Figure 1.
Accles and Shelvoke CASH Small Animal Tool. A 0.22” caliber blank cartridge-powered non-penetrating captive bolt device.
Figure 1.
Accles and Shelvoke CASH Small Animal Tool. A 0.22” caliber blank cartridge-powered non-penetrating captive bolt device.
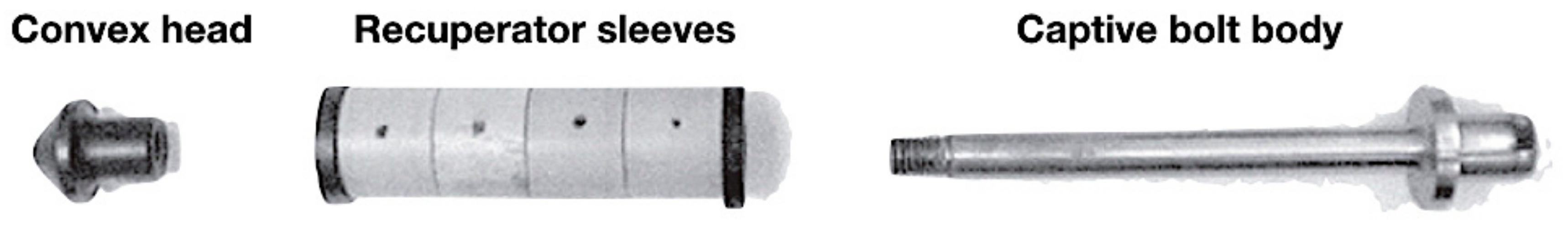
Figure 2.
Bolt components of the non-penetrating captive bolt (adapted from Accles and Shelvoke).
Figure 2.
Bolt components of the non-penetrating captive bolt (adapted from Accles and Shelvoke).
