Free and Nanoencapsulated Tobramycin: Effects on Planktonic and Biofilm Forms of Pseudomonas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Chemicals and Bacteriological Media
2.3. Preparation of Lipid Nanoparticles
2.4. Drug Susceptibility Assay in Planktonic Bacteria
2.5. Effect of Free and Nanoencapsulated Tobramycin on P. aeruginosa Growth
2.6. Antimicrobial Susceptibility of Sessile Bacteria
2.7. Statistical Analysis
3. Results and Discussion
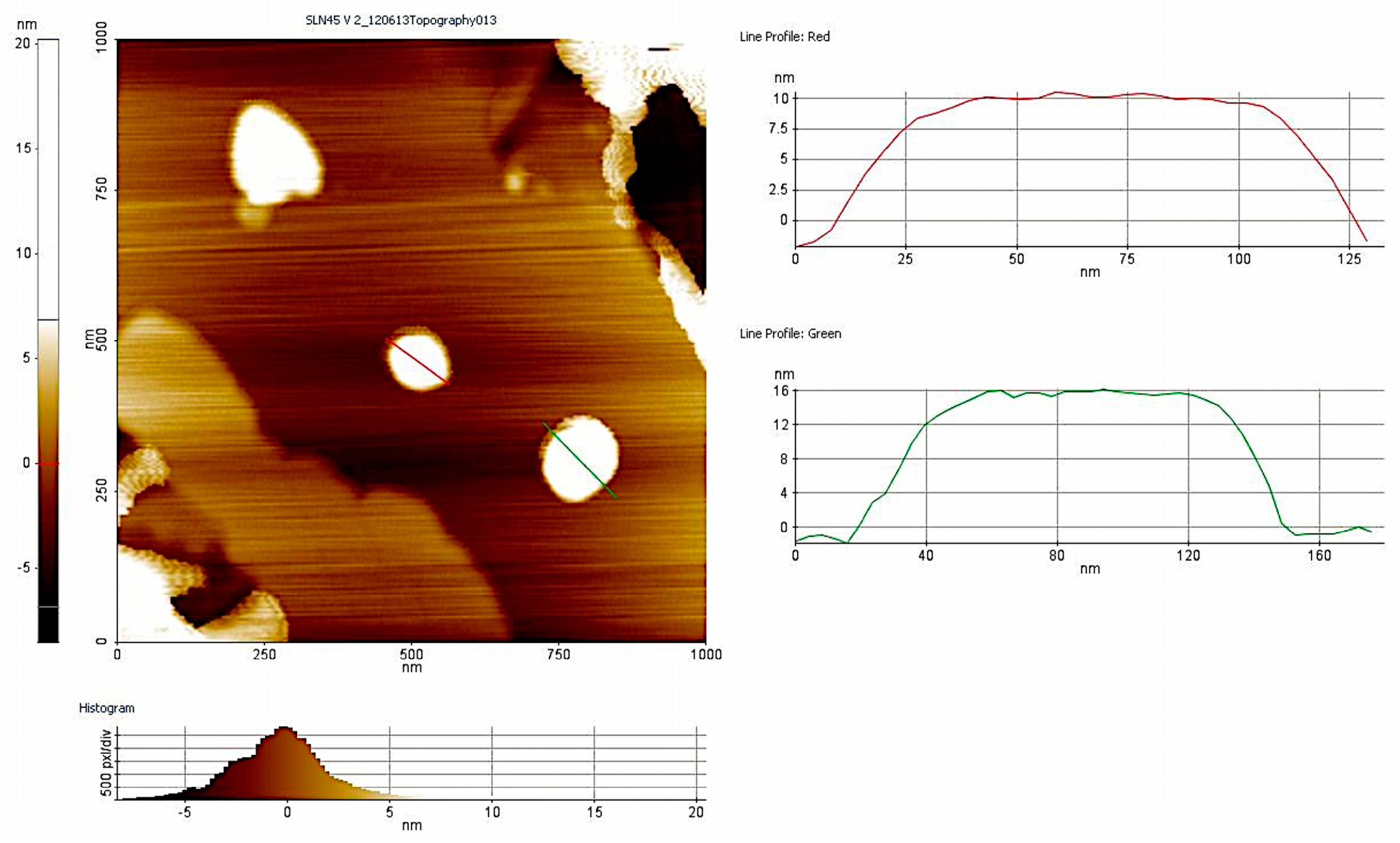
3.1. Nanoparticle Characterization
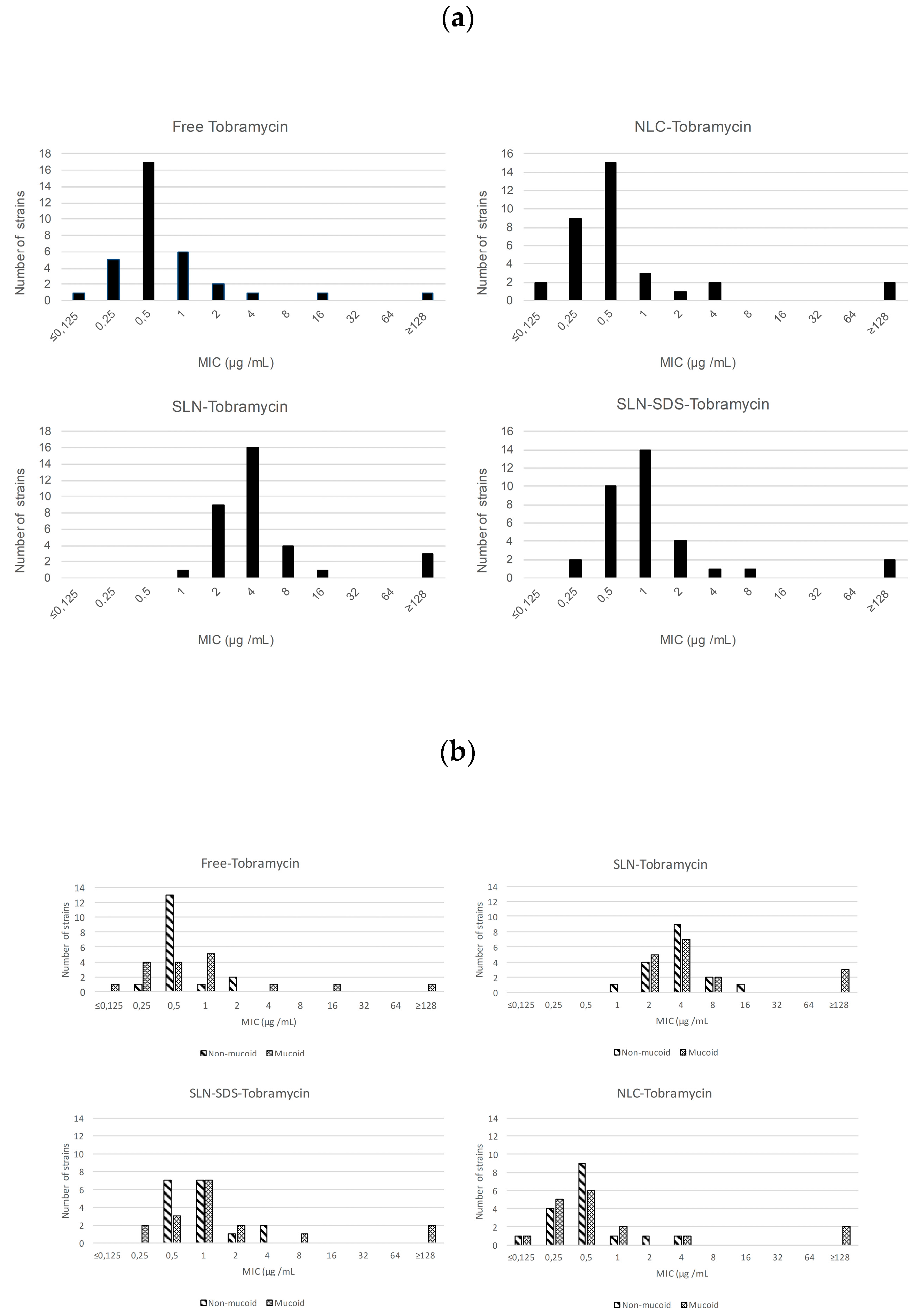
3.2. Antimicrobial Activity of Free and Nanoencapsulated Tobramycin
3.3. Effect of Free and Nanoaencapsulated Tobramycin on Bacterial Growth
3.4. Anti-Biofilm Efficacy of Free and Nanoencapsulated Tobramycin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ma, L.Z. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2013, 14, 20983–21005. [Google Scholar] [PubMed]
- Drenkard, E.; Ausubel, F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar] [PubMed]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Fusté, E.; López-Jiménez, L.; Segura, C.; Gainza, E.; Vinuesa, T.; Viñas, M. Carbapenem-resistance mechanisms of multidrug-resistant Pseudomonas aeruginosa. J. Med. Microbiol. 2013, 62, 1317–1325. [Google Scholar] [PubMed]
- Döring, G.; Flume, P.; Heijerman, H.; Elborn, J.S. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012, 11, 461–479. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.L.; Nelson, L.E.; Shawar, R.M.; Lin, B.B.; Lockwood, L.G.; Dirk, J.E.; Miller, G.H.; Burns, J.L.; Garber, R.L. Aminoglycoside-resistance mechanisms for cystic fibrosis Pseudomonas aeruginosa isolates are unchanged by long-term, intermittent, inhaled tobramycin treatment. J. Infect. Dis. 2000, 181, 1180–1184. [Google Scholar] [PubMed]
