High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 Genes and Their Association with Decreased Susceptibility to Antibiotics and Common Hospital Biocides in Clinical Isolates of Acinetobacter baumannii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antimicrobial Susceptibility Testing
2.3. Phenotypic Detection of MBL
2.4. Determination of Disinfectants/Antiseptics Susceptibility by the Broth Macrodilution Method
2.5. PCR and DNA Sequencing
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Isolates and Their Antibiotics Susceptibility
3.2. Detection of MBL-Encoding Genes
3.3. Correlation of Efflux Pump Genes with MIC of Tested Biocides
3.4. Correlation of blaVIM and blaNDM-1 Genes with qac Genes
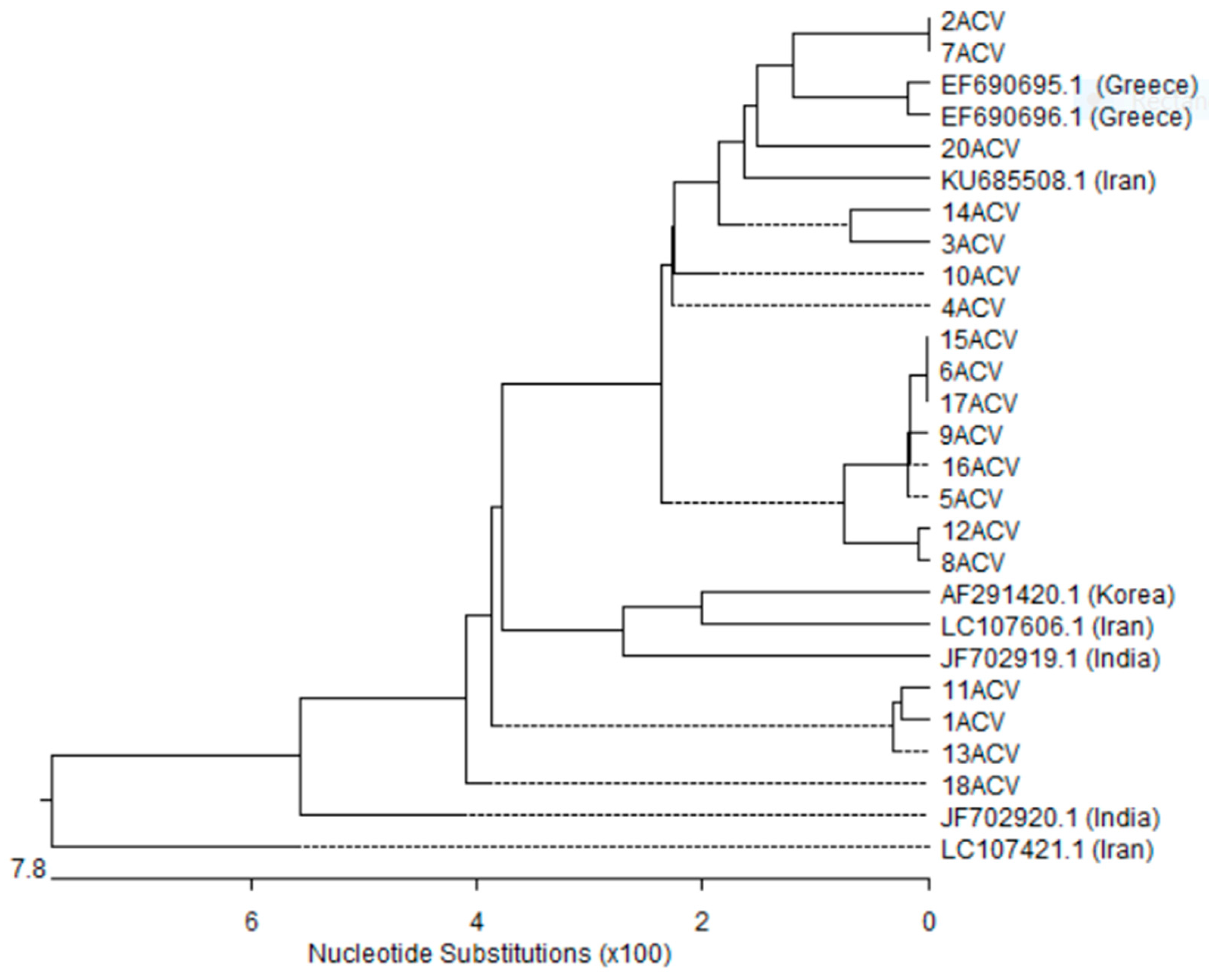
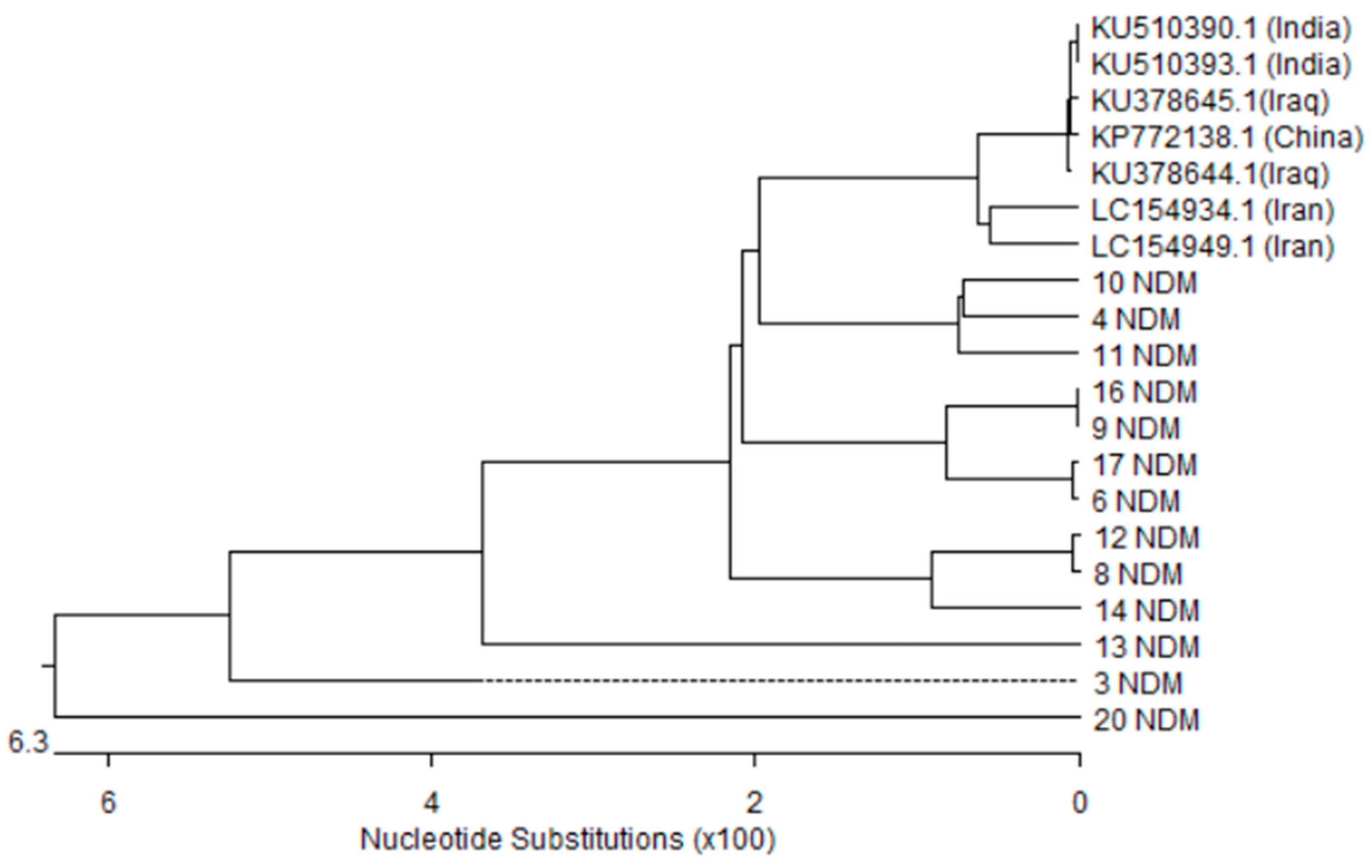
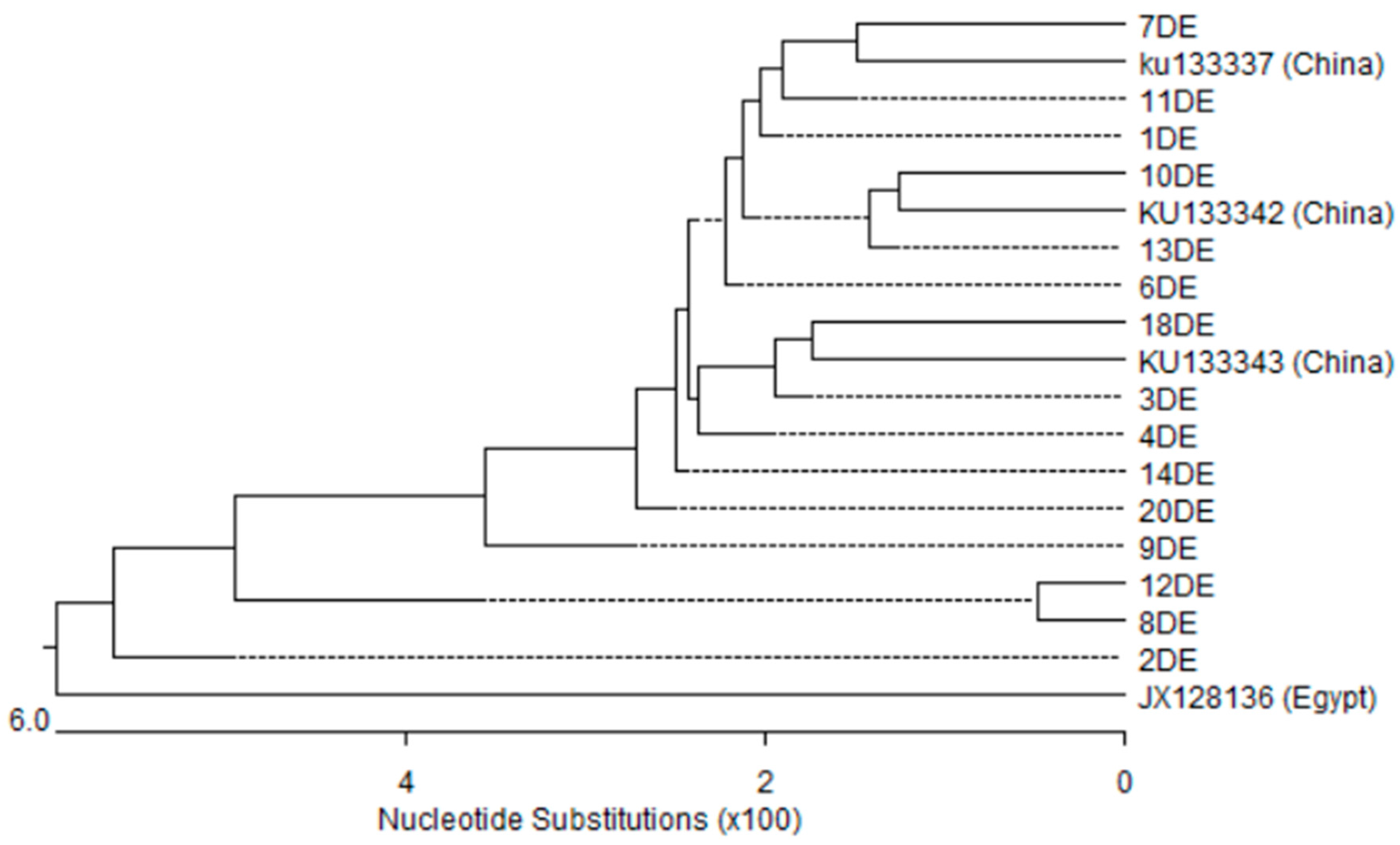
3.5. Nucleotides Sequence and Phylogenetic Analysis
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatterjee, S.; Datta, S.; Roy, S.; Ramanan, L.; Saha, A.; Viswanathan, R.; Som, T.; Basu, S. Carbapenem Resistance in Acinetobacter baumannii and Other Acinetobacter spp. Causing Neonatal Sepsis: Focus on NDM-1 and Its Linkage to ISAba125. Front. Microbiol. 2016, 7, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, Z. Integrons: Epidemiological molecular markers for identifying and surveying metallo-β‑lactamase genes in Gram-negative bacilli. Future Microbiol. 2014, 9, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Zahedi-bialvaei, A.; Samadi-kafil, H.; Ebrahimzadeh-leylabadlo, H.; aghazadeh, M.; Asgharzadeh, M. Dissemination of carbapenemases producing Gram negative bacteria in the Middle East. Iran. J. Microbiol. 2015, 7, 226–246. [Google Scholar] [PubMed]
- Moghadam, M.N.; Motamedifar, M.; Sarvari, J.; Sedigh, E.S.H.; Mousavi, S.M.; Moghadam, F.N. Emergence of multidrug resistance and metallo-beta-lactamase producing Acinetobacter baumannii isolated from patients in Shiraz, Iran. Ann. Med. Health Sci. Res. 2016, 6, 162–167. [Google Scholar] [PubMed]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Z.; Jiang, Y.; Yu, Y. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Antimicrob. Chemother. 2011, 66, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, L.; Riccio, M.L.; Mazzariol, A.; Cornaglia, G.; Amicosante, G.; Fontana, R.; Rossolini, G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999, 43, 1584–1590. [Google Scholar] [PubMed]