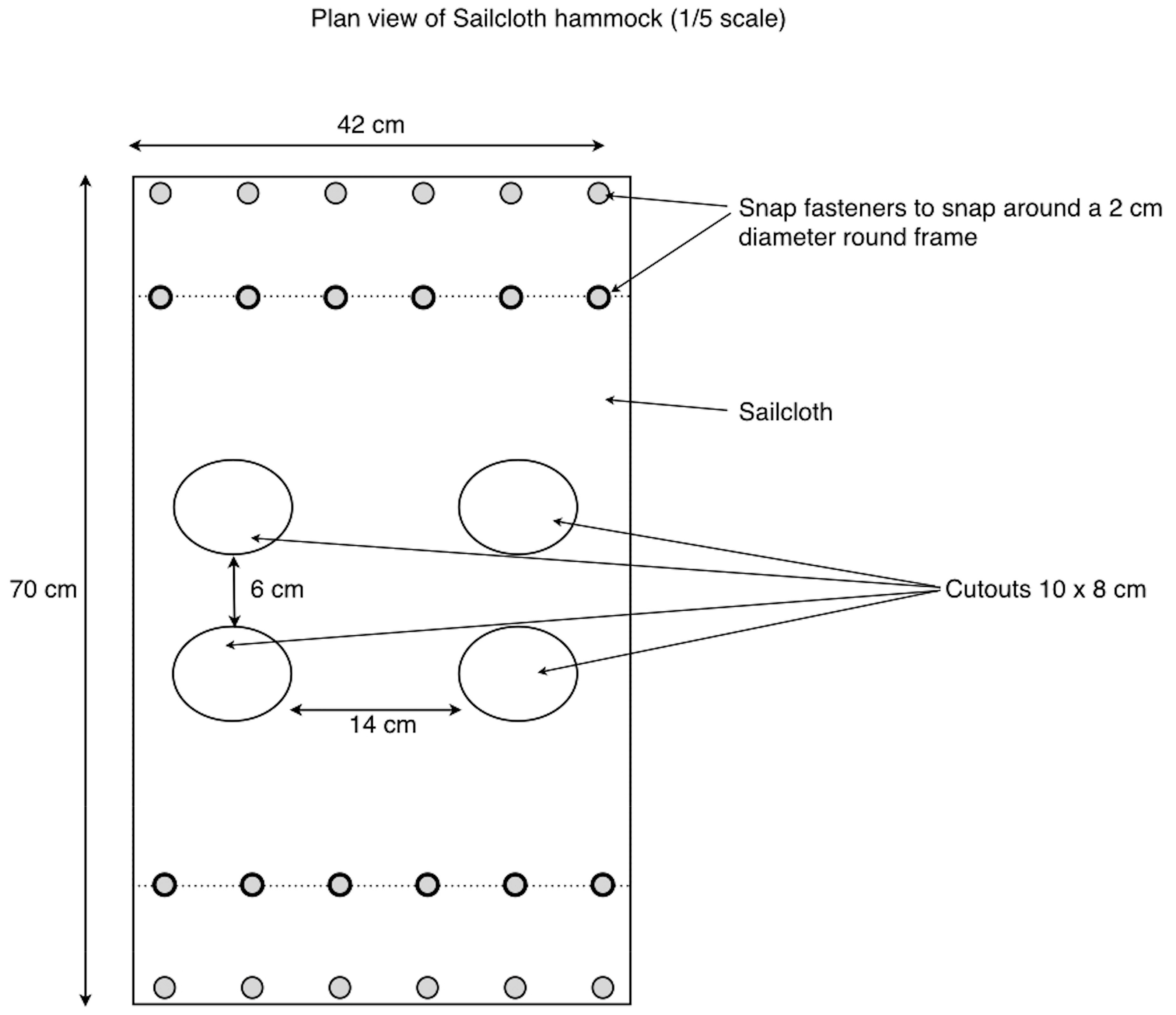
Figure 3.
Sailcloth hammock design.
Figure 3.
Sailcloth hammock design.
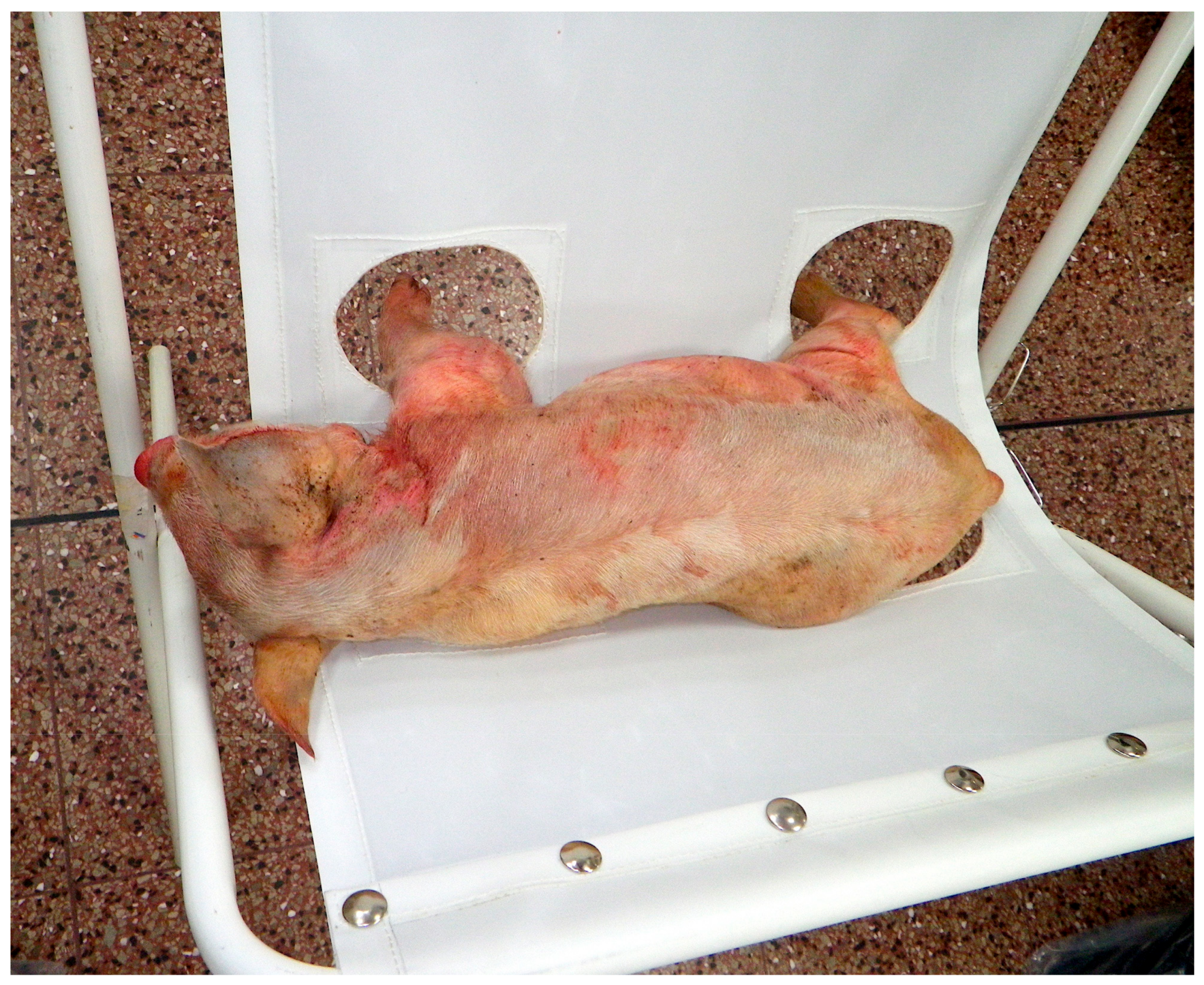
Figure 4.
Sailcloth hammock in use (cadaver used to demonstrate restraint).
Figure 4.
Sailcloth hammock in use (cadaver used to demonstrate restraint).
Figure 5.
Shooting position for piglets on the midline on the frontal/parietal bone.
Figure 5.
Shooting position for piglets on the midline on the frontal/parietal bone.
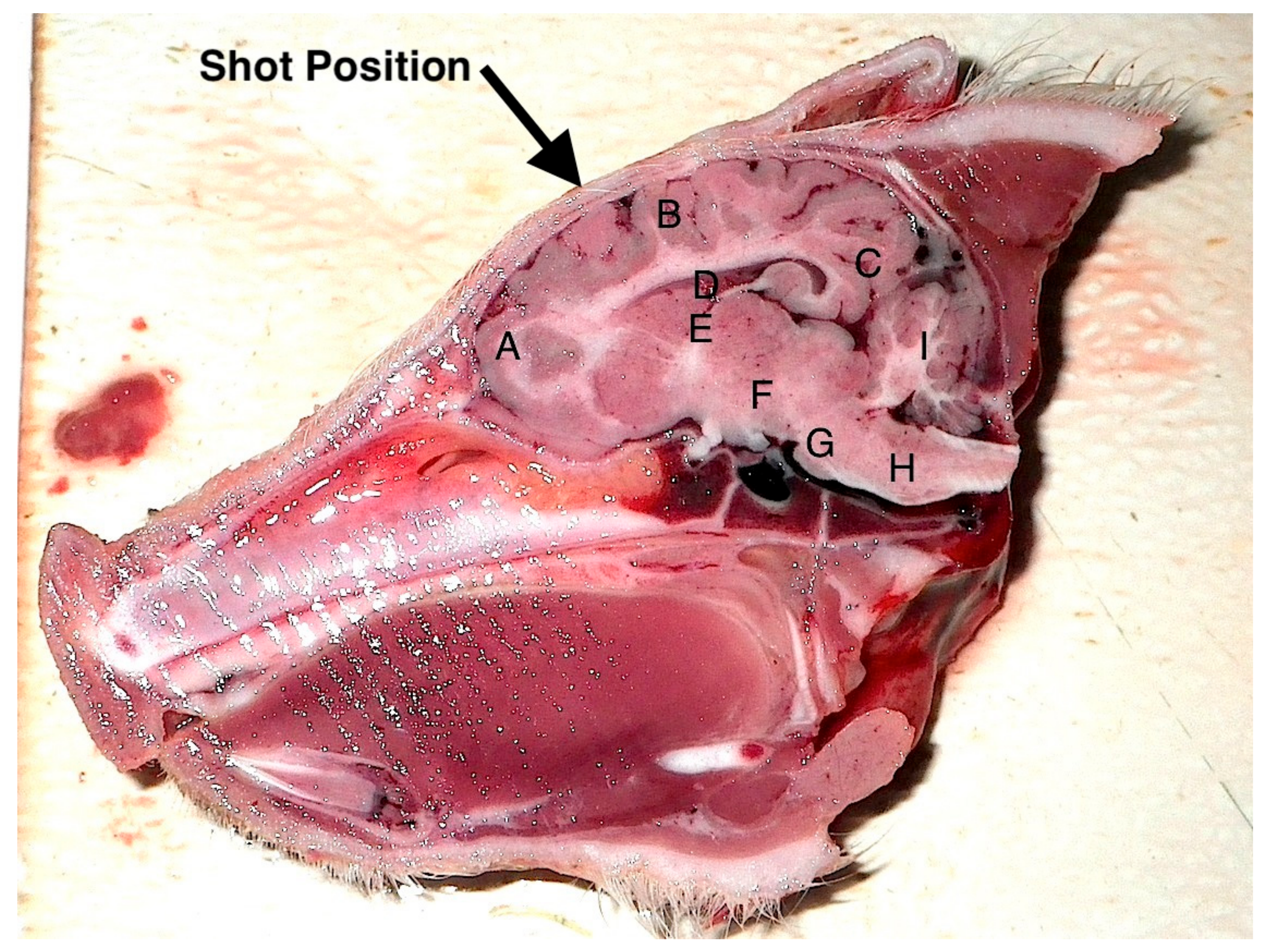
Figure 6.
Sagittal section of an unshot piglet head (died on farm) illustrating shot position and the areas examined for macroscopic damage. A—frontal cerebrum, B—parietal cerebrum, C—occipital cerebrum, D—lateral ventricle. These were scored on the basis of 0 = no damage, 1 = slight deformation, 2 = moderate deformation and 3 = severe deformation of the area. Areas E–I (thalamus, midbrain, pons, medulla and cerebellum respectively) were assessed for the presence or absence of haemorrhage.
Figure 6.
Sagittal section of an unshot piglet head (died on farm) illustrating shot position and the areas examined for macroscopic damage. A—frontal cerebrum, B—parietal cerebrum, C—occipital cerebrum, D—lateral ventricle. These were scored on the basis of 0 = no damage, 1 = slight deformation, 2 = moderate deformation and 3 = severe deformation of the area. Areas E–I (thalamus, midbrain, pons, medulla and cerebellum respectively) were assessed for the presence or absence of haemorrhage.
