Equine Welfare during Exercise: An Evaluation of Breathing, Breathlessness and Bridles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Some Key Attributes of Breathing-Related Functions in the Horse
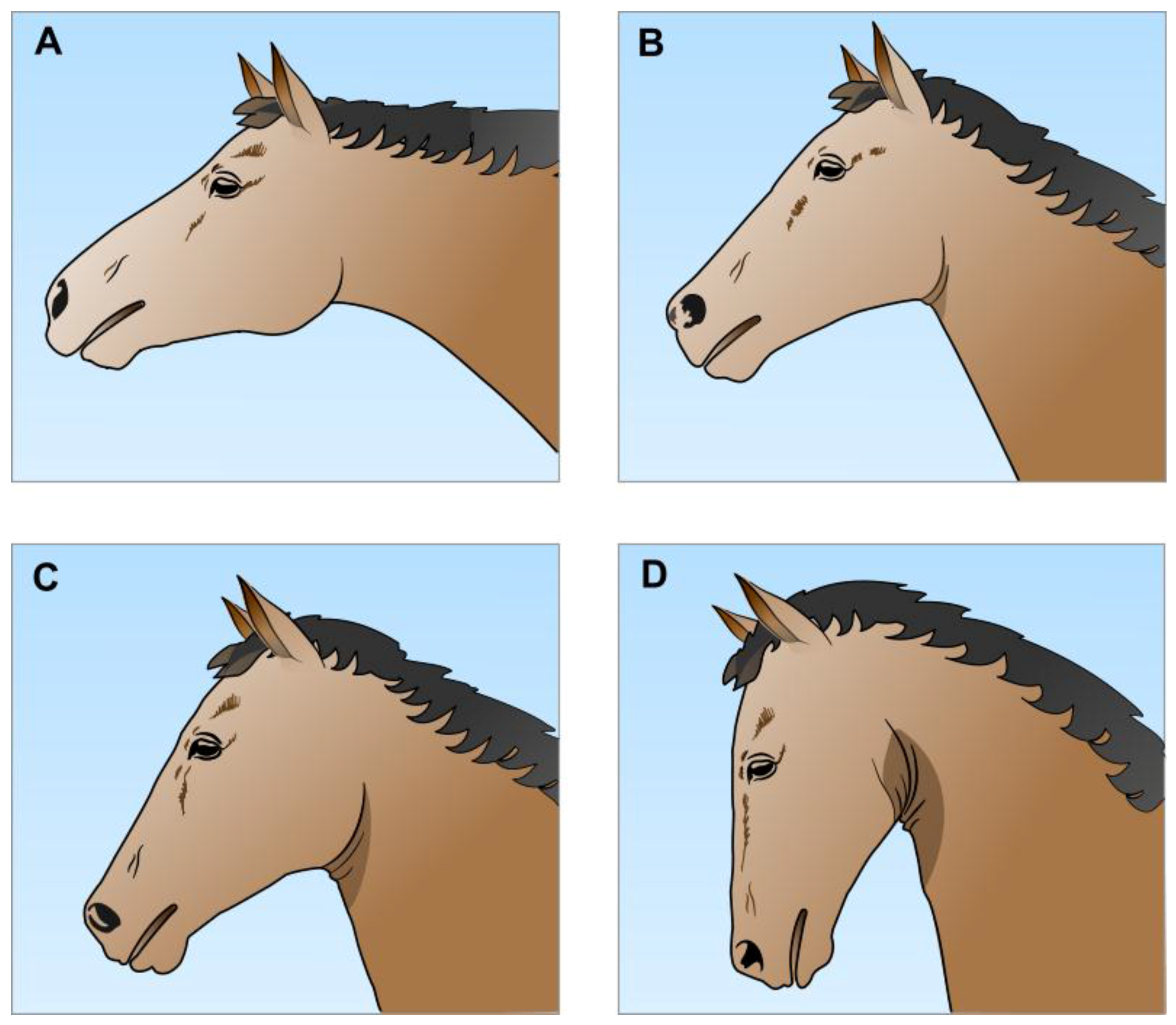
2.1. Upper Airway Anatomy and Obligate Nasal Breathing
2.2. Upper Respiratory Tract Airflow Capacity and Resistance
2.2.1. Jowl Angle and Airflow Resistance
2.2.2. Airflow Resistance and Disorders of the Soft Palate and Nasopharyngeal Walls
2.2.3. Other Pathophysiological Impediments to Airflow in the Upper Respiratory Tract
2.2.4. Clustering of Multiple Upper Respiratory Tract Airflow Impediments
2.3. Oxygen, Carbon Dioxide and Other Key Features of Respiratory Function in Galloping Horses
2.3.1. Oxygen and Carbon Dioxide Partial Pressures, pH and Chemoreceptor Function
2.3.2. Hypoxaemia, Hypercapnia and Acidaemia in Galloping Horses
2.4. Lower Respiratory Tract Pathophysiology and Exercise
2.4.1. Exercise-Induced Pulmonary Haemorrhage (EIPH)
2.4.2. Negative Pressure Pulmonary Oedema (NPPO)
2.4.3. Inflammatory Airway Disease (IAD)
3. Breathing Mechanisms That Underlie Breathlessness
4. Likely Forms of Breathlessness in Exercising Horses
4.1. “Respiratory Effort” and High Airflow Resistance
4.2. “Air Hunger” and the Chemoreceptor-Induced Drive to Breathe
4.3. Potential for Unpleasant “Respiratory Effort” and “Air Hunger” to Occur Simultaneously
4.4. “Chest Tightness” and Lower Respriatory Tract Inflammatroy Processes
4.5. Assessment of the Potential for Breathlessness to Occur in Freely Running Horses
5. Some Implications of Bitted and Bitless Bridle Use
5.1. Bits, Control of Behaviour, and Pain
5.2. Behavioural Signs of Bit-Induced Pain and Discomfort
5.3. Mouth Behaviour of Feral Horses and Horses Wearing Bitless Bridles or Halters
5.4. Respiratory Functionality of a Closed Mouth in Exercising Horses
5.5. Potential Impacts of Bitless Bridles on Breathlessness
5.5.1. Bridle Type in Treadmill and Other Studies
5.5.2. Bridle Type and Potential Impacts on Breathlessness
6. Some Animal Welfare Implications
6.1. Breathlessness and Urging Racehorses to Sustain Maximal Exercise
6.2. Fatigue, Exercise Intolerance and Breathlessness
6.3. Behaviours that Validly Indicate Horses’ Aversion to Bits
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramey, D.W. A historical survey of human-equine interactions. In Equine Welfare; McIlwraith, C.W., Rollin, B.E., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 22–58. [Google Scholar]
- Barrey, E. Genetic basis of equine performance. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 43–58. [Google Scholar]
- Hinchcliff, K.W. Exercise-induced pulmonary haemorrhage (EIPH). In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 633–647. [Google Scholar]
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J. (Eds.) Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Duncan, I.J.H. “Pleasures”, “pains” and animal welfare: Towards a natural history of affect. Anim. Welfare 1998, 7, 383–396. [Google Scholar]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D. Understanding Animal Welfare: The Science in Its Cultural Context; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Yeates, J.W.; Main, D.C.J. Assessment of positive welfare: A review. Vet. J. 2008, 175, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kiley-Worthington, M. Equine psychological needs and quality of life. In Equine Welfare; McIlwraith, C.W., Rollin, B.E., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 94–112. [Google Scholar]
