Antibacterial and Antibiofilm Activities of Makaluvamine Analogs
Abstract
:1. Introduction
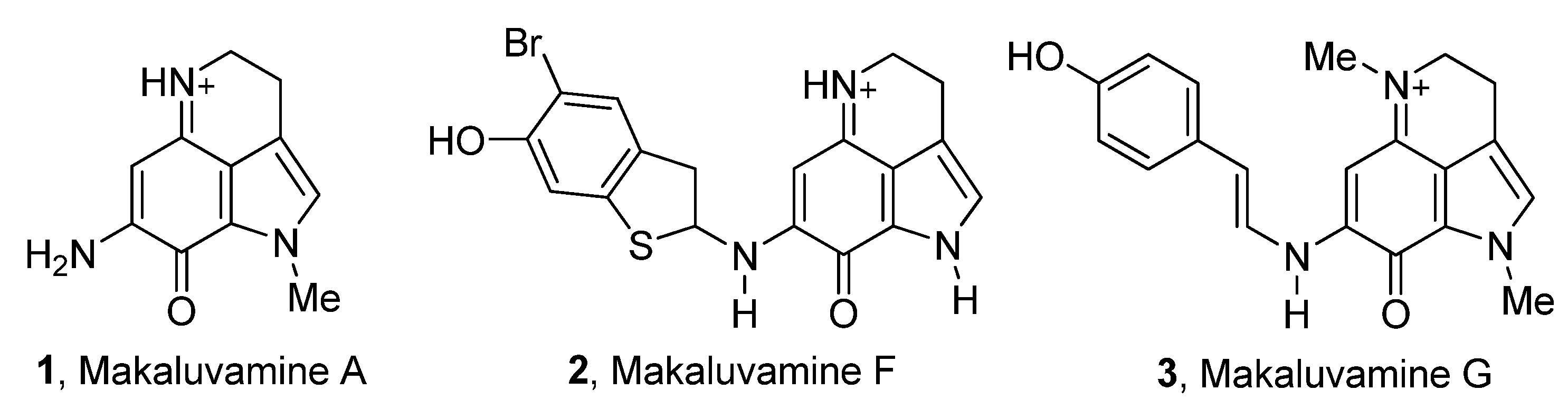
2. Experimental Section
2.1. Synthesis of Makaluvamine Analogs
2.1.1. General Considerations
2.1.2. General Procedure for Amination
2.1.3. General Procedure for Detosylation Using NaOMe
2.2. Biological Assays
2.2.1. Bacterial Strains, Culture Conditions and Chemicals
2.2.2. S. Mutans Biofilm Inhibition Assay
2.2.3. S. Mutans Growth Inhibition Assay
3. Results and Discussion
3.1. Synthesis of Makaluvamine Analogs

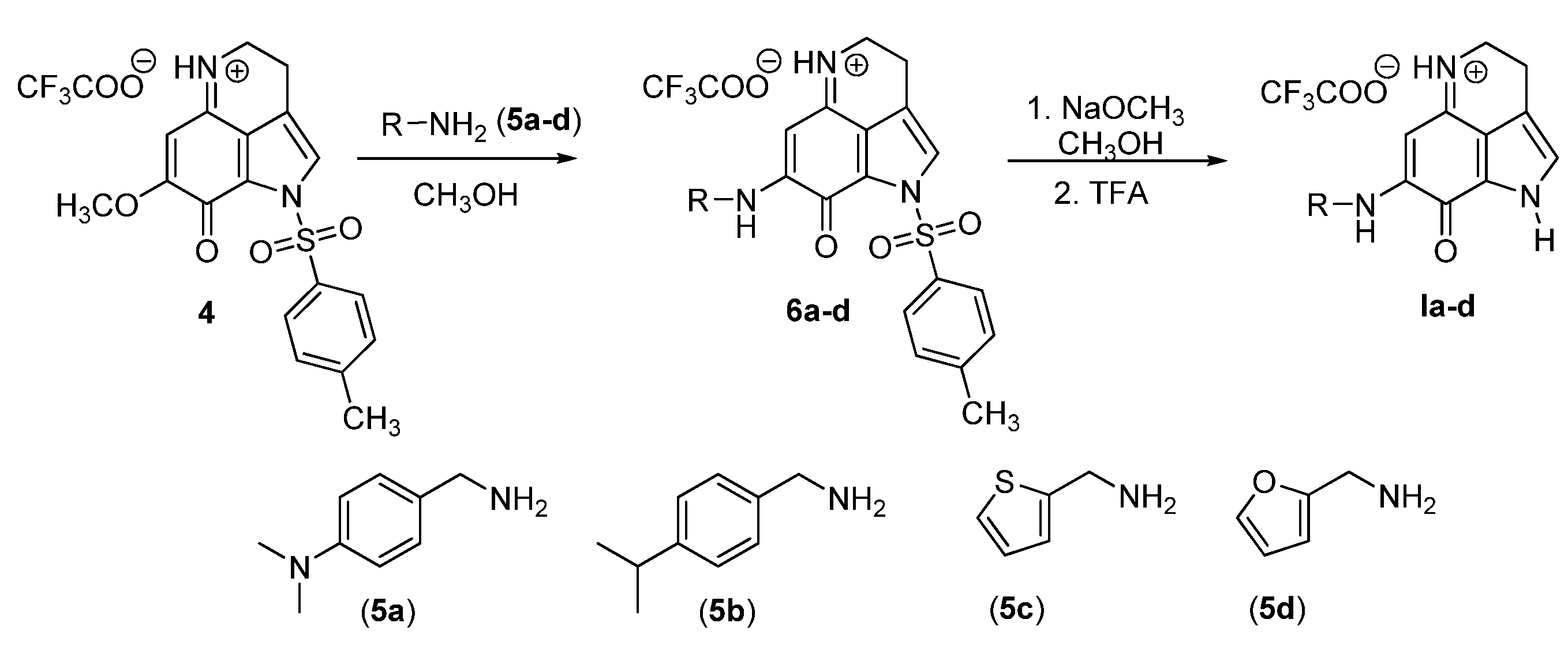
3.2. Biological Evaluation
 | |||||
|---|---|---|---|---|---|
| Entry | Compound No. | R1 | R2 | Antibiofilm Activity IC50 (μM) a | S. mutans MIC50 (μM) b |
| 1 | Ia |  | H | 38.3 ± 3.6 | 32.6 ± 0.1 |
| 2 | Ib |  | H | 13.5 ± 0.8 | 23.1 ± 0.2 |
| 3 | Ic |  | H | 30.9 ± 4.6 | 34.2 ± 0.8 |
| 4 | Id |  | H | 84.7 ± 7.5 | 63.9 ± 13.6 |
| 5 | Ie | 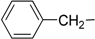 | H | 22.4 ± 2.6 | 28.1 ± 0.4 |
| 6 | If |  | H | 13.9 ± 1.7 | 24.4 ± 0.2 |
| 7 | Ig |  | H | 30.4 ± 7.6 | 36.3 ± 0.8 |
| 8 | Ih | 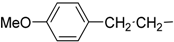 | H | 70.1 ± 1.0 | 68.0 ± 4.1 |
| 9 | Ii |  | H | 88.2 ± 7.6 | 111.6 ± 27.4 |
| 10 | IIa |  | 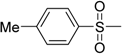 | 16.3 ± 3.4 | 26.4 ± 0.6 |
| 11 | IIb |  |  | 2.5 ± 0.1 | 22.7 ± 0.6 |
| 12 | IIc | 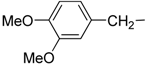 |  | 2.6 ± 0.1 | 28.0 ± 1.9 |
| 13 | IId | 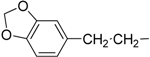 |  | 0.4 ± 0.1 | 1.7 ± 0.1 |
| 14 | IIe |  |  | 2.0 ± 0.1 | 5.8 ± 2.8 |
| 15 | 2A4 c | 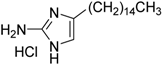 | 0.94 ± 0.02 | 2.0 ± 0.5 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Bowden, G.H.; Li, Y.H. Nutritional influences on biofilm development. Adv. Dent. Res. 1997, 11, 81–99. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Rozen, R.; Steinberg, D.; Bachrach, G. Streptococcus mutans fructosyltransferase interactions with glucans. FEMS Microbiol. Lett. 2004, 232, 39–43. [Google Scholar] [CrossRef]