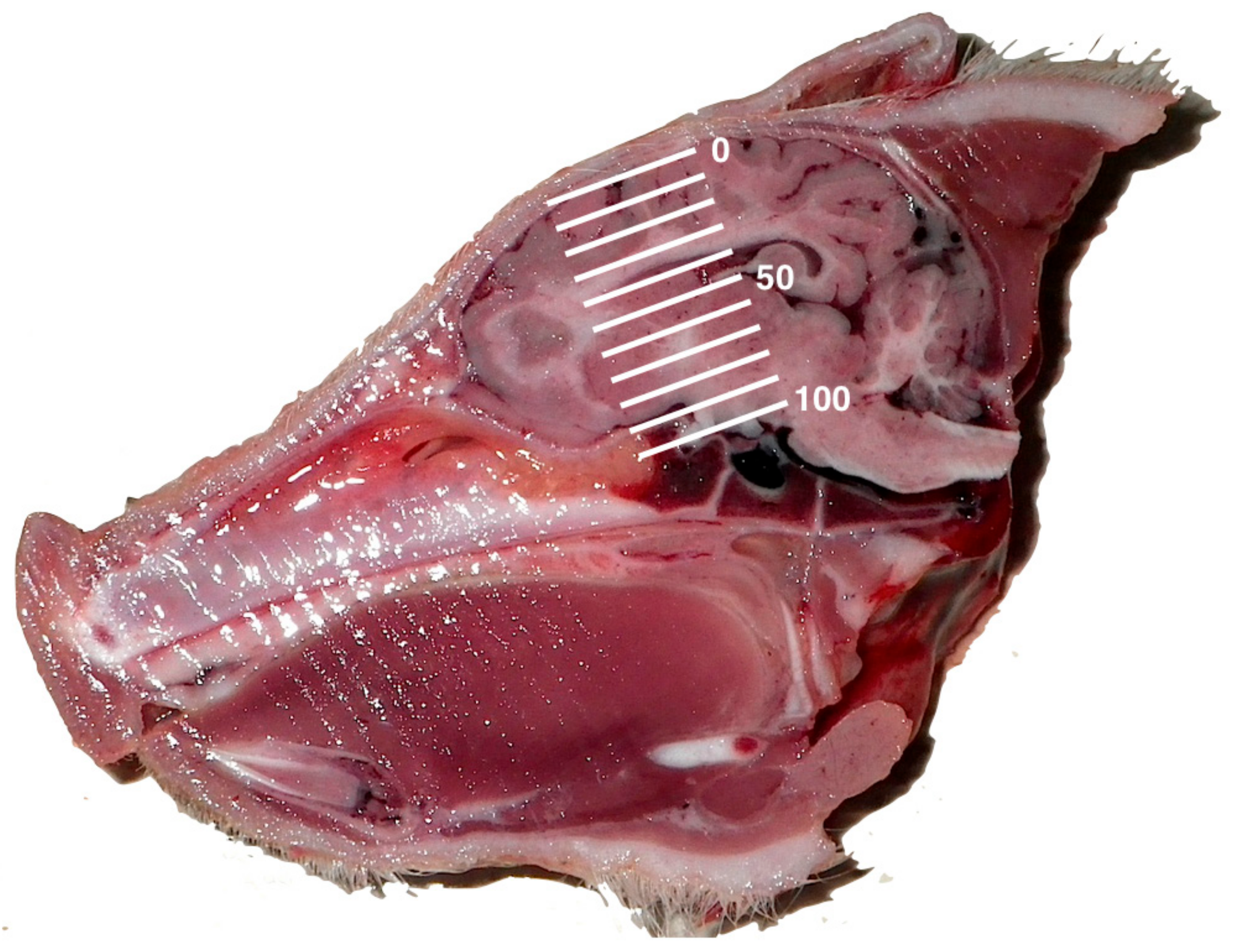
Figure 7.
Skull-plate displacement diagram. The level of skull-plate displacement following the shot is expressed as a percentage, with 0 being no displacement and 100% being a case where the skull fragment was observed at the base of the cranial cavity. Each sagittal plane piglet photograph had the scale expanded proportionately for this assessment, with 0 located at the cranial surface and 100% located at the bone peak between the optic chiasma and mammillary body.
Figure 7.
Skull-plate displacement diagram. The level of skull-plate displacement following the shot is expressed as a percentage, with 0 being no displacement and 100% being a case where the skull fragment was observed at the base of the cranial cavity. Each sagittal plane piglet photograph had the scale expanded proportionately for this assessment, with 0 located at the cranial surface and 100% located at the bone peak between the optic chiasma and mammillary body.