- Stehling, F.; Büscher, R.; Grosse-Onnebrink, J.; Hoyer, P.F.; Mellies, U. Glomerular and tubular renal function after repeated once-daily tobramycin courses in cystic fibrosis patients. Pulm. Med. 2017, 2017, 1–6. [Google Scholar]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-Induced Nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Comparative nephrotoxicity of two aminoglycosides: Gentamicin and tobramycin. Med. J. Aust. 1982, 2, 129–132. [Google Scholar] [PubMed]
- Cao, B.; Christophersen, L.; Kolpen, M.; Jensen, P.Ø.; Sneppen, K.; Høiby, N.; Moser, C.; Sams, T. Diffusion retardation by binding of tobramycin in an alginate biofilm model. PLoS ONE 2016, 11, e0153616. [Google Scholar] [CrossRef] [PubMed]
- Oglesby-Sherrouse, A.G.; Djapgne, L.; Nguyen, A.T.; Vasil, A.I.; Vasil, M.L. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014, 70, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharm. Sci. Tech. 2011, 12, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Hwang, T.L.; Aljuffali, I.A.; Lin, C.F.; Chang, Y.T.; Fang, J.Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Varshosaz, J.; Saadat, A.; Atyabi, F. Stability and antimicrobial effect of amikacin-loaded solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 35–43. [Google Scholar]
- Sans-Serramitjana, E.; Fusté, E.; Martínez-Garriga, B.; Merlos, A.; Pastor, M.; Pedraz, J.L.; Esquisabel, A.; Bachiller, D.; Vinuesa, T.; Viñas, M. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.; Moreno-sastre, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Asensio, V.J.; Pozo, A.D.; Gainza, E.; Pedraz, J.L. Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2014, 477, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.; Momoh, M.A.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. Recent Adv. Nov. Drug Carr. Syst. 2012, 107–140. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 27th Informational Supplement CLSI Document M100-S27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically Feasible Biofilm Susceptibility Assay for Isolates of Pseudomonas aeruginosa from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery—Liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Pedraz, J.L. Stability study of sodium colistimethate-loaded lipid nanoparticles. J. Microencapsul. 2016, 33, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Khatak, S.; Vir, U.; Sara, S. Solid lipid nanoparticles—A review. Int. J. Appl. Pharm. 2013, 5, 8–18. [Google Scholar]
