Effect of Environmental Factors on Intra-Specific Inhibitory Activity of Carnobacterium maltaromaticum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Production Kinetics of Inhibitory Compounds by C. maltaromaticum D0h
2.3. Effect of Environmental Factors on the Sensitivity of C. maltaromaticum D8c to C. maltaromaticum D0h CFS
2.4. Effect of PH, Lactic Acid, Glucose and Atmosphere on Inhibition Level of D0h CFS
2.5. Data Analysis
3. Results
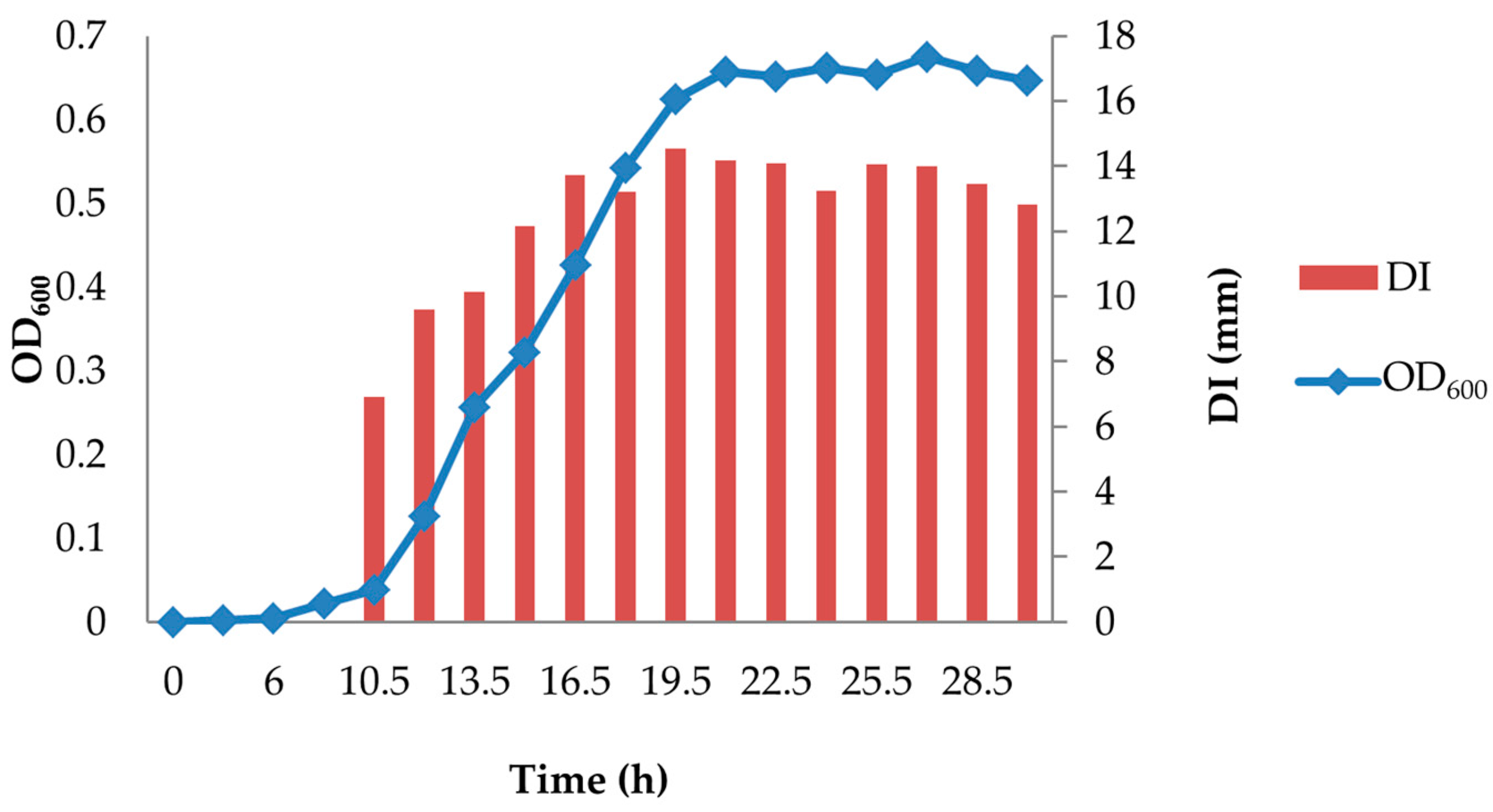
3.1. Kinetics of D0h Inhibitory Activity Production
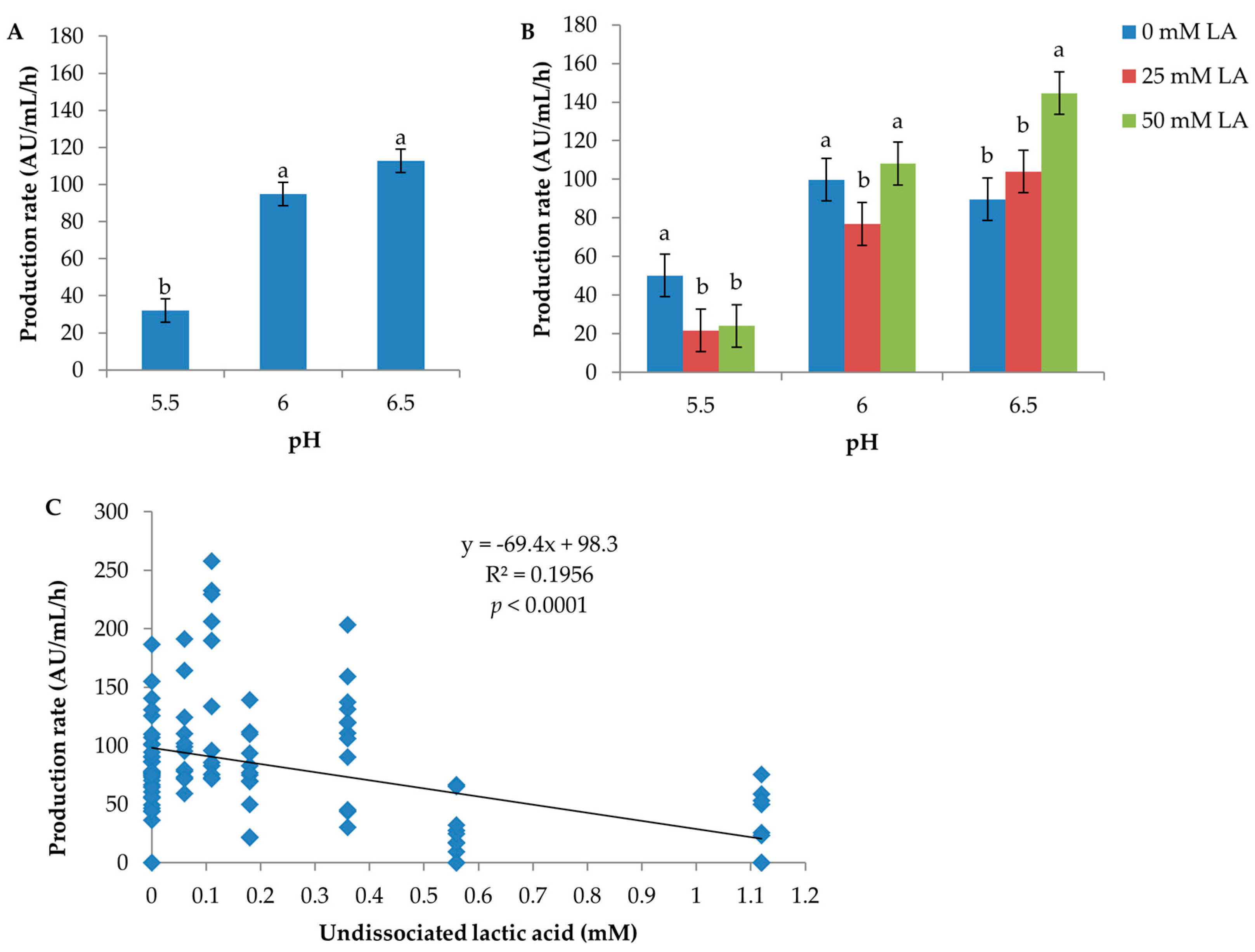
3.2. Environmental Effects on D0h Inhibitor Production
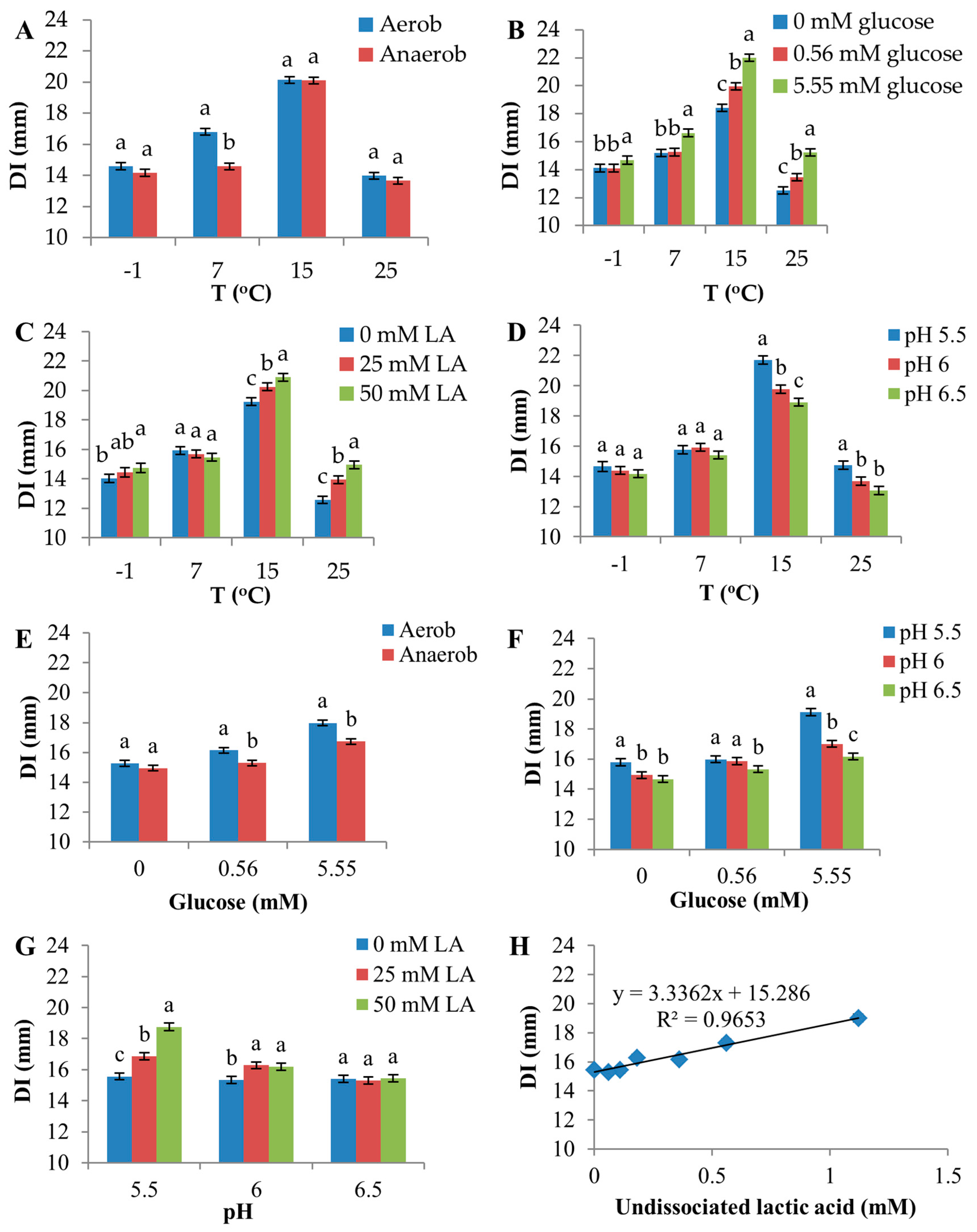
3.3. Influence of Environmental Factors on D8c Sensitivity to D0h CFS
3.4. Interactions among Environmental Factors on D8c Sensitivity
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Koskiniemi, S.; Ruhe, Z.C.; Poole, S.J.; Low, D.A. Mechanisms and biological roles of contact-dependent growth inhibition systems. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.; Fichant, G.; Berthod, J.; Vandenbrouck, Y.; Attree, I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: What can be learned from available microbial genomic resources? BMC Genom. 2009, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Shitut, S.; Freund, L.; Westermann, M.; Bertels, F.; Colesie, C.; Bischofs, I.B.; Kost, C. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat. Commun. 2015, 6, 6238. [Google Scholar] [CrossRef] [PubMed]
- Buckling, A.; Harrison, F.; Vos, M.; Brockhurst, M.A.; Gardner, A.; West, S.A.; Griffin, A. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol. Ecol. 2007, 62, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, P.N.; Nychas, G.J. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wu, X.; Yu, X.; He, L.; Shah, N.P.; Xu, F. Mutual growth-promoting effect between bifidobacterium bifidum WBBI03 and Listeria monocytogenes CMCC 54001. J. Dairy Sci. 2017, 100, 3448–3462. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Crawford, J.M.; Stewart, E.J.; Witt, K.; Gavrish, E.; Epstein, S.; Clardy, J.; Lewis, K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 2010, 17, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.E. A discussion of the dynamics of Salmonella enrichment. J. Hyg. (Lond.) 1962, 60, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.; Dalgaard, P.; Tienungoon, S. Predictive modelling of the growth and survival of Listeria in fishery products. Int. J. Food Microbiol. 2000, 62, 231–245. [Google Scholar] [CrossRef]
- Moller, C.O.; Ilg, Y.; Aabo, S.; Christensen, B.B.; Dalgaard, P.; Hansen, T.B. Effect of natural microbiota on growth of Salmonella spp. In fresh pork—A predictive microbiology approach. Food Microbiol. 2013, 34, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.J.; Dourou, D.; Skandamis, P.; Koutsoumanis, K.; Baranyi, J.; Sofos, J. Effect of microbial cell-free meat extract on the growth of spoilage bacteria. J. Appl. Microbiol. 2009, 107, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Laursen, B.G.; Bay, L.; Cleenwerck, I.; Vancanneyt, M.; Swings, J.; Dalgaard, P.; Leisner, J.J. Carnobacterium divergens and Carnobacterium maltaromaticum as spoilers or protective cultures in meat and seafood: Phenotypic and genotypic characterization. Syst. Appl. Microbiol. 2005, 28, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Zendo, T. Screening and characterization of novel bacteriocins from lactic acid bacteria. Biosci. Biotechnol. Biochem. 2013, 77, 893–899. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Button, J.E.; Santarelli, M.; Dutton, R.J. Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell 2014, 158, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gutierrez, R.A.; Lopez-Ramirez, V.; Islas, A.; Alcaraz, L.D.; Hernandez-Gonzalez, I.; Olivera, B.C.; Santillan, M.; Eguiarte, L.E.; Souza, V.; Travisano, M.; et al. Antagonism influences assembly of a Bacillus guild in a local community and is depicted as a food-chain network. ISME J. 2013, 7, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Blana, V.A.; Nychas, G.J. Presence of quorum sensing signal molecules in minced beef stored under various temperature and packaging conditions. Int. J. Food Microbiol. 