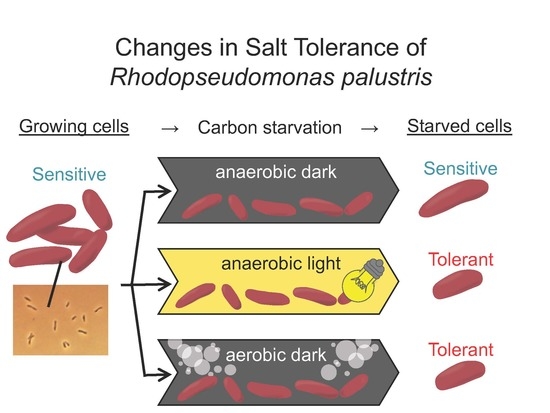
Increase of Salt Tolerance in Carbon-Starved Cells of Rhodopseudomonas palustris Depending on Photosynthesis or Respiration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Growth and Preparation of Carbon-Starved Cells
2.2. Cellular ATP Contents
2.3. Evaluation of Salt Stress Tolerance
2.4. Microscopic Analysis of Cells
3. Results
3.1. Cellular ATP Contents
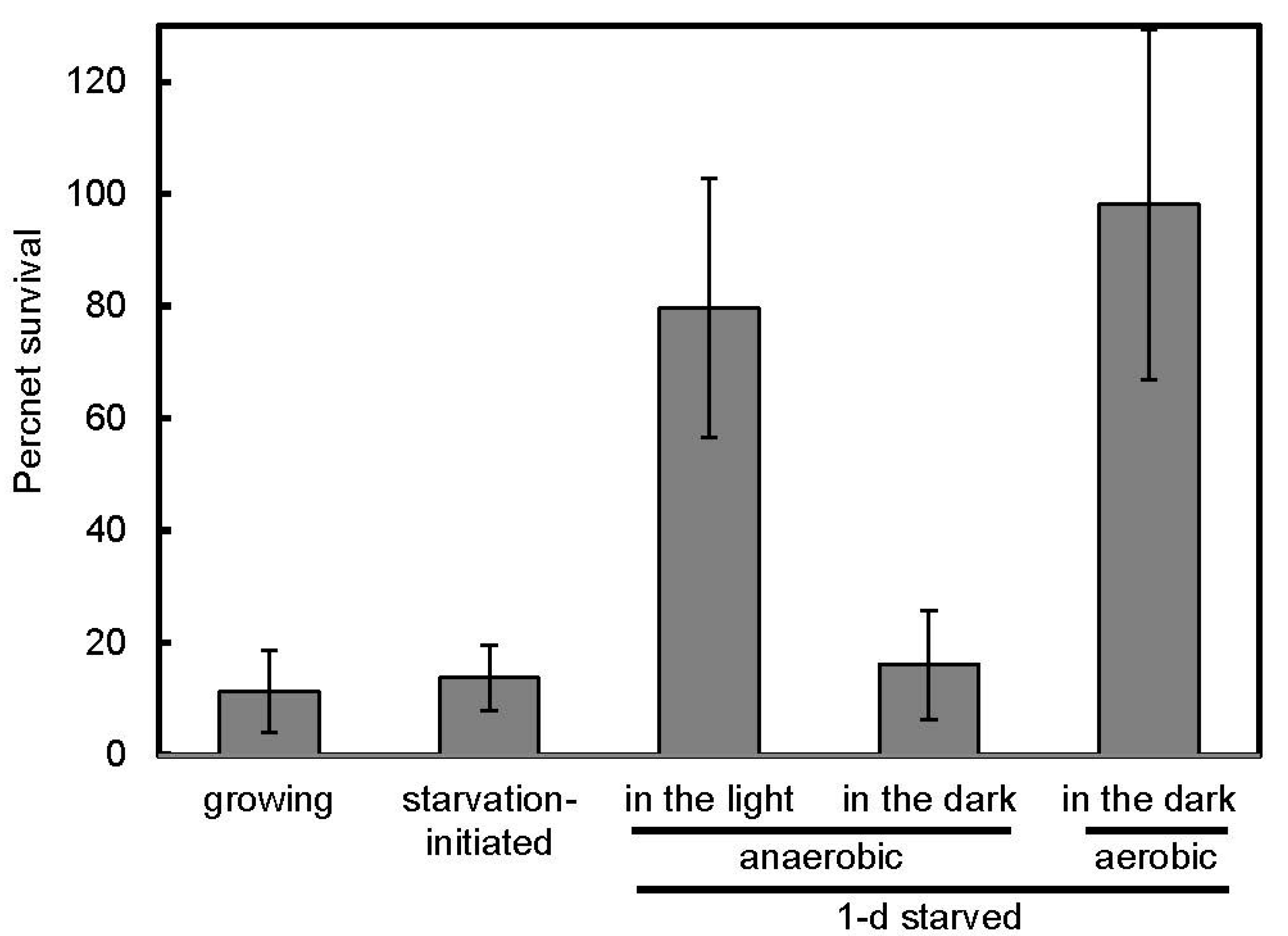
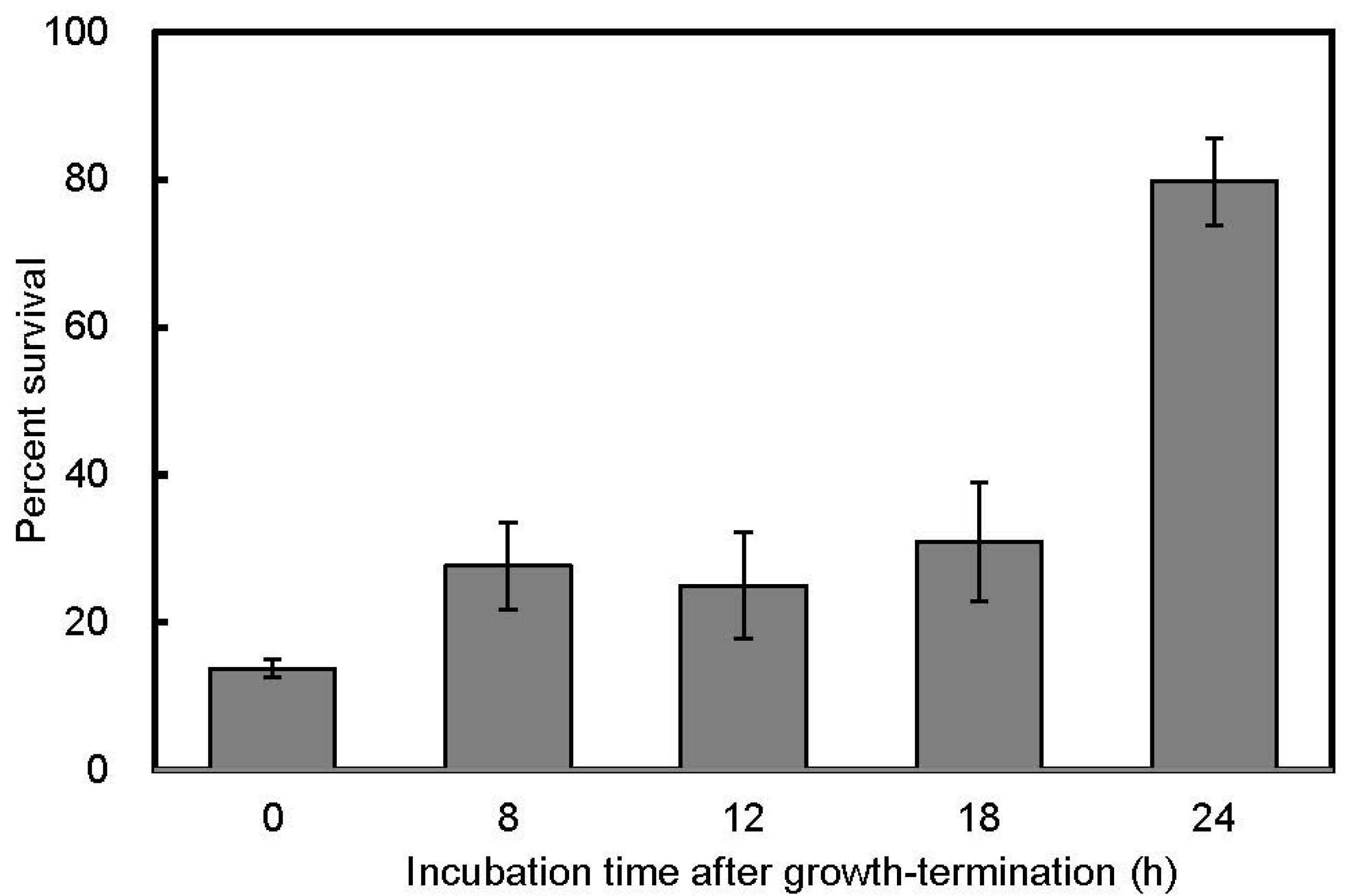
3.2. Salt Stress Tolerance
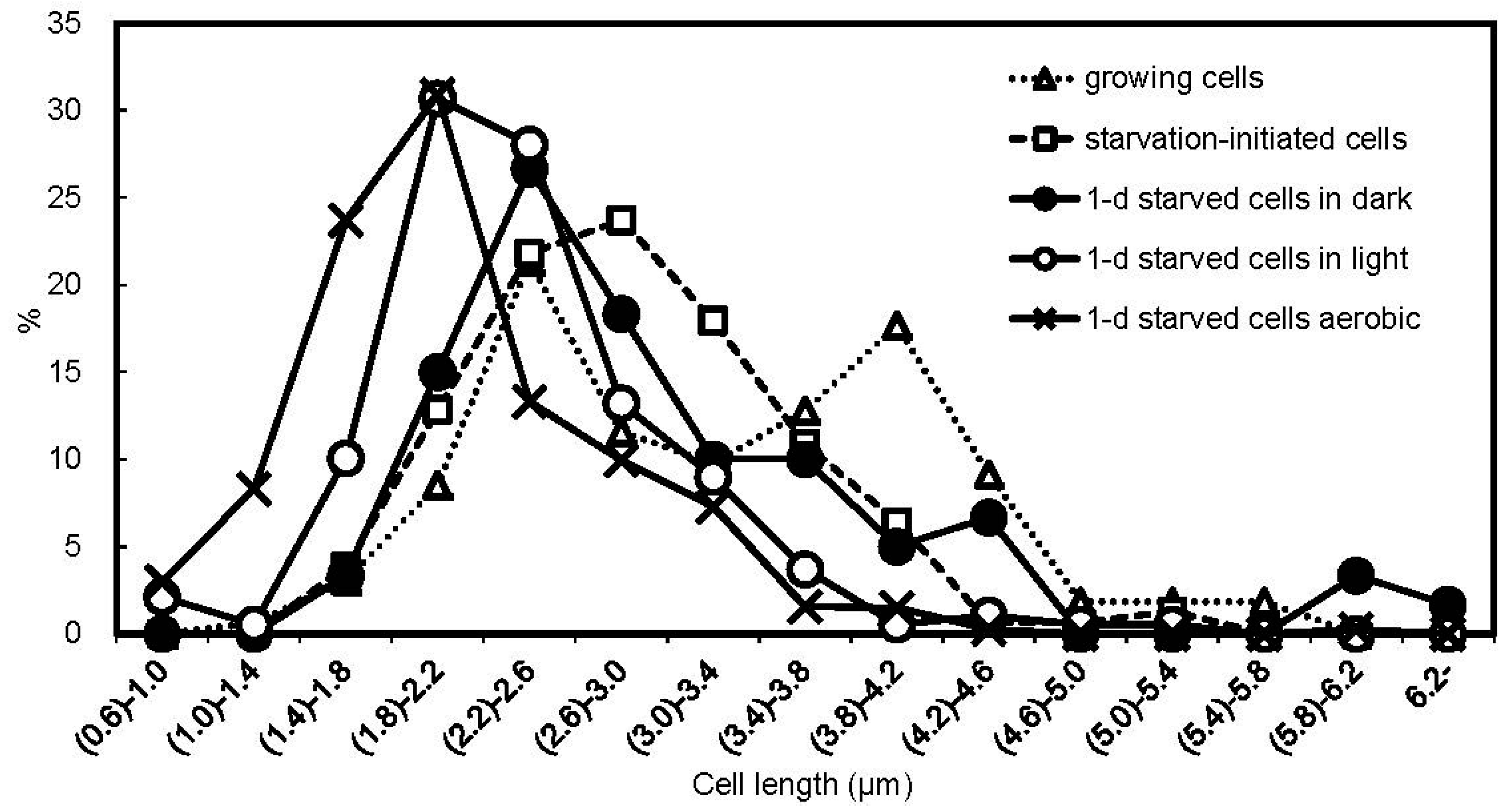
3.3. Cell Size
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roszak, D.B.; Colwell, R.R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [PubMed]
- Kjelleberg, S.; Albertson, N.; Flärdh, K.; Holmquist, L.; Jouper-Jaan, A.; Marouga, R.; Ostling, J.; Svenblad, B.; Weichart, D. How do non-differentiating bacteria adapt to starvation? Antonie Leeuwenhoek 1993, 63, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.E.N. Stress induced cross-protection against environmental challenges on prokaryotic and eukaryotic microbes. World J. Microbiol. Biotechnol. 2011, 27, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, E.S.C.; Baek, S.-H.; Sassetti, C.M. The Normalcy of dormancy: Common themes in microbial quiescence. Cell Host Microbe 2013, 13, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Roy, S.; Rosenkrands, I.; Lindenstrøm, T.; Filskov, J.; Rasmussen, E.M.; Cassidy, J.; Andersen, P. Differential influence of nutrient-starved Mycobacterium tuberculosis on adaptive immunity results in progressive tuberculosis disease and pathology. Infect. Immun. 2015, 83, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Kanno, N. Survivability of microbes in natural environments and their ecological impacts. Microbes Environ. 2015, 30, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Jane, W.