- Green, T.C.; Mellor, D.J. Extending ideas about animal welfare assessment to include “quality of life” and related concepts. N. Z. Vet. J. 2011, 59, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Lee, C. How assessing relationships between emotions and cognition can improve farm animal welfare. Rev. Sci. Tech. Off. Int. Epizoot. 2014, 33, 103–110. [Google Scholar] [CrossRef]
- Broom, D.M. Animal Welfare in the European Union; Policy Department C Citizens: Rights and Constitutional Affairs, Directorate General for Internal Policies, European Parliament: Brussels, Belgium, 2017; Available online: http://www.europarl.europa.eu/RegData/etudes/workshop/join/2013/474410/IPOL-FEMM_AT(2013)474410_EN.pdf (accessed on 14 April 2017).
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “A Life worth Living. Animals 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.G. Physiology and Behaviour of Animal Suffering; Blackwell Science: Oxford, UK, 2004. [Google Scholar]
- Denton, D.A.; McKinley, M.J.; Farrell, M.; Egan, G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cognit. 2009, 18, 500–514. Available online: http://dx.doi.org/10.1016/j.concog.2008.06.009 (accessed on 22 May 2017). [CrossRef] [PubMed]
- Vinuela-Fernandz, I.; Weary, D.M.; Flecknell, P. Pain. In Animal Welfare, 2nd ed.; Appleby, M.C., Mench, J., Olsson, I.A., Hughes, B.O., Eds.; CABI: Oxford, UK, 2011; pp. 64–77. [Google Scholar]
- Art, T.; Scrteyn, D.; Lekeux, P. Effect of exercise on the partitioning of equine respiratory resistance. Equine Vet. J. 1988, 20, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Art, T.; Anderson, I.; Woakes, A.I.; Roberts, C.; West, P.J.; Snow, D.H.; Lekeux, P. Mechanics of breathing during strenuous exercise in thoroughbred horses. Respir. Physiol. 1990, 82, 279–294. [Google Scholar] [CrossRef]
- Ducharme, N.G.; Hackett, R.P.; Ainsworth, D.A.; Erb, H.N.; Shannon, K.J. Repeatability and normal values for measurement of pharyngeal and tracheal pressures in exercising horses. Am. J. Vet. Res. 1994, 55, 369–374. [Google Scholar]
- Odeh, M.; Schnall, R.; Gavriely, N.; Oliven, A. Dependency of upper airway patency on head position: The effect of muscle contraction. Respir. Physiol. 1995, 100, 239–244. [Google Scholar] [CrossRef]
- Cook, W.R. Pathophysiology of bit control in the horse. J. Equine Vet. Sci. 1999, 19, 196–204. [Google Scholar] [CrossRef]
- Cehak, A.; Rohn, K.; Barton, A.; Stadler, P.; Ohnesorge, B. Effect of head and neck position on pharyngeal diameter in horses. Vet. Radiol. Ultrasound 2010, 51, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bayly, W.M.; Grant, B.D.; Breeze, R.G. Arterial blood gas tension and acid base balance during exercise in horses with pharyngeal lymphoid hyperplasia. Equine Vet. J. 1984, 16, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Bayly, W.M.; Hodgson, D.R.; Schulz, D.A.; Dempseu, J.A.; Gollnick, P.D. Exercise-induced hypercapnia in the horse. J. Appl. Physiol. 1989, 67, 1958–1966. [Google Scholar] [PubMed]
- Butler, P.J.; Woakes, A.J.; Smale, K.; Roberts, C.A.; Hillidge, C.J.; Snow, D.H.; Marlin, D.J. Respiratory and cardiovascular adjustments during exercise of increasing intensity and during recovery in thoroughbred racehorses. J. Exp. Biol. 1993, 179, 159–180. [Google Scholar] [PubMed]
- Tate, L.P.; Corbett, W.T.; Bishop, B.J.; Foreman, J.H. Blood gas tensions, acid-base status, heart rates and venous profiles in exercising horses with laryngeal hemiplegia before and after corrective surgery. Vet. Surg. 1993, 22, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Durando, M.M.; Martin, B.B.; Hammer, E.J.; Langsam, P.P.; Birks, E.K. Dynamic upper airway changes and arterial blood gas parameters during treadmill exercise. Equine Exercise Physiology 6. Equine Vet. J. 2002, 34, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Art, T.; Bayly, W. Lower airway function: Responses to exercise and training. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 587–603. [Google Scholar]