2014, 173, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.; Gill, C.O.; Tran, F.; Yang, X. Unusual compositions of microflora of vacuum-packaged beef primal cuts of very long storage life. J. Food Prot. 2014, 77, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Leisner, J.J.; Laursen, B.G.; Prevost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Baranyi, J.; Tamplin, M. Interstrain interactions between bacteria isolated from vacuum-packaged refrigerated beef. Appl. Environ. Microbiol. 2015, 81, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Small, A.H.; Jenson, I.; Kiermeier, A.; Sumner, J. Vacuum-packed beef primals with extremely long shelf life have unusual microbiological counts. J. Food Prot. 2012, 75, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Bowman, J.P.; Porteus, B.; Dann, A.L.; Tamplin, M. Effect of abattoir and cut on variations in microbial communities of vacuum-packaged beef. Meat Sci. 2017, 131, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Aween, M.M.; Hassan, Z.; Muhialdin, B.J.; Eljamel, Y.A.; Al-Mabrok, A.S.; Lani, M.N. Antibacterial activity of Lactobacillus acidophilus strains isolated from honey marketed in malaysia against selected multiple antibiotic resistant (mar) gram-positive bacteria. J. Food Sci. 2012, 77, M364–M371. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from sturgeon fish. Food Control. 2013, 32, 379–385. [Google Scholar] [CrossRef]
- Russo, F.; Ercolini, D.; Mauriello, G.; Villani, F. Behaviour of Brochothrix thermosphacta in presence of other meat spoilage microbial groups. Food Microbiol. 2006, 23, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Nattress, F.M.; Baker, L.P.; Dilts, B.D. Survival of Campylobacter jejuni on beef and pork under vacuum packaged and retail storage conditions: Examination of the role of natural meat microflora on C. Jejuni survival. Food Microbiol. 2011, 28, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Scherwitzel, M.; Paulsen, P.; Szostak, M.P. Survival of Campylobacter jejuni under conditions of atmospheric oxygen tension with the support of Pseudomonas spp. Appl. Environ. Microbiol. 2010, 76, 5911–5917. [Google Scholar] [CrossRef] [PubMed]