-N.; Chen, Y.-S.; Wong, H. Morphological changes of Vibrio parahaemolyticus under cold and starvation stresses. Int. J. Food Microbiol. 2009, 129, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Givskov, M.; Eberl, L.; Møller, S.; Poulsen, L.K.; Molin, S. Responses to nutrient starvation in Pseudomonas putida KT2442: Analysis of general cross-protection, cell shape, and macromolecular content. J. Bacteriol. 1994, 176, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kaberdin, V.R.; Montánchez, I.; Parada, C.; Orruño, M.; Arana, I.; Barcina, I. Unveiling the metabolic pathways associated with the adaptive reduction of cell size during Vibrio harveyi persistence in seawater microcosms. Microb. Ecol. 2015, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Lappin-Scott, H.M.; Costerton, J.W. Starvation and penetration of bacteria in soils and rocks. Experientia 1990, 46, 807–812. [Google Scholar] [CrossRef]
- Morita, R.Y. Bacteria in Oligotrophic Environments; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Petrova, O.; Gorshkov, V.; Daminova, A.; Ageeva, M.; Moleleki, L.N.; Gogolev, Y. Stress response in Pectobacterium atrosepticum SCRI1043 under starvation conditions: Adaptive reactions at a low population density. Res. Microbiol. 2014, 165, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.H.; Williams, H.D. Adaptation to nutrient starvation in Rhizobium leguminosarum bv. phaseoli: Analysis of survival, stress resistance, and changes in macromolecular synthesis during entry to and exit from stationary phase. J. Bacteriol. 1997, 179, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Gengenbacher, M.; Dick, T. Mild nutrient starvation triggers the development of a small-cell survival morphotype in Mycobacteria. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.; Kline, L.; Kenyon, W. Carbon-starvation induces cross-resistance to thermal, acid, and oxidative stress in Serratia marcescens. Microorganisms 2015, 3, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Hurst, G.B.; Lu, T.-Y.S.; Perry, L.M.; Pan, C.; Lankford, P.K.; Pelletier, D.A. Rhodopseudomonas palustris CGA010 proteome implicates extracytoplasmic function sigma factor in stress response. J. Proteome Res. 2015, 14, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Francez-Charlot, A.; Kaczmarczyk, A.; Fischer, H.-M.; Vorholt, J.A. The general stress response in Alphaproteobacteria. Trends Microbiol. 2015, 23, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Landini, P.; Egli, T.; Wolf, J.; Lacour, S. sigmaS, a major player in the response to environmental stresses in Escherichia coli: Role, regulation and mechanisms of promoter recognition. Environ. Microbiol. Rep. 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, J.B.; Oda, Y.; Rühl, M.; Posto, A.L.; Sauer, U.; Harwood, C.S. Non-growing Rhodopseudomonas palustris increases the hydrogen gas yield from acetate by shifting from the glyoxylate shunt to the tricarboxylic acid cycle. J. Biol. Chem. 2014, 289, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Breznak, J.A.; Potrikus, C.J.; Pfennig, N.; Ensign, J.C. Viability and endogenous substrates used during starvation survival of Rhodospirillum rubrum. J. Bacteriol. 1978, 134, 381–388. [Google Scholar] [PubMed]
- Kanno, N.; Matsuura, K.; Haruta, S. Differences in survivability under starvation conditions among four species of purple nonsulfur phototrophic bacteria. Microbes Environ. 2014, 29, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Odaa, Y.; Slagmana, S.; Meijerb, W.G.; Forneya, L.J.; Gottschala, J.C. Influence of growth rate and starvation on fluorescent in situ hybridization of Rhodopseudomonas palustris. FEMS Microbiol. Ecol. 2000, 32, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pechter, K.B.; Gallagher, L.; Pyles, H.; Manoil, C.S.; Harwood, C.S. Essential genome of the metabolically versatile alphaproteobacterium Rhodopseudomonas palustris. J. Bacteriol. 