- Mugabe, C.; Azghani, A.O.; Omri, A. Liposome-mediated gentamicin delivery: Development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J. Antimicrob. Chemother. 2005, 55, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Ly, N.S.; Landersdorfer, C.B.; Wanigaratne, N.A.; Velkov, T.; Yadav, R.; Oliver, A.; Martin, L.; Shin, B.S.; Forrest, A.; et al. Two mechanisms of killing of Pseudomonas aeruginosa by tobramycin assessed at multiple inocula via mechanism-based modeling. Antimicrob. Agents Chemother. 2015, 59, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Bernier, S.P.; Kuchma, S.L.; Hammond, J.H.; Hasan, F.; O’Toole, G.A. Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. Int. Microbiol. 2010, 13, 207–212. [Google Scholar] [PubMed]
- Fernández-Olmos, A.; García-Castillo, M.; Maiz, L.; Lamas, A.; Baquero, F.; Cantón, R. In vitro prevention of Pseudomonas aeruginosa early biofilm formation with antibiotics used in cystic fibrosis patients. Int. J. Antimicrob. Agents 2012, 40, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Messiaen, A.S.; Raemdonck, K.; Deschout, H.; Rejman, J.; De Baets, F.; Nelis, H.; De Smedt, S.C.; Demeester, J.; Coenye, T.; et al. Transport of nanoparticles in cystic fibrosis sputum and bacterial biofilms by single-particle tracking microscopy. Nanomedicine 2013, 8, 935–949. [Google Scholar] [CrossRef] [PubMed]
 ); Free tobramycin (
); Free tobramycin (  ); SLN-Tobramycin (
); SLN-Tobramycin (  ); NLC-Tobramycin (
); NLC-Tobramycin (  ).
).
 ); Free tobramycin (
); Free tobramycin (  ); SLN-Tobramycin (
); SLN-Tobramycin (  ); NLC-Tobramycin (
); NLC-Tobramycin (  ).
).
| Source | Patient | Characteristics | Antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Age | Gender | Mucoid | Hemolysis | PIPER/TZ | CAZ | AZT | IMP | MERO | GNT | TOBRA | AMK | COL | CPFX |