- Lavieri, N.; Williams, S.K. Effects of packaging systems and fat concentrations on microbiology, sensory and physical properties of ground beef stored at 4 +/− 1 °C for 25 days. Meat Sci. 2014, 97, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.J. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J. Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum-packaged beef. Int. J. Food Microbiol. 2004, 90, 273–282. [Google Scholar] [CrossRef]
- Ganzle, M.G.; Weber, S.; Hammes, W.P. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 1999, 46, 207–217. [Google Scholar] [CrossRef]
- Abriouel, H.; Valdivia, E.; Galvez, A.; Maqueda, M. Influence of physico-chemical factors on the oligomerization and biological activity of bacteriocin AS-48. Curr. Microbiol. 2001, 42, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Balciunas, E.M.; Martinez, F.A.C.; Todorov, S.D.; de Melo Franco, B.D.; Converti, A.; Oliveira, R.P.D. Novel biotechnological applications of bacteriocins: A review. Food Control. 2013, 32, 134–142. [Google Scholar] [CrossRef]
- Rosengren, A.; Lindblad, M.; Lindqvist, R. The effect of undissociated lactic acid on Staphylococcus aureus growth and enterotoxin a production. Int. J. Food Microbiol. 2013, 162, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Balannec, B.; Bouguettoucha, A.; Amrane, A. Unstructured model for batch cultures without pH control of Lactobacillus helveticus—Inhibitory effect of the undissociated lactic acid. Biochem. Eng. J. 2007, 35, 289–294. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Fang, W.; Pradhan, A.K.; Li, Y. A predictive model for assessment of decontamination effects of lactic acid and chitosan used in combination on Vibrio parahaemolyticus in shrimps. Int. J. Food Microbiol. 2013, 167, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.A. Antimicrobial effects of lactates—A review. J. Food Prot. 1994, 57, 445–450. [Google Scholar] [CrossRef]
- Lindblad, M.; Lindqvist, R. Modelling time to growth of Escherichia coli as a function of water activity and undissociated lactic acid. Lett. Appl. Microbiol. 2010, 50, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Biesta-Peters, E.G.; Reij, M.W.; Gorris, L.G.M.; Zwietering, M.H. Comparing nonsynergistic gamma models with interaction models to predict growth of emetic Bacillus cereus when using combinations of pH and individual undissociated acids as growth-limiting factors. Appl. Environ. Microbiol. 