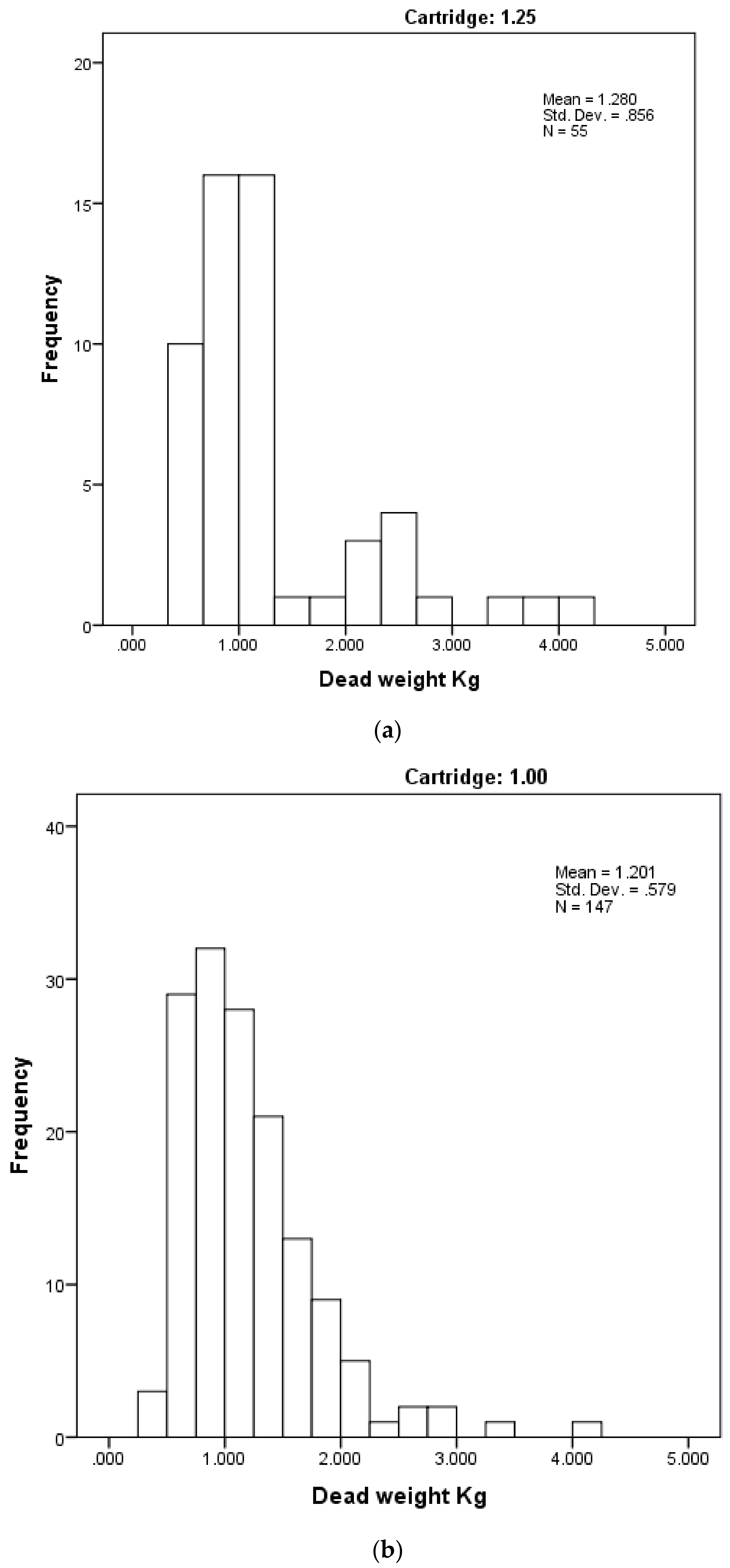
Figure 8.
Distribution of piglet dead weights (kg) by cartridge used. (a) with 1.25 grain cartridge and (b) with 1 grain cartridge.
Figure 8.
Distribution of piglet dead weights (kg) by cartridge used. (a) with 1.25 grain cartridge and (b) with 1 grain cartridge.