- Widdicombe, J. Lung afferent activity: Implications for respiratory sensation. Respir. Physiol. Neurobiol. 2009, 167, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, J.-P.; Leguillette, R.; Richard, E.A. Inflammatory airway disease of horses—Revised consensus statement. J. Vet. Int. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, K.E.; Greet, T.R.C. Collection and evaluation of tracheobronchial washes in the horse. Equine Vet. J. 1984, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.R.; Soma, L.R.; Maxson, A.D.; Thompson, J.E.; Holcombe, S.J.; Spencer, P.A. Effects of furosemide on the racing times of Thoroughbreds. Am. J. Vet. Res. 1990, 51, 772–778. [Google Scholar] [PubMed]
- Lapointe, J.M.; Vrins, A.; McCarvill, E. A survey of exercise-induced pulmonary haemorrhage in Quebec standardbred racehorses. Equine Vet. J. 1994, 26, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Erickson, H.H. Exercise-induced pulmonary hemorrhage: Where are we now? VMRR 2016, 7, 133–148. [Google Scholar] [CrossRef]
- Cook, W.R.; Mills, D.S. Preliminary study of jointed snaffle vs. crossunder bitless bridles: Quantified comparison of behaviour in four horses. Equine Vet. J. 2009, 41, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Quick, J.S.; Warren-Smith, A.K. Preliminary investigations of horses’ (Equus caballus) responses to different bridles during foundation training. J. Vet. Behav. 2009, 4, 169–176. [Google Scholar] [CrossRef]
- Cook, W.R. An endoscopic test for bit-induced nasopharyngeal asphyxia as a cause of exercise-induced pulmonary haemorrhage in the horse. Equine Vet. J. 2014, 46, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.R. Hypothesis article: Bit-induced asphyxia in the racehorse as a cause of sudden death. Equine Vet. J. 2016, 28, 405–409. [Google Scholar] [CrossRef]
- Lyle, C.H.; Uzal, F.A.; McGorum, B.C.; Aida, H.; Blissitt, K.J.; Case, J.T.; Charles, J.T.; Gardner, I.; Horadagoda, N.; Kusano, K.; et al. Sudden death in racing Thoroughbred horses: An international multicentre study of post mortem findings. Equine Vet. J. 2011, 43, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.R. A hypothetical, aetiological relationship between the horse’s bit, nasopharyngeal oedema and negative pressure pulmonary oedema. Equine Vet. Educ. 2014, 26, 381–389. [Google Scholar] [CrossRef]
- Cheetham, J.; Holcombe, S.J.; Ducharme, N.G. Upper airway function of normal horses during exercise. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 529–547. [Google Scholar]
- Cook, W.R. Some observations on form and function of the equine upper airway in health and disease, Part I: The pharynx. Proc. AAEP 1981, 27, 355–392. [Google Scholar]
- Lane, J.G.; Blandon, B.; Little, D.R.M.; Naylor, J.R.J.; Franklin, S.H. Dynamic obstruction of the equine upper respiratory tract. Part 1. Observations during high-speed treadmill endoscopy in 600 Thoroughbred racehorses. Equine Vet. J. 2006, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Malony, G.M.; Welbel, E.R.; Langman, V.A.; Kamau, J.M.; Seeherman, H.J.; Heglund, N.C. Design of the mammalian respiratory system. Respir. Physiol. 1981, 44, 25–37. [Google Scholar] [PubMed]
- Weber, J.M.; Dobson, G.P.; Parkhouse, W.S.; Wheeldon, D.; Harman, J.C.; Snow, D.H.; Hochachka, P.W. Cardiac output and oxygen consumption in exercising Thoroughbred horses. Am. J. Appl. Physiol. 1987, 253, R890–R895. [Google Scholar]
