structures. Prog. Aerosp. Sci. 2018, 98, 74–90. [Google Scholar] [CrossRef]
- Jyri, O.; Pentti, M. Mechanical properties of structural steel at elevated temperatures and after cooling down. Fire Mater. 2010, 28, 237–251. [Google Scholar]
- International Iron and Steel Institute. State of the Art and Process Technology in Clean Steelmaking; International Iron and Steel Institute: Beijing, China, 2006. [Google Scholar]
- Xu, Z. Secondary Refining; Metallurgical Industry Press: Beijing, China, 1994. [Google Scholar]
- Gao, H.C.; Yan, G.F. Problems about process theory in production pf clean steel by electromagnetic stirring in ladle. Res. Iron Steel 2003, 3, 14–18. [Google Scholar]
- Miki, Y.; Kitaoka, H.; Bessho, N. Inclusion separation from molten steel in tundish with rotating electromagnetic field. Tetsu Hagane 2009, 82, 498–503. [Google Scholar] [CrossRef]
- Nakanishi, K. Japanese State of the art continuous casting process. Trans. Iron Steel Inst. Jpn. 2007, 36, S14–S17. [Google Scholar] [CrossRef]
- Zhan, D.P.; Song, J.X.; Jiang, Z.H. Application effect of electromagnetic brake in continuous casting mold. China Metall. 2006, 16, 23–25. [Google Scholar]
- Mazumdar, D.; Guthrie, R.I.L. The physical and mathematical modelling of gas stirred ladle systems. ISIJ Int. 1995, 35, 1–20. [Google Scholar] [CrossRef]
- Wang, L.; LEE, H.G.; Hayes, P. A new approach to molten steel refining using fine gas bubbles. ISIJ Int. 1996, 36, 17–24. [Google Scholar] [CrossRef]
- Zheng, X.; Hayes, P.C.; Lee, H.G. Particle removal from liquid phase using fine gas bubbles. ISIJ Int. 1997, 37, 1091–1097. [Google Scholar] [CrossRef]
- Zhan, S.H.; Wang, J.J.; Qiu, S.T.; Xiao, Z.Q.; Gan, Y. Flow control and inclusion removal in continuous casting tundish with bottom gas blowing. J. Anhui Univ. Technol. 2006, 23, 367–372. [Google Scholar]
- Turkdogan, E.T. Novel concept of cleansing liquid steel of solid oxide inclusions by cullet injection in ladle. Ironmak. Steelmak. 2004, 31, 131–134. [Google Scholar] [CrossRef]
- Matsuno, H.; Kikuchi, Y.; Komatsu, M. Development of a new deoxidation technique for rh degassers. Iron Steelmak. 1993, 20, 35–38. [Google Scholar]
- Bai, X.Q.; Li, D.H.; Zhang, L. Ultrasonic separation of inclusions from flow liquid. Acta. Metall. Etall. Sin. 2002, 38, 483–489. [Google Scholar]
- Li, K.W.; Liu, J.H.; Zhou, J.B. Micro non-metallic inclusion removal from molten steel with gas bubbles generated by the nitrogen absorbing and releasing method. Chin. J. Eng. 2015, 37, 1124–1129. [Google Scholar]
- Wang, L.; LEE, H.G.; Hayes, P. Prediction of the optimum bubble size for inclusion removal from molten steel by flotation. ISIJ Int. 1996, 36, 7–16. [Google Scholar] [CrossRef]
- Bessho, N.; Yamasaki, H.; Fujii, T. Removal of inclusion from molten steel in continuous casting tundish. ISIJ Int. 1992, 32, 157–163. [Google Scholar] [CrossRef]
- Wang, L.T.; Zhanq, Q.Y.; Peng, S.H. Fundamental of inclusion removal from molten steel by rising bubble. J. Iron Steel Res Int. 2004, 11, 5–9. [Google Scholar]
- Zhu, M.Y.; Xiao, Z.Q. Physical and Mathematical Simulation of Steel Refining Process; Metallurgical Industry Press: Beijing, China, 1998. [Google Scholar]
- Zhang, L.F.; Taniguchi, S. Fundamentals of inclusion removal from liquid steel by bubble flotation. Int. Mater. Rev. 2000, 45, 59–82. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Michelic, S.K. Characterization and analysis of non-metallic inclusions in low-carbon Fe–Mn–Si–Al TWIP steels. Steel Res. Int. 2016, 87, 1723–1732. [Google Scholar] [CrossRef]
- Han, Z.J.; Lin, P.; Liu, L.; Cui, J.Y.; Zhou, D.G. Thermodynamics of calcium treatment for 20CrMnTiH1. Iron Steel 2007, 9, 32–36. [Google Scholar]
- Diederichs, R.; Bleck, W. Modelling of manganese sulphide formation during solidification, part I: Description of mns formation parameters. Steel Res. Int. 2006, 77, 202–209. [Google Scholar] [CrossRef]
- Turkdogan E, T. Fundamentals of Steelmaking; The Institute of Materials: London, UK, 1996. [Google Scholar]
- Li, K.W.; Liu, J.H.; Zhang, J. Theoretical analysis of bubble nucleation in molten steel supersaturated with nitrogen or hydrogen. Metall. Mater. Trans. B 2017, 48, 2136–2146. [Google Scholar] [CrossRef]
- Huang, X.H. Principal of Iron and Steel Metallurgy, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Szekely, J.; Martins, G.P. Non-equilibrium effects in the growth of spherical gas bubbles due to solute diffusion. Chen. Eng. Sci. 1971, 26, 147–159. [Google Scholar] [CrossRef]
- Turnbull, D. Formation of crystal nuclei in liquid metals. J. Appl. Phys. 1950, 21, 1022–1028. [Google Scholar] [CrossRef]
- Cui, Z.Y. Metallography and Heat Treatment; Harbin Institute of Technology Press: Harbin, China, 1998. [Google Scholar]
- Tiller, W.A.; Jackson, K.A.; Rutter, J.W. The redistribution of solute atoms during the solidification of metals. Acta Metal. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Xue, Z.L.; Wang, Y.F.; Wang, L.T. Inclusion removal from molten steel by attachment small bubbles. Acta Metall. Sin. 2003, 39, 431–434. [Google Scholar]





| Element | C | Si | Mn | P | S | Cr | Als |
|---|---|---|---|---|---|---|---|
| % | 0.208 | 0.225 | 0.557 | 0.016 | 0.005 | 0.907 | 0.02 |
| Element | CaO | Al2O3 | SiO2 | MgO | Other |
|---|---|---|---|---|---|
| % | 50.91 | 30.81 | 9.75 | 4.92 | 3.61 |
| Parameters | ||||
|---|---|---|---|---|
| Values | 8.314 | 7000 | 1.5 [30] | 28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, J.; Yu, S.; Dong, D.; Wang, G.; Li, S. Production of Clean Steel Using the Nitrogen Elevating and Reducing Method. Metals 2018, 8, 560. https://doi.org/10.3390/met8070560
Zhang J, Liu J, Yu S, Dong D, Wang G, Li S. Production of Clean Steel Using the Nitrogen Elevating and Reducing Method. Metals. 2018; 8(7):560. https://doi.org/10.3390/met8070560
Chicago/Turabian StyleZhang, Jie, Jianhua Liu, Saijian Yu, Daxi Dong, Gongliang Wang, and Shiqi Li. 2018. "Production of Clean Steel Using the Nitrogen Elevating and Reducing Method" Metals 8, no. 7: 560. https://doi.org/10.3390/met8070560