| PA 056 SJD | 14 | Male | – | ß | R | S | R | I | R | S | S | S | S | R |
| PA 086 SJD | 13 | Female | + | ß | S | S | S | S | S | R | R | s | s | S |
| PA 571.1 SJD | 10 | Male | + | – | S | S | S | S | S | S | S | S | S | S |
| PA 571.2 SJD | 10 | Male | + | – | S | S | S | S | S | S | S | S | S | S |
| PA 288 SJD | 13 | Male | – | – | S | S | R | R | R | I | S | S | S | S |
| PA 596 SJD | 9 | Male | – | ß | S | S | S | S | S | S | S | S | S | S |
| PA 666 SJD | 13 | Male | – | ß | S | S | S | S | S | S | S | S | S | R |
| PA 686 SJD | 13 | Male | – | ß | S | S | S | S | S | S | S | S | S | S |
| PA 744 SJD | 14 | Female | – | – | S | S | S | S | S | S | S | S | S | S |
| PA 668 SJD | 2 | Female | – | – | S | S | S | S | S | S | S | S | S | S |
| PA 721 SJD | 7 | Female | – | ß | S | S | S | S | S | S | S | S | S | S |
| PA 122 SJD | 11 | Male | + | ß | S | S | S | S | S | S | S | S | S | S |
| PA 788 SJD | 7 | Female | – | ß | S | S | S | S | S | R | S | S | S | S |
| PA 768 SJD | 14 | Male | – | ß | S | S | R | I | R | S | S | S | S | R |
| 594 SJD | 9 | Male | + | ß | S | S | S | S | S | S | S | S | S | S |
| 2881M SJD | 13 | Male | + | – | S | I | R | R | R | S | S | S | S | R |
| 610M SJD | 13 | Male | + | – | S | S | R | R | R | S | S | S | S | R |
| 610 SJD | 13 | Male | – | ß | S | S | R | I | R | S | S | S | S | R |
| 805 SJD | 15 | Female | – | ß | S | S | S | S | S | R | S | S | S | S |
| 555.1 SJD | 7 | Female | + | – | S | S | S | S | S | S | S | S | S | S |
| PA 417 VH | 17 | Female | – | ß | R | R | R | S | S | S | S | S | S | R |
| PA 362 VH | 36 | Male | + | ß | S | S | S | S | S | S | R | S | S | S |
| PA 684 VH | 32 | Male | – | – | S | S | I | R | R | S | S | I | S | I |
| PA 103 VH | 29 | Female | + | – | R | S | S | S | S | R | S | R | S | S |
| 023 VH | 15 | Male | + | – | S | R | I | R | R | R | R | S | S | |
| 852 VH | 17 | Male | – | – | S | S | S | S | S | S | S | S | S | S |
| 153 VH | 17 | Female | + | – | S | S | S | S | S | S | S | S | S | R |