- Art, T.; Lekeux, P. Training-induced modification of cardiorespiratory and ventilatory measurements in thoroughbred horses. Equine Vet. J. 1993, 25, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.G.; Hackett, R.P.; Gleed, R.D.; Ainsworth, D.M.; Erickson, B.K.; Erb, H.N.; Soderholm, L.V.; Mitchell, L.M. Pulmonary capillary pressure in horses undergoing alternation to pleural pressure by imposition of upper airway resistive loads. Equine Vet. J. 1999, 30, 27–33. [Google Scholar]
- Wilson, W.D.; Lofstedt, J.; Lakritz, J. Abnormal respiratory noise (stridor). In Large Animal Internal Medicine, 5th ed.; Smith, B.P., Ed.; Elsevier: St Louis, MI, USA, 2009; pp. 66–70. [Google Scholar]
- Gerring, E.L. Differential diagnosis of equine respiratory noises. Practce 1985, 7, 109–117. [Google Scholar] [CrossRef]
- Petsche, V.M.; Derksen, F.J.; Berney, C.E.; Robinson, N.E. Effect of head position on upper airway function in exercising horses. Equine Vet. J. 1995, 18, 18–22. [Google Scholar] [CrossRef]
- Strand, E.; Fjordbakk, C.T.; Holcombe, S.J.; Risberg, A.; Chalmers, H.J. Effect of poll flexion and dynamic laryngeal collapse on tracheal pressure in Norwegian Coldblooded Trotter racehorses. Equine Vet. J. 2009, 41, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Linford, R.L.; O’Brien, T.R.; Wheat, J.D.; Meagher, D. Radiographic assessment of epiglotic length and pharyngeal and laryngeal diameters in the thoroughbred. Am. J. Vet. Res. 1983, 44, 1660–1666. [Google Scholar] [PubMed]
- Allen, K.J.; Terron-Canedo, N.; Hillyer, M.H.; Franklin, S.H. Equitation and exercise factors affecting dynamic upper respiratory tract function: A review illustrated by case reports. Equine Vet. Educ. 2011, 23, 361–368. [Google Scholar] [CrossRef]
- Go, L.-M.; Barton, A.K.; Ohnesorge, B. Objective classification of different head and neck positions and their influence on the radiographic pharyngeal diameter in sport horses. BMC Vet. Res. 2014, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Houghton Mifflin Company. Poiseuille’s Law: Dictionary.com “poiseuille’s law”. In The American Heritage® Science Dictionary; Houghton Mifflin Company: Boston, MA, USA, 2017; Available online: http://www.dictionary.com/browse/poiseuille-s-law (accessed on 10 March 2017).
- Poiseuille’s Law: IV Fluids, Open Anaesthesia. 2017. Available online: https://www.openanesthesia.org/poiseuilles_law_iv_fluids/ (accessed on 10 March 2017).
- Meyer, H. “Rollkur”, “Hyperflexion” and “LDR”—The natural position of the head and neck of the horse and the modification by the rider. Pferdeheilkunde 2010, 26, 388–413. [Google Scholar] [CrossRef]
- Zebisch, A.; May, A.; Reese, S.; Gehlen, H. Effects of different head-neck positions on the larynges of ridden horses. J. Anim. Physiol. Anim. Nutr. 2014, 98, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Zebisch, A.; May, A.; Reese, S.; Gehlen, H. Effect of different head-neck positions on physical and psychological stress parameters in the ridden horse. J. Anim. Physiol. Anim. Nutr. 2014, 98, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Sleutjens, J.; Smiet, E.; Van Weeren, R.; Van der Kolk, J.; Back, W.; Wijnberg, I. Effect of head and neck position on intrathoracic pressure and arterial blood gas values in Dutch Warmblood riding horses during moderate exercise. Am. J. Vet. Res. 2012, 73, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.R. A solution to respiratory and other problems caused by the bit. Pferdeheilkunde 2003, 16, 333–351. [Google Scholar] [CrossRef]
- Carey, C.; Moriarty, S.H.; Brennan, R. The impact of bitted and bitless bridles on the Therapeutic Riding Horse. In Proceedings of the 12th International Equitation Science Conference on Understanding Horses to Improve Training and Performance, Wagga Wagga, Australia, 22–25 November 2016; p. 99. Available online: http://www.equitationscience.com/documents/Conferences/2016/Proceedings%20ISES%202016.pdf (accessed on 9 October 2016).