- Tsakris, A.; Pournaras, S.; Woodford, N.; Palepou, M.F.; Babini, G.S.; Douboyas, J.; Livermore, D.M. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 2000, 38, 1290–1292. [Google Scholar] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Mcglone, S.M.; Doi, Y.; Bailey, R.R.; Harrison, L.H. Economic value of Acinetobacter baumannii screening in the intensive care unit. Clin. Microbiol. Infect. 2011, 17, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Weinbren, M.J. New Delhi metallo-beta-lactamase: A cautionary tale. J. Hosp. Infect. 2010, 75, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Mochon, A.B.; Garner, O.B.; Hindler, J.A.; Krogstad, P.; Ward, K.W.; Lewinski, M.A.; Rashee, J.K.; Anderson, K.F.; Limbago, B.M.; Humphries, R.M. New Delhi metallo-beta-lactamase (NDM-1)-producing Klebsiella pneumoniae: Case report and laboratory detection strategies. J. Clin. Microbiol. 2011, 49, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Koljalg, S.; Naaber, P.; Mikelsaar, M. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J. Hosp. Infect. 2002, 51, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kucken, D.; Heinz-Hubert, F.; Kaukfers, P.M. Association of qacE and qacEΔ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol. Lett. 2000, 183, 95–98. [Google Scholar] [CrossRef]
- Higgins, C.S.; Murtough, S.M.; Williamson, E.; Hiom, S.J.; Payne, D.J.; Russell, A.D.; Walsh, T.R. Resistance to antibiotics and biocides among non-fermenting Gram-negative bacteria. Clin. Microbiol. Infect. 2001, 7, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ogbulie, J.N.; Adieze, I.E.; Nwankwo, N.C. Susceptibility pattern of some clinical bacterial isolates to selected antibiotics and disinfectants. Polish J. Microbiol. 2008, 57, 199–204. [Google Scholar]
- Kazama, H.; Hamashima, H.; Sasatsu, M.; Arai, T. Distribution of the antiseptic-resistance genes qacE and qacEΔ1 in Gram-negative bacteria. FEMS Microbiol. Lett. 1998, 159, 173–178. [Google Scholar] [CrossRef]
- Chang, Y.C.; Shih, D.Y.C.; Wang, J.Y.; Yang, S.S. Molecular characterization of class 1 integrons and antimicrobial resistance in Aeromonas strains from foodborne outbreak-suspect samples and environmental sources in Taiwan. Diagnos. Microbiol. Infect. Dis. 2007, 59, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Riano, I.; Moreno, M.A.; Teshager, T.; Saenz, Y.; Dominguez, L.; Torres, C. Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 2006, 58, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Pitout, J.D.; Nordmann, P. Carbapenemases: Molecular diversity and clinical consequences. Future Microbiol. 