| 516 VH | 20 | Female | + | – | S | S | S | S | S | S | S | S | S | S |
| 547 VH | 15 | Male | + | – | R | R | R | R | R | R | R | R | S | R |
| 861 VH | 23 | Male | + | ß | S | S | S | S | S | S | S | S | S | S |
| 639 VH | 18 | Male | + | – | S | S | S | S | S | S | S | S | S | R |
| 897 VH | 26 | Female | – | – | S | S | S | S | S | S | S | S | S | I |
| 697 VH | 10 | Female | – | ß | R | S | S | S | S | R | R | R | S | R |
| 458 VH | 32 | Male | + | ß | S | S | S | R | R | R | S | R | S | R |
| Formulation | Mean Size (nm) | PDI | Zeta-Potential (mV) | Percentage EE (Encapsulation Efficiency) |
|---|---|---|---|---|
| TB-SLN | 302 ± 20.5 | 0.361 ± 0.02 | −20.5 ± 6.09 | ND |
| TB-NLC | 254.05 ± 14.5 | 0.311 ± 0.01 | −23.03 ± 2.76 | 93.15 ± 0.65 |
| ATCC 27853 | PAO1 | 056SJD | 362VH | |||||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBEC (μg/mL) | MIC (μg/mL) | MBEC (μg/mL) | MIC (μg/mL) | MBEC (μg/mL) | MIC (μg/mL) | MBEC (μg/mL) | |
| Free Tobramicin | 0.5 | 8 | 0.5 | 16 | 1 | 16 | 16 | 32 |
| SLN-SDS-Tobramicin | 0.25 | 4 | 0.25 | 8 | 0.5 | 8 | 16 | 32 |
| NLC- Tobramicin | ≤0.0625 | 2 | 0.25 | 4 | 0.25 | 4 | 16 | 16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sans-Serramitjana, E.; Jorba, M.; Fusté, E.; Pedraz, J.L.; Vinuesa, T.; Viñas, M. Free and Nanoencapsulated Tobramycin: Effects on Planktonic and Biofilm Forms of Pseudomonas. Microorganisms 2017, 5, 35. https://doi.org/10.3390/microorganisms5030035
Sans-Serramitjana E, Jorba M, Fusté E, Pedraz JL, Vinuesa T, Viñas M. Free and Nanoencapsulated Tobramycin: Effects on Planktonic and Biofilm Forms of Pseudomonas. Microorganisms. 2017; 5(3):35. https://doi.org/10.3390/microorganisms5030035
Chicago/Turabian StyleSans-Serramitjana, Eulalia, Marta Jorba, Ester Fusté, José Luis Pedraz, Teresa Vinuesa, and Miguel Viñas. 2017. "Free and Nanoencapsulated Tobramycin: Effects on Planktonic and Biofilm Forms of Pseudomonas" Microorganisms 5, no. 3: 35. https://doi.org/10.3390/microorganisms5030035