- Lenander-Lumikari, M.; Loimaranta, V. Saliva and dental caries. Adv. Dent. Res. 2000, 14, 40–47. [Google Scholar] [CrossRef]
- Onisi, M. Streptococcus mutans, caries producing bacteria, and mecanism of its infection. Kokubyo Gakkai Zasshi. 1971, 38, 217–234. [Google Scholar] [CrossRef]
- Beighton, D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 2005, 33, 248–255. [Google Scholar] [CrossRef]
- Dye, B.A.; Tan, S.; Smith, V.; Lewis, B.G.; Barker, L.K.; Thornton-Evans, G.; Eke, P.I.; Beltran-Aguilar, E.D.; Horowitz, A.M.; Li, C.H. Trends in Oral Health Stzatus: United States, 1988–1994 and 1999–2004; U.S. Department of Health and Human Services: Washington, DC, USA, 2007. [Google Scholar]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial interactions in dental biofilm development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Edwardsson, S. The caries-inducing property of variants of Streptococcus mutans. Odontol. Rev. 1970, 21, 153–157. [Google Scholar]
- Hamada, S.; Slade, H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar]
- Biral, R.R.; Bertolini, P. Bacteriologic identification of caries-inducing streptococci (Streptococcus mutans). Rev. Bras. Odontol. 1970, 27, 291–298. [Google Scholar]
- Bradshaw, D.J.; Lynch, R.J. Diet and the microbial aetiology of dental caries: New paradigms. Int. Dent. J. 2013, 63 (Suppl 2), 64–72. [Google Scholar] [CrossRef]
- Durso, S.C.; Vieira, L.M.; Cruz, J.N.; Azevedo, C.S.; Rodrigues, P.H.; Simionato, M.R. Sucrose Substitutes Affect the Cariogenic Potential of Streptococcus mutans Biofilms. Caries Res. 2014, 48, 214–222. [Google Scholar]
- Hada, L.S.; Kakiuchi, N.; Hattori, M.; Namba, T. Identification of antibacterial principles against Streptococcus mutans and inhibitory principles against glucosyltransferase from the seed of Areca catechu L. Phytother. Res. 1989, 3, 140–144. [Google Scholar] [CrossRef]