2007, 2, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R. Clinically significant carbapenemases: An update. Curr. Opin. Infect. Dis. 2008, 21, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Colinon, C.; Jocktane, D.; Brothier, E.; Rossolini, G.M.; Cournoyer, B.; Nazaret, S. Genetic analyses of Pseudomonas aeruginosa isolated from healthy captive snakes: Evidence of high inter- and intrasite dissemination and occurrence of antibiotic resistance genes. Environ. Microbiol. 2010, 12, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Chen, G.L.; Ito, R.; Kimura, S.; Hu, Z.Q. Identification of a plasmid-borne bla(IMP-11) gene in clinical isolates of Escherichia coli and Klebsiella pneumoniae. J. Med. Microbiol. 2012, 61, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 2000, 52, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Martro, E.; Hernandez, A.; Ariza, J.; Matas, L.; Argerich, M.J.; Martin, R.; Ausina, V. Assessment of Acinetobacter baumannii susceptibility to antiseptics and disinfectants. J. Hosp. Infect. 2003, 55, 39–46. [Google Scholar] [CrossRef]
- Kawamura-Sato, K.; Wachino, J.; Kondo, T.; Ito, H.; Arakawa, Y. Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. J. Antimicrob. Chemother. 2010, 65, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Schmitt, R.; Wöhrmann, A.; Stefanik, D.; Seifert, H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J. Hosp. Infect. 2007, 66, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014; p. 34. [Google Scholar]
- Mazzola, P.G.; Jozala, A.F.; Novaes, L.C.; Moriel, P.; Penna, T.C. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009, 45, 241–248. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Q.; Mi, Z.; Huang, Z.; Chen, G. Distribution of the antiseptic-resistance gene qacEΔ1 in 331 clinical isolates of Pseudomonas aeruginosa in China. J. Hosp. Infect. 2007, 66, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.T.; Chen, H.C.; Chuang, Y.P.; Chang, S.C.; Wang, J.T. Cloning of a cation efflux pump gene associated with chlorhexidine resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2002, 46, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Doi, Y.; Yamane, K.; Yagi, T.; Kurokawa, H.; Shibayama, K.; Kato, H.; Kai, K.; Arakawa, Y. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by Gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 2003, 41, 5407–5413. [Google Scholar] [CrossRef] [PubMed]
- Senda, K.; Arakawa, Y.; Ichiyama, S.; Nakashima, K.; Ito, H.; Ohsuka, S.; Shimokata, K.; Kato, N.; Ohta, M. PCR detection of metallo-betalactamase gene (blaIMP) in Gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 1996, 34, 2909–2913. [Google Scholar] [PubMed]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Abdelwahab, S.F.; Hasanen, A.M.; Mohammed, D.S. Multidrug resistant Egyptian isolates of Acinetobacter baumannii. J. Am. Sci. 2011, 7, 1013–1019. [Google Scholar]