2010, 76, 5791–5801. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; Scarpellini, M.; Franzetti, L.; Colombo, S.; Galli, A. Reclassification of Lactobacillus maltaromicus (Miller et al. 1974) DSM 20342(T) and DSM 20344 and Carnobacterium piscicola (Collins et al. 1987) dsm 20730(T) and DSM 20722 as Carnobacterium maltaromaticum comb. Nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.I.; Boulahya, K.A.; Paris, C.; Delaunay, S.; Cailliez-Grimal, C. Effect of oxygen on the biosynthesis of flavor compound 3-methylbutanal from leucine catabolism during batch culture in Carnobacterium maltaromaticum lma 28. J. Dairy Sci. 2013, 96, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Borch, E.; Molin, G. The aerobic growth and product formation of Lactobacillus, Leuconostoc, Brochothrix, and Carnobacterium in batch cultures. Appl. Microbiol. Biotechnol. 1989, 30, 81–88. [Google Scholar] [CrossRef]
- Castellano, P.; Raya, R.; Vignolo, G. Mode of action of lactocin 705, a two-component bacteriocin from Lactobacillus casei crl705. Int. J. Food Microbiol. 2003, 85, 35–43. [Google Scholar] [CrossRef]
- McAuliffe, O.; Ryan, M.P.; Ross, R.P.; Hill, C.; Breeuwer, P.; Abee, T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 1998, 64, 439–445. [Google Scholar] [PubMed]
- Jacquet, T.; Cailliez-Grimal, C.; Francius, G.; Borges, F.; Imran, M.; Duval, J.F.; Revol-Junelles, A.M. Antibacterial activity of class IIa bacteriocin Cbn BM1 depends on the physiological state of the target bacteria. Res. Microbiol. 2012, 163, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.B.; Skaugen, M.; Salehian, Z.; Holo, H.; Nes, I.F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 2007, 104, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Hechard, Y.; Sahl, H.G. Mode of action of modified and unmodified bacteriocins from gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef]
- Henning, S.; Metz, R.; Hammes, W.P. Studies on the mode of action of nisin. Int. J. Food Microbiol. 1986, 3, 121–134. [Google Scholar] [CrossRef]