2016, 198, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Hiraishi, A.; Shimada, K.; Matsuura, K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int. J. Syst. Bacteriol. 1995, 45, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Rao, S.P.S.; Pethe, K.; Dick, T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 2010, 156, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Blair, K.M.; Salama, N.R. Staying in shape: The impact of cell shape on bacterial survival in diverse environments. Microbiol. Mol. Biol. Rev. 2016, 80, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999, 63, 334–348. [Google Scholar] [PubMed]
- Pastor, J.M.; Bernal, V.; Salvador, M.; Argandoña, M.; Vargas, C.; Csonka, L.; Sevilla, Á.; Iborra, J.L.; Nieto, J.J.; Cánovas, M. Role of central metabolism in the osmoadaptation of the halophilic bacterium Chromohalobacter salexigens. J. Biol. Chem. 2013, 288, 17769–17781. [Google Scholar] [CrossRef] [PubMed]
- Denich, T.J.; Beaudette, L.A.; Lee, H.; Trevors, J.T. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- López, C.S.; Garda, H.A.; Rivas, E.A. The effect of osmotic stress on the biophysical behavior of the Bacillus subtilis membrane studied by dynamic and steady-state fluorescence anisotropy. Arch. Biochem. Biophys. 2002, 408, 220–228. [Google Scholar] [CrossRef]
- Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Disalvo, E.A. Influence of the growth at high osmolality on the lipid composition, water permeability and osmotic response of Lactobacillus bulgaricus. Arch. Biochem. Biophys. 2005, 443, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.; Oh, D.-C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiol. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Botteldoorn, N.; Herman, L.; Možina, S.S. Survival and stress induced expression of groEL and rpoD of Campylobacter jejuni from different growth phases. Int. J. Food Microbiol. 2006, 112, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Rockabrand, D.; Arthur, T.; Korinek, G.; Livers, K.; Blum, P. An essential role for the Escherichia coli DnaK protein in starvation-induced thermotolerance, H2O2 resistance, and reductive division. J. Bacteriol. 1995, 177, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Van Overbeek, L.S.; Eberl, L.; Givskov, M.; Molin, S.; van Elsas, J.D. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl. Environ. Microbiol. 1995, 61, 4202–4208. [Google Scholar] [PubMed]
| Growing Cells | Starvation-Initiated Cells | 1-d Anaerobic Starved Cells in the Light | 1-d Anaerobic Starved Cells in the Dark | 1-d Aerobic Starved Cells in the Dark | |
|---|---|---|---|---|---|
| × 10−16 mol CFU−1 | 4.12 ± 0.52 | 6.45 ± 0.37 | 12.9 ± 0.87 | 4.06 ± 0.45 | 7.49 ± 0.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasai, S.; Kanno, N.; Matsuura, K.; Haruta, S. Increase of Salt Tolerance in Carbon-Starved Cells of Rhodopseudomonas palustris Depending on Photosynthesis or Respiration. Microorganisms 2018, 6, 4. https://doi.org/10.3390/microorganisms6010004
Wasai S, Kanno N, Matsuura K, Haruta S. Increase of Salt Tolerance in Carbon-Starved Cells of Rhodopseudomonas palustris Depending on Photosynthesis or Respiration. Microorganisms. 2018; 6(1):4. https://doi.org/10.3390/microorganisms6010004
Chicago/Turabian StyleWasai, Sawa, Nanako Kanno, Katsumi Matsuura, and Shin Haruta. 2018. "Increase of Salt Tolerance in Carbon-Starved Cells of Rhodopseudomonas palustris Depending on Photosynthesis or Respiration" Microorganisms 6, no. 1: 4. https://doi.org/10.3390/microorganisms6010004