- Mohamed, N.M.; Raafat, D. Phenotypic and genotypic detection of metallo-betalactamases in imipenem-resistant Acinetobacter baumannii isolated from a tertiary hospital in Alexandria, Egypt. Res. J. Microbiol. 2011, 6, 750–760. [Google Scholar] [CrossRef]
- Al-Hassan, L.; El Mehallawy, H.; Amyes, S.G. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin. Microbiol. Infect. 2013, 19, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.; Attia, A.S.; Tawakkol, W.M.; Hashem, A.M. Emergence of carbapenem resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Int. J. Infect. Dis. 2013, 17, e1252–e1254. [Google Scholar] [CrossRef] [PubMed]
- Al-Agamy, M.H.; Khalaf, N.G.; Tawfick, M.M.; Shibl, A.M.; El Kholy, A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int. J. Infect. Dis. 2014, 22, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweih, N.A.; Al-Hubail, M.; Rotimi, V.O. Three distinct clones of carbapenem resistant Acinetobacter baumannii with high diversity of carbapenemases isolated from patients in two hospitals in Kuwait. J. Infect. Public Health 2012, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Touati, A.; Sahli, F.; Ameur, A.A.; Haouchine, D.; Rolain, J.M. Antibiotic resistance determinants of multidrug-resistant Acinetobacter baumannii clinical isolates in Algeria. Diagn. Microbiol. Infect. Dis. 2013, 76, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species; critical review. IUBMB Life 2011, 63, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.; Harms, K.; Fricke, W.F.; Johnsen, P.J.; da Silva, G.J.; Nielsen, K.M. Natural Transformation Facilitates Transfer of Transposons, Integrons and Gene Cassettes between Bacterial Species. PLoS Pathog. 2012, 8, e1002837. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Thirunarayan, M.A.; Krishnan, P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 2010, 65, 2253–2254. [Google Scholar] [CrossRef] [PubMed]
- Gottig, S.; Pfeifer, Y.; Wichelhaus, T.A.; Zacharowski, K.; Bingold, T.; Averhoff, B.; Brandt, C.; Kempf, V.A. Global spread of New Delhi metallo-b lactamase 1. Lancet Infect. Dis. 2010, 10, 828–829. [Google Scholar] [CrossRef]
- Kaase, M.; Nordmann, P.; Wichelhaus, T.A.; Gatermann, S.G.; Bonnin, R.A.; Poirel, L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 2011, 66, 1260–1262. [Google Scholar] [CrossRef] [PubMed]