- Henry, C.; Koumanov, F.; Ghezzi, C.; Mathieu, J.P.; Hamant, S.; De Leiris, J.; Comet, M. Experimental models, protocols, and reference values for evaluation of iodinated analogues of glucose. Nucl. Med. Biol. 1995, 22, 875–885. [Google Scholar] [CrossRef]
- Stoffels, G.; Nes, I.F.; Guthmundsdottir, A. Isolation and properties of a bacteriocin-producing Carnobacterium piscicola isolated from fish. J. Appl. Bacteriol. 1992, 73, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Khouiti, Z.; Simon, J.P. Carnocin kz213 produced by Carnobacterium piscicola 213 is adsorbed onto cells during growth. Its biosynthesis is regulated by temperature, pH and medium composition. J. Ind. Microbiol. Biotechnol. 2004, 31, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Sergelidis, D. Chemostat production of pediocin SM-1 by Pediococcus pentosaceus mees 1934. Biotechnol. Prog. 2015, 31, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Stiles, M.E.; Holzapfel, W.H. Bacteriocin production by Carnobacterium piscicola LV 61. Int. J. Food Microbiol. 1993, 20, 131–147. [Google Scholar] [CrossRef]
- Aryani, D.C.; den Besten, H.M.; Hazeleger, W.C.; Zwietering, M.H. Quantifying strain variability in modeling growth of Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.; Ratkowsky, D.A.; Mellefont, L.A.; McMeekin, T.A. Modelling the effects of temperature, water activity, pH and lactic acid concentration on the growth rate of escherichia coli. Int. J. Food Microbiol. 2003, 82, 33–43. [Google Scholar] [CrossRef]
- Campos, D.T.; Marks, B.P.; Powell, M.R.; Tamplin, M.L. Quantifying the robustness of a broth-based Escherichia coli o157:H7 growth model in ground beef. J. Food Prot. 2005, 68, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Mejlholm, O.; Gunvig, A.; Borggaard, C.; Blom-Hanssen, J.; Mellefont, L.; Ross, T.; Leroi, F.; Else, T.; Visser, D.; Dalgaard, P. Predicting growth rates and growth boundary of Listeria monocytogenes—An international validation study with focus on processed and ready-to-eat meat and seafood. Int. J. Food Microbiol. 2010, 141, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Tienungoon, S.; Ratkowsky, D.A.; McMeekin, T.A.; Ross, T. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl. Environ. Microbiol. 2000, 66, 4979–4987. [Google Scholar] [CrossRef] [PubMed]