- Ooshima, T.; Fujiwara, T.; Takei, T.; Izumitani, A.; Sobue, S.; Hamada, S. The caries inhibitory effects of GOS-sugar in vitro and in rat experiments. Microbiol. Immunol. 1988, 32, 1093–1105. [Google Scholar] [CrossRef]
- Ooshima, T.; Izumitani, A.; Minami, T.; Yoshida, T.; Sobue, S.; Fujiwara, T.; Hamada, S. Noncariogenicity of maltitol in specific pathogen-free rats infected with mutans streptococci. Caries Res. 1992, 26, 33–37. [Google Scholar]
- Hamada, S.; Horikoshi, T.; Minami, T.; Kawabata, S.; Hiraoka, J.; Fujiwara, T.; Ooshima, T. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect. Immun. 1991, 59, 4161–4167. [Google Scholar]
- Kakiuchi, N.; Hattori, M.; Nishizawa, M.; Yamagishi, T.; Okuda, T.; Namba, T. Studies on dental caries prevention by traditional medicines. VIII. Inhibitory effect of various tannins on glucan synthesis by glucosyltransferase from Streptococcus mutans. Chem. Pharm. Bull. 1986, 34, 720–725. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Fitzgerald, R.J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J. Bacteriol. 1969, 98, 341–346. [Google Scholar]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar]
- Radisky, D.C.; Radisky, E.S.; Barrows, L.R.; Copp, B.R.; Kramer, R.A.; Ireland, C.M. Novel cytotoxic topoisomerase II inhibiting pyrroloiminoquinones from Fijian sponges of the genus Zyzzya. J. Am. Chem. Soc. 1993, 115, 1632–1638. [Google Scholar]
- Carney, J.R.; Scheuer, P.J.; Kelly-Borges, M. Makaluvamine G, a cytotoxic pigment from an an Indonesian Sponge Histodermella sp. Tetrahedron 1993, 49, 8483–8486. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Harper, M.K.; Faulkner, D.J. Makaluvamines H–M and damirone C from the pohnpeian sponge Zyzzya fuliginosa. J. Nat. Prod. 1995, 58, 1861–1867. [Google Scholar] [CrossRef]
- Hu, J.F.; Schetz, J.A.; Kelly, M.; Peng, J.N.; Ang, K.K.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P.; et al. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef]
- Shinkre, B.A.; Raisch, K.P.; Fan, L.; Velu, S.E. Synthesis and antiproliferative activity of benzyl and phenethyl analogs of makaluvamines. Bioorganic Med. Chem. 2008, 16, 2541–2549. [Google Scholar] [CrossRef]