- Alshara, J.M.R.; Alsehlawi, Z.S.R.; Aljameel, D.S.A. First Report of New Delhi Metallo-beta-Lactamase (NDM-1) Producing Pseudomonas aeruginosa in Iraq. J. Biol. Agric. Healthc. 2014, 4, 40–47. [Google Scholar]
- Bjorland, J.; Steinum, T.; Kvitle, B.; Waage, S.; Sunde, M.; Heir, E. Widespread distribution of disinfectant resistance genes among Staphylococci of bovine and caprine origin in Norway. J. Clin. Microbiol. 2005, 43, 4363–4368. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Gemmell, C.G.; Hunter, I.S. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J. Antimicrob. Chemother. 2008, 61, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jaglic, Z.; Cervinkova, D. Genetic basis of resistance to quaternary ammonium compounds—The qac genes and their role: A review. Vet. Med. 2012, 57, 275–281. [Google Scholar]
- Wang, C.; Zhan, Q.; Mi, Z.; Huang, Z.; Chen, G. Distribution of the antiseptic-resistance gene qacEΔ1 in 283 clinical isolates of Gram-negative bacteria in China. J. Hosp. Infect. 2008, 69, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; O’Donoghue, M.M.; Ito, T.; Hiramatsu, K.; Boost, M.V. Prevalence of antiseptic-resistance genes in Staphylococcus aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J. Hosp. Infect. 2011, 78, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.R.; Sulong, A.; Hamat, R.A.; Nordin, S.A.; Neela, V.K. Extremely high prevalence of antiseptic resistant Quaternary Ammonium Compound E gene among clinical isolates of multiple drug resistant Acinetobacter baumannii in Malaysia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Tattawasart, U.; Maillard, J.Y.; Furr, J.R. Possible link between bacterial resistance and use of antibiotics and biocides. Antimicrob. Agents Chemother. 1998, 42, 2151. [Google Scholar] [PubMed]
- Langsrud, S.; Sundheim, G.; Holck, A.L. Cross resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress inducers. J. Appl. Microbiol. 2004, 96, 201. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef] [PubMed]
- White, D.G.; McDermott, P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001, 4, 313–317. [Google Scholar] [CrossRef]
- Boost, M.V.; Chan, J.; Shi, G.; Cho, P. Effect of Multipurpose Solutions against Acinetobacter Carrying QAC Genes. Optom. Vis. Sci. 2016, 91, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tamura, Y.; Yokota, T. Antiseptic and antibiotic resistance plasmid in Staphylococcus aureus that possesses ability to confer chlorhexidine and acrinol resistance. Antimicrob. Agents Chemother. 1988, 32, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Davies, R.H. Co-Selection of Resistance to Antibiotics, Biocides and Heavy Metals, and Its Relevance to Foodborne Pathogens; Review. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [PubMed]