- Franklin, S.H.; Naylor, J.R.; Lane, J.G. Videoendoscopic evaluation of the upper respiratory tract in 93 sport horses during exercise testing on a high-speed treadmill. Equine Exerc. Physiol. 7 Equine Vet. J. 2006, 36, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Fjordbakk, C.T.; Strand, E.; Hanche-Olsen, S. Surgical and conservative management of bilateral dynamic laryngeal collapse associated with poll flexion in harness race horses. Vet. Surg. 2008, 37, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.H.; Allen, K.J. Assessment of dynamic upper respiratory tract function in the equine athlete. Equine Vet. Educ. 2017, 29, 92–103. [Google Scholar] [CrossRef]
- Parente, E.J.; Martin, B.B.; Tulleners, E.P.; Ros, M.W. Dorsal displacement of the soft palate in 92 horses during high-speed treadmill examination (1993–1998). Vet. Surg. 2002, 31, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Christley, R.M.; Birchall, M.A.; Franklin, S.H. A systematic review of the efficacy of interventions for dynamic intermittent dorsal displacement of the soft palate. Equine Vet. J. 2012, 44, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Franklin, S. Characteristics of palatal instability in Thoroughbred racehorses and their association with the development of dorsal displacement of the soft palate. Equine Vet. J. 2013, 45, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, N.G.; Cheetham, J. Abnormalities of the upper airway. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 549–586. [Google Scholar]
- Kannegieter, N.J.; Dore, M.L. Endoscopy of the upper respiratory tract during treadmill exercise: A clinical study of 100 horses. Aust. Vet. J. 1995, 72, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.H.; Burn, J.F.; Allen, K.J. Clinical trials using a telemetric endoscope for use during over-ground exercise: A preliminary study. Equine Vet. J. 2008, 40, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Strand, E.; Fjordbakk, C.T.; Sundberg, L.; Spangen, H.; Lunde, H.; Hanche-Olsen, S. Relative prevalence of upper respiratory tract obstructive disorders in two breeds of harness racehorses (185 cases: 1998–2006). Equine Vet. J. 2012, 44, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ahern, T.J. Pharyngeal dysfunction during exercise. J. Equine Vet. Sci. 1999, 19, 226–231. [Google Scholar] [CrossRef]
- Boyle, A.G.; Martin, B.B., Jr.; Davidson, E.J.; Durando, M.M.; Birks, E.K. Dynamic pharyngeal collapse in racehorses. Equine Exerc. Physiol. 2006, 36, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Taylor, R.J.; Dixon, P.M. Unilateral displacement of the roof of the nasopharynx as a cause of stridor in a poney. Vet. Rec. 1994, 134, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Strand, E.; Skjerve, E. Complex dynamic upper airway collapse: Association between abnormalities in 99 harness racehorses with one or more dynamic disorders. Equine Vet. J. 2012, 44, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Strand, E.; Hanche-Olsen, S.; Grøvold, A.M.R.; Mellum, C.N. Dynamic bilateral arytenoid and vocal fold collapse associated with head flexion in 5 Norwegian Coldblooded Trotter racehorses. Equine Vet. Educ. 2004, 16, 242–250. [Google Scholar] [CrossRef]
- Votion, D. Metabolic responses to exercise and training. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elseier: New York, NY, USA, 2014; pp. 747–767. [Google Scholar]
- Duffin, J. The chemoreflex control of breathing. Can. J. Anaesth. 1990, 37, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.D.; Gillespie, J.R.; Landgren, G.L.; Fedde, M.R.; Jones, B.W.; DeBowes, R.M.; Pieschl, R.L.; Erickson, H.H. Mechanisms of exercise-induced hypoxemia in horses. J. Appl. Physiol. 1989, 66, 1227–1233. [Google Scholar] [PubMed]
- Parks, C.M.; Manohar, M. Blood-gas tensions and acid-base status in ponies during treadmill exercise. Am. J. Vet. Res. 1984, 45, 15–19. [Google Scholar] [PubMed]
- Katz, L.M.; Bayly, W.M.; Roeder, M.J.; Kingston, J.K.; Hines, M.T. Effects of training on maximum oxygen consumption of ponies. Am. J. Vet. Res. 2000, 61, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Bayly, W.M.; Hines, M.T.; Sides, R.H. Ventilatory responses of ponies and horses to exercise. Equine Comp. Exerc. Physiol. 2005, 2, 229–240. [Google Scholar] [CrossRef]
- Franklin, S.H.; Van Erck-Westergren, E.; Bayly, W.M. Respiratory responses to exercise in the horse. Equine Vet. J. 2012, 44, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.A.; Kusano, K.; Evans, D.L. Observations on respiratory flow strategies during and after intense treadmill exercise to fatigue in thoroughbred racehorses. Equine Vet. J. 2006, 36, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Padilla, D.J.; McDonough, P.; Kindig, C.A.; Erickson, H.H.; Poole, D.C. Ventilatory dynamics and control of blood gases after maximal exercise in the Thoroughbred horse. J. Appl. Physiol. 2004, 96, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Thiel, M.; Tolkmitt, G.; Hoernicke, H. Body temperature changes in horses during riding: Time course and effects on heart rate and respiratory frequency. In Equine Exercise Physiology; 2. ICEEP Publications: Davis, CA, USA, 1987; pp. 183–193. [Google Scholar]