| Medium (No.) | Lactic Acid (mM) | Glucose (mM) | pH | UndisLA (mM) 1 |
|---|---|---|---|---|
| 1 | 0 | 0 | 5.5 | 0 |
| 2 | 0 | 0 | 6 | 0 |
| 3 | 0 | 0 | 6.5 | 0 |
| 4 | 25 | 0 | 5.5 | 0.56 |
| 5 | 25 | 0 | 6 | 0.18 |
| 6 | 25 | 0 | 6.5 | 0.06 |
| 7 | 50 | 0 | 5.5 | 1.12 |
| 8 | 50 | 0 | 6 | 0.36 |
| 9 | 50 | 0 | 6.5 | 0.11 |
| 10 | 0 | 0.56 | 5.5 | 0 |
| 11 | 0 | 0.56 | 6 | 0 |
| 12 | 0 | 0.56 | 6.5 | 0 |
| 13 | 25 | 0.56 | 5.5 | 0.56 |
| 14 | 25 | 0.56 | 6 | 0.18 |
| 15 | 25 | 0.56 | 6.5 | 0.06 |
| 16 | 50 | 0.56 | 5.5 | 1.12 |
| 17 | 50 | 0.56 | 6 | 0.36 |
| 18 | 50 | 0.56 | 6.5 | 0.11 |
| 19 | 0 | 5.55 | 5.5 | 0 |
| 20 | 0 | 5.55 | 6 | 0 |
| 21 | 0 | 5.55 | 6.5 | 0 |
| 22 | 25 | 5.55 | 5.5 | 0.56 |
| 23 | 25 | 5.55 | 6 | 0.18 |
| 24 | 25 | 5.55 | 6.5 | 0.06 |
| 25 | 50 | 5.55 | 5.5 | 1.12 |
| 26 | 50 | 5.55 | 6 | 0.36 |
| 27 | 50 | 5.55 | 6.5 | 0.11 |
| Temperature (°C) | Atmosphere | Medium No. 1 | Incubation Time (Days) | |
|---|---|---|---|---|
| Aerobic | Anaerobic | |||
| 25 | + | + | 1–27 | 10 |
| 15 | + | + | 1–27 | 19 |
| 7 | + | + | 1–27 | 45 |
| −1 | + | + | 1–3, 5, 6, 8–12, 14, 15, 17–21, 23, 24, 26, 27 | 145 |
| −1 | + | + | 13 | 263 |
| −1 | + | 4, 16, 22 | 263 | |
| −1 | + | + | 7, 25 | NG 2 |
| −1 | + | 4, 16, 22 | NG | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Kaur, M.; Bowman, J.P.; Ratkowsky, D.A.; Tamplin, M. Effect of Environmental Factors on Intra-Specific Inhibitory Activity of Carnobacterium maltaromaticum. Microorganisms 2017, 5, 59. https://doi.org/10.3390/microorganisms5030059
Zhang P, Kaur M, Bowman JP, Ratkowsky DA, Tamplin M. Effect of Environmental Factors on Intra-Specific Inhibitory Activity of Carnobacterium maltaromaticum. Microorganisms. 2017; 5(3):59. https://doi.org/10.3390/microorganisms5030059
Chicago/Turabian StyleZhang, Peipei, Mandeep Kaur, John P. Bowman, David A. Ratkowsky, and Mark Tamplin. 2017. "Effect of Environmental Factors on Intra-Specific Inhibitory Activity of Carnobacterium maltaromaticum" Microorganisms 5, no. 3: 59. https://doi.org/10.3390/microorganisms5030059