| Chemical Agents | Recommended Dilution * | Starting Concentration | Dilutions |
|---|---|---|---|
| Chlorhexidine digluconate (CLX) | 0.0075% | 0.3% | 0.15%, 0.075%, 0.0375%, 0.0187%, 0.00937%, 0.00468%, 0.00234%, 0.00172% |
| Cetrimide | 0.075% | 3% | 1.5%, 0.75%, 0.375%, 0.187%, 0.094%, 0.046%, 0.023%. |
| Povidone-iodine (PVP-I2) | 5% | 10% | 5%, 2.5%, 1.25%, 0.625%, 0.3125%, 0.156%, 0.078% and 0.039% |
| Gene Name | Sequence 5′-3′ | PCR Products | Reference | Annealing Temperature |
|---|---|---|---|---|
| qacE | Forward-CCCGAATTCATGAAAGGCTGGCTT Reverse-TAAGCTTTCACCATGGCGTCGG | 350 bp | [14] | 55 °C |
| qacΔE1 | Forward-TAGCGAGGGCTTTACTAAGC Reverse-ATTCAGAATGCCGAACACCG | 300 bp | [32] | 55 °C |
| cepA | Forward-CAACTCCTTCGCCTATCCCG Reverse-TCAGGTCAGACCAAACGGCG | 1058 bp | [33] | 62 °C |
| blaOxa51-like | Forward-TAATGCTTTGATCGGCCTTG Reverse-TGGATTGCACTTCATCTTGG | 353 bp | [34] | 60 °C |
| Intl1 | Forward-GCATCCTCGGTTTTCTGG Reverse-GGTGTGGCGGGCTTCGTG | 457 bp | [35] | 60 °C |
| blaIMP | Forward-CTACCGCAGCAGAGTCT TTG Reverse-AACCAGTTTTGCCTTACCAT | 587 bp | [36] | 55 °C |
| blaNDM-1 | Forward-GGCGGAATGGCTCATCACGA Reverse-CGCAAC ACAGCCTGACTTTC | 287 bp | [6] | 58 °C |
| balVIM | Forward-GATGGTGTTTGGTCGCATA Reverse-CGAATGCGCAGCACCAG | 390 bp | [37] | 52 °C |
| blaSPM | Forward-AAAATCTGGGTACGCAAACG Reverse-ACATTATCCGCTGGAACAGG | 271 bp | ||
| blaGIM | Forward-TCGACACACCTTGGTCTGAA Reverse-AACTTCCAACTTTGCCATGC | 477 bp | ||
| blaSIM | Forward-TACAAGGGATTCGGCATCG Reverse-TAATGGCCTGTTCCCATGTG | 570 bp |
| Antimicrobial Agent | A. baumannii (56 Isolates) | ||
|---|---|---|---|
| S No (%) | I No (%) | R No (%) | |
| Amikacin | 9 (16.1) | 3 (5.3) | 44 (78.6) |
| Amoxicillin/clavulanic acid | 0 (0.0) | 0 (0.0) | 56 (100) |
| Cefazolin | 0 (0.0) | 0 (0.0) | 56 (100) |
| Cefepime | 4 (7.1) | 0 (0.0) | 52 (92.9) |
| Cefotaxime | 1 (1.8) | 5 (8.9) | 50 (89.3) |
| Cefotetan | 1 (1.8) | 2 (2.6) | 53 (94.6) |
| Ceftazidime | 4 (7.1) | 1 (1.8) | 51 (91.1) |
| Ceftriaxone | 5 (8.9) | 2 (2.6) | 49 (87.5) |
| Cefuroxime | 1 (1.8) | 1 (1.8) | 54 (96.4) |
| Imipenem | 16 (28.6) | 3 (5.3) | 37 (66.1) |
| Levofloxacin | 13 (23.2) | 7 (12.5) | 36 (64.3) |
| Meropenem | 16 (28.6) | 1 (1.8) | 39 (69.6) |
| Gifloatxacin | 8 (14.3) | 5 (8.9) | 33 (58.9) |
| Nitrofurantoin | 0 (0.0) | 0 (0.0) | 56 (100) |
| Ticarcillin/clavulanic acid | 6 (10.7) | 1 (1.8) | 49 (87.5) |
| Tobramycin | 16 (28.6) | 6 (10.7) | 34 (60.7) |