- Hodgson, D.R.; Davis, R.E.; McConaghy, F.F. Thermoregulation in the horse in response to exercise. Br. Vet. J. 1994, 150, 219–235. [Google Scholar] [CrossRef]
- Foreman, J.H. Thermoregulation in the horse exercising under hot and humid conditions. Pferdeheilkunde 1996, 12, 405–408. [Google Scholar]
- Jackson, J.A.; Ducharme, N.G.; Hackett, R.P.; Rehder, R.S.; Ainsworth, D.M.; Shannon, K.J.; Erickson, B.K.; Erb, H.N.; Jansson, N.; Soderholm, L.V.; et al. Effects of airway obstruction on transmural pulmonary artery pressure in exercising horses. Am. J. Vet. Res. 1997, 58, 897–903. [Google Scholar] [PubMed]
- Cook, W.R.; Williams, R.M.; Kirker-Head, C.A.; Verbridge, D.J. Upper airway obstruction (partial asphyxia) as the possible cause of exercise-induced pulmonary haemorrhage in the horse: An hypothesis. Equine Vet. Sci. 1988, 8, 11–26. [Google Scholar] [CrossRef]
- Gunson, D.E.; Sweeney, C.R.; Soma, L.R. Sudden death attributable to exercise-induced pulmonary hemorrhage in racehorses: Nine cases (1981–1983). JAVMA 1988, 193, 102–106. [Google Scholar] [PubMed]
- Hinchcliff, K.W.; Couetil, L.L.; Knight, P.K.; Morley, P.S.; Robinson, N.E.; Sweeney, C.R.; Van Ercj, E. Exercise induced pulmonary haemorrhage in horses: American College of Veterinary Internal Medicine consensus statement. J. Vet. Intern. Med. 2015, 29, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.P.; Ducharme, N.G.; Ainsworth, D.M.; Erickson, B.K.; Erb, H.N.; Soderholm, L.; Thorson, L.M. Effects of extrathoracic airway obstruction on intrathoracic pressure and pulmonary artery pressure in exercising horses. Am. J. Vet. Res. 1999, 60, 485–494. [Google Scholar] [PubMed]
- West, J.B.; Mathieu-Costello, O.; Jones, J.H.; Birks, E.K.; Logemann, R.B.; Pascoe, J.R.; Tyler, W.S. Stress failure of pulmonary capillaries in racehorses with exercise-induced pulmonary hemorrhage. J. Appl. Physiol. 1993, 75, 1097–1109. [Google Scholar] [PubMed]
- Deepika, K.; Barrocas, Q.M.; Fonseca, J.J.; Bikasi, G.B. Negative pressure pulmonary edema after acute upper airway obstruction. J. Clin. Anaesth. 1997, 9, 403–408. [Google Scholar] [CrossRef]
- Bhaskar, B.; Fraser, J.F. Negative pressure pulmonary oedema revisited: Pathophysiology and review of management. Saudi J. Anaesth. 2011, 5, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, E.J.; Bohanon, T.C.; Bednarski, R.M.; Hubbell, J.A.E.; Muir, W.W. Bilateral arytenoid cartilage paralysis after inhalation anaesthesia in a horse. J. Am. Vet. Med. Assoc. 1990, 197, 1363–1365. [Google Scholar] [PubMed]
- Lang, S.A.; Duncan, P.G.; Shephard, D.A.E.; Ha, H.C. Pulmonary oedema associated with airway obstruction. Can. J. Anaesth. 1990, 37, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.M.; Railton, D.I.; McGorum, B.C. Temporary bilateral laryngeal paralysis in a horse associated with general anaesthesia and post anaesthetic myositis. Vet. Rec. 1993, 132, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kollias-Baker, C.A.; Pipers, F.S.; Heard, D.; Seeherman, H. Pulmonary edema associated with transient airway obstruction in three horses. JAVMA 1993, 202, 1116–1118. [Google Scholar] [PubMed]
- Tute, A.S.; Wilkins, P.A.; Gleed, R.D.; Credille, K.M.; Murphy, D.J.; Ducharme, N.G. Negative pressure pulmonary edema as a post-anesthetic complication associated with upper airway obstruction in a horse. Vet. Surg. 1996, 25, 519–523. [Google Scholar] [PubMed]
- Senior, M. Postanaesthetic pulmonary oedema in horses: A review. Vet. Anaes. Analg. 2005, 32, 193–200. [Google Scholar]
- Wilson, R.C.; Jones, P.W. Long-term reproducibility of Borg scale estimates of breathlessness during exercise. Clin. Sci. Lond 1991, 80, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, S.J.; Derksen, F.J.; Stick, J.A.; Robinson, N.E. Effect of nasal occlusion on tracheal and pharyngeal pressures in horses. Am. J. Vet. Res. 1996, 57, 1258–1260. [Google Scholar] [PubMed]
- Leclere, M.; Lavoie-Lamoureux, A.; Lavoie, J.-P. Heaves, an asthma-like disease of horses. Respirology 2011, 16, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Bullone, M.; Lavoie, J.-P. Asthma, “of horses and men”–how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol. Immunol. 2015, 66, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.L.; Denicola, D.B. Blood gas, plasma lactate and bronchoalveolar lavage cytology analyses in racehorses with respiratory disease. Equine Vet. J. Suppl. 1999, 30, 77–82. [Google Scholar] [CrossRef]
- Sanchez, A.; Couetil, L.L.; Ward, M.P.; Clark, S.P. Effect of airway disease on blood gas exchange in racehorses. J. Vet. Intern. Med. 2005, 19, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Courouce-Malblanc, A.; Deniau, V.; Rossignol, F.; Corde, R.; Leleu, C.; Maillard, K.; Pitel, P.-H.; Pronost, S.; Fortier, G. Physiological measurements and prevalence of lower airway diseases in Trotters with dorsal displacement of the soft palate. Equine Vet. J. Suppl. 2010, 38, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.; et al. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Corfield, D.R.; Murphy, K.; Guz, A. Does the motor cortical control of the diaphragm “bypass” the brain stem respiratory centres in man? Respir. Physiol. 1998, 114, 109–117. [Google Scholar] [CrossRef]