- Shinkre, B.A.; Raisch, K.P.; Fan, L.; Velu, S.E. Analogs of the marine alkaloid makaluvamines: Synthesis, topoisomerase II inhibition, and anticancer activity. Bioorganic Med. Chem. Lett. 2007, 17, 2890–2893. [Google Scholar]
- Nag, S.; Nadkarni, D.H.; Qin, J.-J.; Voruganti, S.; Nguyen, T.; Xu, S.; Wang, W.; Wang, H.; Velu, S.E.; Zhang, R. Anticancer Activity and Molecular Mechanisms of Action of Makaluvamines and Analogues. Mol. Cell. Pharmacol. 2012, 4, 69–81. [Google Scholar]
- Sadanandan, E.V.; Pillai, S.K.; Lakshmikantham, M.V.; Billimoria, A.D.; Culpepper, J.S.; Cava, M.P. Efficient Syntheses of the Marine Alkaloids Makaluvamine D and Discorhabdin C: The 4,6,7-Trimethoxyindole Approach. J. Org. Chem. 1995, 60, 1800–1805. [Google Scholar]
- Wen, Z.T.; Burne, R.A. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 2002, 68, 1196–1203. [Google Scholar] [CrossRef]
- Liu, C.; Worthington, R.J.; Melander, C.; Wu, H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 2011, 55, 2679–2687. [Google Scholar] [CrossRef]
- Ajdic, D.; McShan, W.M.; McLaughlin, R.E.; Savic, G.; Chang, J.; Carson, M.B.; Primeaux, C.; Tian, R.; Kenton, S.; Jia, H.; et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 2002, 99, 14434–14439. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nijampatnam, B.; Nadkarni, D.H.; Wu, H.; Velu, S.E. Antibacterial and Antibiofilm Activities of Makaluvamine Analogs. Microorganisms 2014, 2, 128-139. https://doi.org/10.3390/microorganisms2030128
Nijampatnam B, Nadkarni DH, Wu H, Velu SE. Antibacterial and Antibiofilm Activities of Makaluvamine Analogs. Microorganisms. 2014; 2(3):128-139. https://doi.org/10.3390/microorganisms2030128
Chicago/Turabian StyleNijampatnam, Bhavitavya, Dwayaja H. Nadkarni, Hui Wu, and Sadanandan E. Velu. 2014. "Antibacterial and Antibiofilm Activities of Makaluvamine Analogs" Microorganisms 2, no. 3: 128-139. https://doi.org/10.3390/microorganisms2030128