| Trimethoprim/sulfamethoxazole | 4 (7.1) | 0 (0.0) | 52 (92.9) |
| Tigicyclin | 56 (100) | 0 (0.0) | 0 (0.0) |
| Colistin | 56 (100) | 0 (0.0) | 0 (0.0) |
| Isolate Number | Type of Specimen | Biocide Resistance Genes | MIC of Biocide μg/mL | MBL Genes | Intl1 Gene | Antimicrobial Susceptibility | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qacE | qacEΔ1 | cepA | PVP-I2 | CLX | Cetrimide | blaNDM | blaVIM | Ak | AMC | KZ | FEP | CTX | CTT | CAZ | CRO | CXM | GAT | IMP | MEM | LEV | F | TIM | TOB | SXT | TGC | CL | Category | MBL | |||
| 1 | Throat Swab | + | + | - | 0.625 | 0.075 | 0.187 | - | + | + | R | R | R | R | R | R | R | R | R | I | R | R | I | R | R | R | R | S | S | MDR | + |
| 2 | Sputum | + | + | - | 0.625 | 0.0375 | 0.75 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 3 | Throat Swab | + | + | - | 1.25 | 0.009 | 0.049 | + | + | + | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | R | R | S | S | MDR | + |
| 4 | Blood | + | + | - | 1.25 | 0.0046 | 0.75 | + | + | + | I | R | R | R | R | R | R | R | R | R | R | R | S | R | R | R | R | S | S | MDR | + |
| 5 | Sputum | - | - | - | 1.25 | 0.009 | 0.098 | - | + | + | R | R | R | R | R | R | R | R | R | I | R | R | I | R | R | S | R | S | S | MDR | + |
| 6 | Pus | + | + | - | 0.625 | 0.0375 | 0.098 | + | + | + | S | R | R | R | R | R | R | R | R | R | R | R | I | R | R | S | S | S | S | MDR | + |
| 7 | Blood | + | + | - | 0.625 | 0.009 | 0.75 | - | + | + | R | R | R | R | R | R | R | R | R | I | I | R | I | R | R | I | R | S | S | MDR | + |
| 8 | Sputum | + | + | - | 1.25 | 0.0046 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 9 | Blood | + | + | - | 1.25 | 0.009 | 0.098 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 10 | Pus | - | + | - | 0.625 | 0.075 | 0.187 | + | + | + | S | R | R | R | R | R | R | R | R | S | R | R | R | R | R | R | R | S | S | MDR | + |
| 11 | Pus | - | + | - | 1.25 | 0.009 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 12 | Blood | - | + | - | 1.25 | 0.009 | 0.187 | + | + | + | R | S | R | R | R | R | R | R | R | S | R | R | S | R | R | R | R | S | S | MDR | + |
| 13 | Sputum | - | + | - | 1.25 | 0.0046 | 0.098 | + | + | + | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | R | R | S | S | MDR | + |
| 14 | Pus | + | + | - | 1.25 | 0.009 | 0.187 | + | + | + | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | MDR | + |
| 15 | Pus | - | - | - | 1.25 | 0.0023 | 0.049 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | R | S | S | MDR | + |