- Bianchi, A.L.; Denavit-Saubie, M.; Champagnat, J. Central control of breathing in mammals: Neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 1995, 75, 1–45. [Google Scholar] [PubMed]
- Buchanan, G.F.; Richerson, G.B. Role of chemoreceptors in mediating dyspnea. Respir. Physiol. Neurobiol. 2009, 167, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.; Ofir, D.; O’Donnell, D.E. Effects of pregnancy, obesity and aging on the intensity of perceived breathlessness during exercise in healthy humans. Respir. Physiol. Neurobiol. 2009, 167, 87–100. [Google Scholar] [CrossRef] [PubMed]
- El-Manshawi, A.; Killian, K.J.; Summers, E.; Jones, N.L. Breathlessness during exercise with and without resistive loading. J. Appl. Physiol. 1986, 61, 896–905. [Google Scholar] [PubMed]
- Gandevia, S.C. Roles for perceived voluntary motor commands in motor control. Trends Neurosci. 1987, 10, 81–85. [Google Scholar] [CrossRef]
- Manning, H.L.; Schwartzstein, R.M. Pathophysiology of dyspnea. N. Engl. J. Med. 1995, 333, 1547–1553. [Google Scholar] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J. Peripheral chemoreceptors in health and disease. J. Appl. Physiol. 2004, 96, 359–366. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Ora, J.; Webb, K.A.; Laveneziana, P.; Jensen, D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir. Physiol. Neurobiol. 2009, 167, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; Heigenhauser, G.J.F. Effects of gas exchange on acid-base balance. Compr. Physiol. 2012, 2, 2203–2254. [Google Scholar] [PubMed]
- Banzett, R.B.; Pedersen, S.H.; Schwartzstein, R.M.; Lansing, R.W. The affective dimension of laboratory dyspnea: Air hunger is more unpleasant than work/effort. Am. J. Respir. Crit. Care Med. 2008, 177, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Lansing, R.W.; Gracely, R.H.; Banzett, R.B. The multiple dimensions of dyspnea: Review and hypotheses. Respir. Physiol. Neurobiol. 2009, 167, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Adams, L. The shuttle walking test: A reproducible method for evaluating the impact of shortness of breath on functional capacity in patients with advanced cancer. Thorax 2001, 56, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Australian Lung Foundation. Better Living with Chronic Obstructive Pulmonary Disease: A Patient Guide, 2nd ed.; The Queensland Government: Queensland, Australia, 2012; Available online: http://lungfoundation.com.au/wp-content/uploads/2014/02/Better-Living-with-COPD.pdf (accessed on 15 February 2017).
- Johnson, B.D.; Saupe, K.W.; Dempsey, J.A. Mechanical constraints on exercise hyperpnea in endurance athletes. J. Appl. Physiol. 1992, 73, 874–886. [Google Scholar] [PubMed]
- Reinero, CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet. Rec. 2011, 190, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Undem, B.J.; Nassenstein, C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir. Physiol. Neurobiol. 2009, 167, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Waran, N.; McGreevy, P.D.; Casey, R. Training methods and horse welfare. In The Welfare of Horses; Waran, N., Ed.; Boston Kluwer Academic Publishers: Dordrecht, Netherlands, 2002; pp. 151–180. [Google Scholar]
- Duberstein, K.J.; Johnson, E.L. Bits 101. In University of Georgia Bulletin 1379; University of Georgia: Athens, GA, USA, 2014; pp. 1–6. Available online: http://extension.uga.edu/publications/files/pdf/B%201379_5.PDF (accessed on 10 August 2016).
- McGreevy, P.; McLean, A. Behavioral problems with the ridden horse. In The Domestic Horse: The Origins, Development, and Management of Its Behavior; Mills, D.S., McDonnell, S.M., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 196–211. [Google Scholar]
- Van Lancker, S.; Van der Broeck, W.; Simoens, P. Incidence and morphology of bone irregularities of the equine interdental space (bars of the mouth). Equine Vet. Educ. 2007, 19, 103–106. [Google Scholar] [CrossRef]
- Cook, W.R. Damage by the bit to the equine interdental space and second lower premolar. Equine Vet. J. 2011, 23, 355–360. [Google Scholar] [CrossRef]
- Mata, F.; Johnson, C.; Bishop, C. A cross-sectional epidemiological study of prevalence and severity of bit-induced oral trauma in polo ponies and racehorses. J. Appl. Anim. Welf. Sci. 2015, 18, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Black, J.B.; Frisbie, D. Welfare concerns in the training and competition of cutting, reining and reined cow horses. In Equine Welfare; McIlwraith, C.W., Rollin, B.E., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 302–317. [Google Scholar]
- Fenner, K.; Yoon, S.; White, P.; Starling, M.; McGreevy, P. The effect of noseband tightening on horses’ behaviour, eye temperature, and cardiac responses. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- American Quarter Horse Association Rule Book, Rule 44(h,k). In Show Rules and Regulations; AQHA: Amarillo, TX, USA, 2009.
- Leitch, M. Welfare in the discipline of dressage. In Equine Welfare; McIlwraith, C.W., Rollin, B.E., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 332–340. [Google Scholar]
- AWA, NZ. Animal Welfare Act 1999 and Animal Welfare Amendment Act 2015 No. 2; Government of New Zealand: Wellington, New Zealand, 1999. Available online: http://www.legislation.govt.nz/act/public/1999/0142/latest/DLM49664.html (accessed on 10 August 2016).
- Cook, W.R. Bit-induced asphyxia: Elevation and dorsal displacement of the soft palate at exercise. J. Equine Vet. Sci. 2002, 20, 7–14. [Google Scholar] [CrossRef]
- Hanson, F.; Cook, R. The Bedouin bridle rediscovered: A welfare, safety and performance enhancer. Horse’s Hoof 2015, 60, 1–8. Available online: http://www.bitlessbridle.com/THEBEDOUBRIDLE.pdf (accessed on 10 August 2016).
- Cook, W.R. Bit-induced pain: a cause of fear, flight, fight and facial neuralgia in the horse. Pferdeheilkunde 2003, 19, 1–8. [Google Scholar] [CrossRef]
- Dugdale, D.; Greenwood, R. Some observations on conservative techniques for treatment of laryngopalatal displacement. Equine Vet. Educ. 1993, 5, 177–180. [Google Scholar] [CrossRef]
- Franklin, S.H.; Naylor, J.R.; Lane, J.G. The effect of a tongue-tie in horses with dorsal displacement of the soft palate. Equine Vet. J. 2002, 34, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.; Allen, K. Laboratory exercise testing. In Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Hinchcliff, K.W., Kaneps, A.J., Geor, R.J., Eds.; Elsevier: New York, NY, USA, 2014; pp. 11–24. [Google Scholar]
- Tabernaberri, C.; Cooper, C.; Clemence, J. Transition to a bitless bridle. In Hoofbeats; October/November; Australian Associated Press (AAP); Equine Veterinarians Australia (EVA): Sydney, Australia, 2011; pp. 62–69. Available online: http://www.whisperingacres.com.au/images/articles/Hoofbeats1.pdf (accessed on 17 February 2017).
- McGreevy, P.D.; Corken, R.A.; Salvin, H.; Black, C.M. Whip use by jockeys in a sample of Australian thoroughbred races—An observational study. PLoS ONE 2012, 7, e33398. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.; McDonald, C.; Wilson, B.; McManus, P.; McGreevy, P. Whip rule breaches in major Australian racing jurisdictions: Welfare and regulatory implications. Animals 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- British Horseracing Authority. Responsible Regulation: A Review of the Use of the Whip in Horseracing; British Horseracing Authority: London, UK, 2011; Available online: http://www.britishhorseracing.com/wp-content/uploads/2014/03/WhipReview.pdf (accessed on 27 January 2017).
- Evans, D.; McGreevy, P. An investigation of racing performance and whip use by jockeys in Thoroughbred races. PLoS ONE 2011, 6, e15622. [Google Scholar] [CrossRef] [PubMed]
- Travers, C.W.; Guthrie, A.J.; Lund, R.J. Characterization of a standard exercise to fatigue test in Thoroughbred horse. Pferdeheikunde 1996, 12, 463–465. [Google Scholar]
- Flaminio, M.J.B.F.; Gaighan, E.M.; Gillespie, J.R. Exercise intolerance in endurance horses. Vet. Clin. N. Am. Equine Pract. 1996, 12, 565–580. [Google Scholar] [CrossRef]
- Valberg, S.J. Muscular causes of exercise intolerance in horses. Vet. Clin. N. Am. Equine Prac. 1996, 12, 495–515. [Google Scholar] [CrossRef]
- Noakes, T.D. Fatigue is a brain-derived emotion that regulates the exercise behaviour to ensure the protection of whole body homeostasis. Front. Physiol. 2012, 3, 82. Available online: https://doi.org/10.3389/fphys.2012.00082 (accessed on 22 May 2017). [CrossRef] [PubMed]
- Parente, E.J. Testing methods for exercise intolerance in horses. Vet. Clin. N. Am. Equine Pract. 1996, 12, 421–433. [Google Scholar] [CrossRef]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A scientific conception of animal welfare that reflects ethical concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar]
- Fraser, D. Assessing animal welfare at the farm and group level: The interplay of science and values. Anim. Welf. 2003, 12, 433–443. [Google Scholar]
- Fraser, D. Understanding Animal Welfare: The Science in its Cultural Constext; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Lund, V. Natural living—A precondition for animal welfare in organic farming. Livest. Sci. 2006, 100, 71–83. [Google Scholar] [CrossRef]
- Mellor, D.J. Positive animal welfare states and promoting environment-focused and animal-animal interactive behaviours. N. Z. Vet. J. 2015, 63, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J. Positive welfare states and reference standards for welfare assessment. N. Z. Vet. J. 2015, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mill, J.M.; Ward, W.R. Lameness in dairy cows and farmers’ knowledge, training and awareness. Vet. Rec. 1994, 134, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.M.A.; Hendricks, A.; Burn, C.C. Do dog owners perceive the clinical signs related to conformational inherited disorders as “normal” for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellor, D.J.; Beausoleil, N.J. Equine Welfare during Exercise: An Evaluation of Breathing, Breathlessness and Bridles. Animals 2017, 7, 41. https://doi.org/10.3390/ani7060041
Mellor DJ, Beausoleil NJ. Equine Welfare during Exercise: An Evaluation of Breathing, Breathlessness and Bridles. Animals. 2017; 7(6):41. https://doi.org/10.3390/ani7060041
Chicago/Turabian StyleMellor, David J., and Ngaio J. Beausoleil. 2017. "Equine Welfare during Exercise: An Evaluation of Breathing, Breathlessness and Bridles" Animals 7, no. 6: 41. https://doi.org/10.3390/ani7060041