| 16 | Blood | - | - | - | 0.039 | 0.0023 | 0.187 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 17 | Pus | - | - | - | 0.625 | 0.0046 | 0.049 | + | + | + | S | R | R | R | R | R | R | R | R | S | R | R | I | R | R | R | R | S | S | MDR | + |
| 18 | Sputum | - | + | - | 1.25 | 0.0046 | 0.187 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | S | S | MDR | + |
| 19 | Chest Tube | - | - | - | 0.078 | 0.0046 | 0.187 | - | - | + | R | S | R | R | R | I | R | R | R | S | R | R | R | R | R | R | R | S | S | MDR | + |
| 20 | Chest Tube | + | + | - | 0.039 | 0.075 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 21 | Pus | - | - | - | 0.078 | 0.0023 | 0.049 | - | - | + | R | R | R | R | R | I | R | R | R | R | R | R | I | R | R | R | R | S | S | MDR | + |
| 22 | Nasal Swab | - | - | - | 0.625 | 0.0023 | 0.049 | - | - | + | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | MDR | + |
| Chemical Agent | MIC μg/mL (%) | Number of Isolates (n = 22) | qac Genes | Chi-Square Tests | p-Value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Povidone-iodine | >50,000 (5%) | 0 | 0 | 0 | - | - |
| <50,000 (5%) | 22 | 15 | 7 | |||
| Chlorhexidine | >75 (0.0075%) | 12 | 11 | 1 | 6.712 | 0.0096 * |
| <75 (0.0075%) | 10 | 4 | 6 | |||
| Cetrimide | >750 (0.075%) | 17 | 14 | 3 | 6.924 | 0.0085 * |
| <50 (0.075%) | 5 | 1 | 4 | |||
| MBL Genes | blaVIM | blaNDM-1 | |||
|---|---|---|---|---|---|
| qac Genes | Positive | Negative | Positive | Negative | |
| Positive | 15 | 0 | 11 | 4 | |
| Negative | 4 | 3 | 2 | 5 | |
| p-value | 0.0064 * | 0.0467 * | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomaa, F.A.M.; Helal, Z.H.; Khan, M.I. High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 Genes and Their Association with Decreased Susceptibility to Antibiotics and Common Hospital Biocides in Clinical Isolates of Acinetobacter baumannii. Microorganisms 2017, 5, 18. https://doi.org/10.3390/microorganisms5020018
Gomaa FAM, Helal ZH, Khan MI. High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 Genes and Their Association with Decreased Susceptibility to Antibiotics and Common Hospital Biocides in Clinical Isolates of Acinetobacter baumannii. Microorganisms. 2017; 5(2):18. https://doi.org/10.3390/microorganisms5020018
Chicago/Turabian StyleGomaa, Fatma Alzahraa M., Zeinab H. Helal, and Mazhar I. Khan. 2017. "High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 Genes and Their Association with Decreased Susceptibility to Antibiotics and Common Hospital Biocides in Clinical Isolates of Acinetobacter baumannii" Microorganisms 5, no. 2: 18. https://doi.org/10.3390/microorganisms5020018