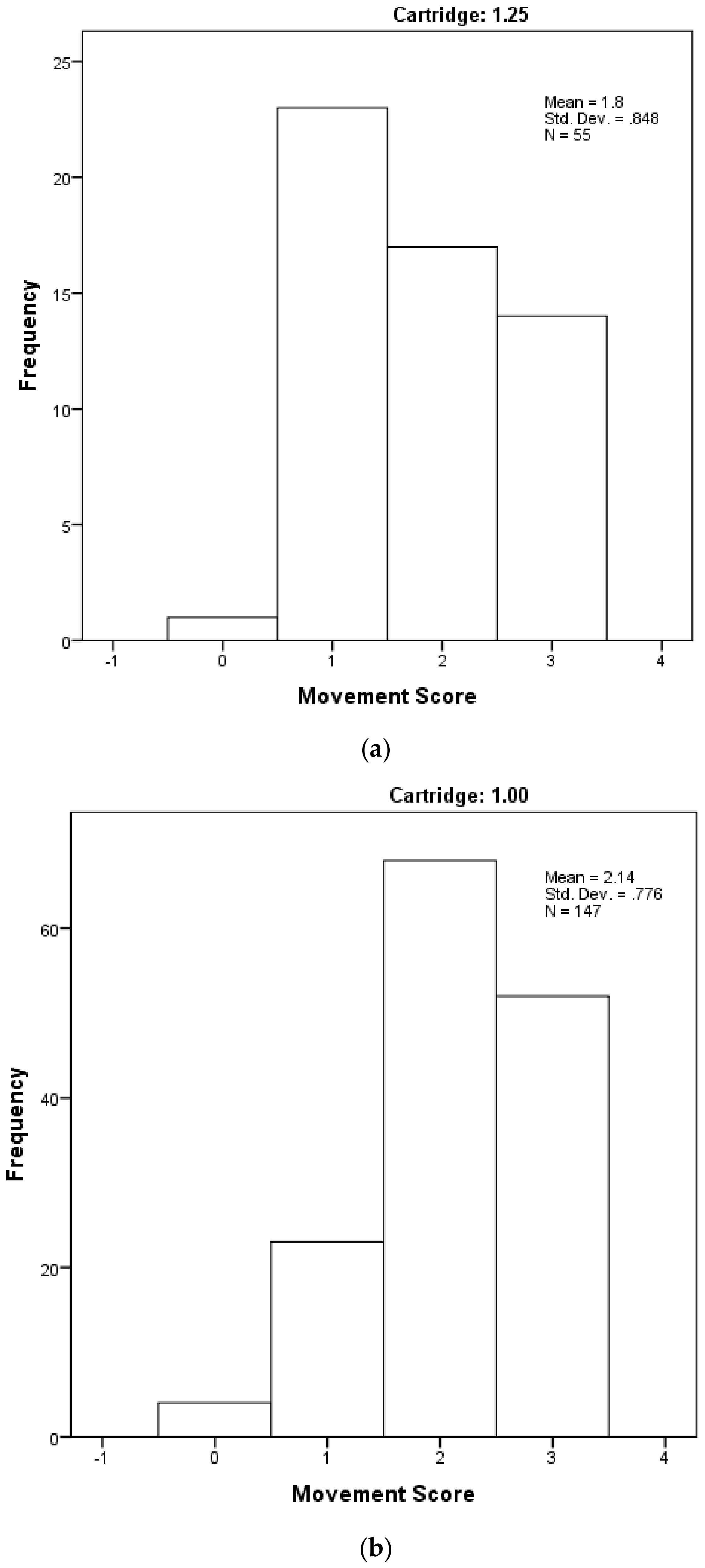
Figure 9.
The distribution of piglet movement scores by cartridge-size used, based on a subjective scoring where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; Score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement. (a) with 1.25 grain cartridge and (b) with 1 grain cartridge.
Figure 9.
The distribution of piglet movement scores by cartridge-size used, based on a subjective scoring where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; Score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement. (a) with 1.25 grain cartridge and (b) with 1 grain cartridge.
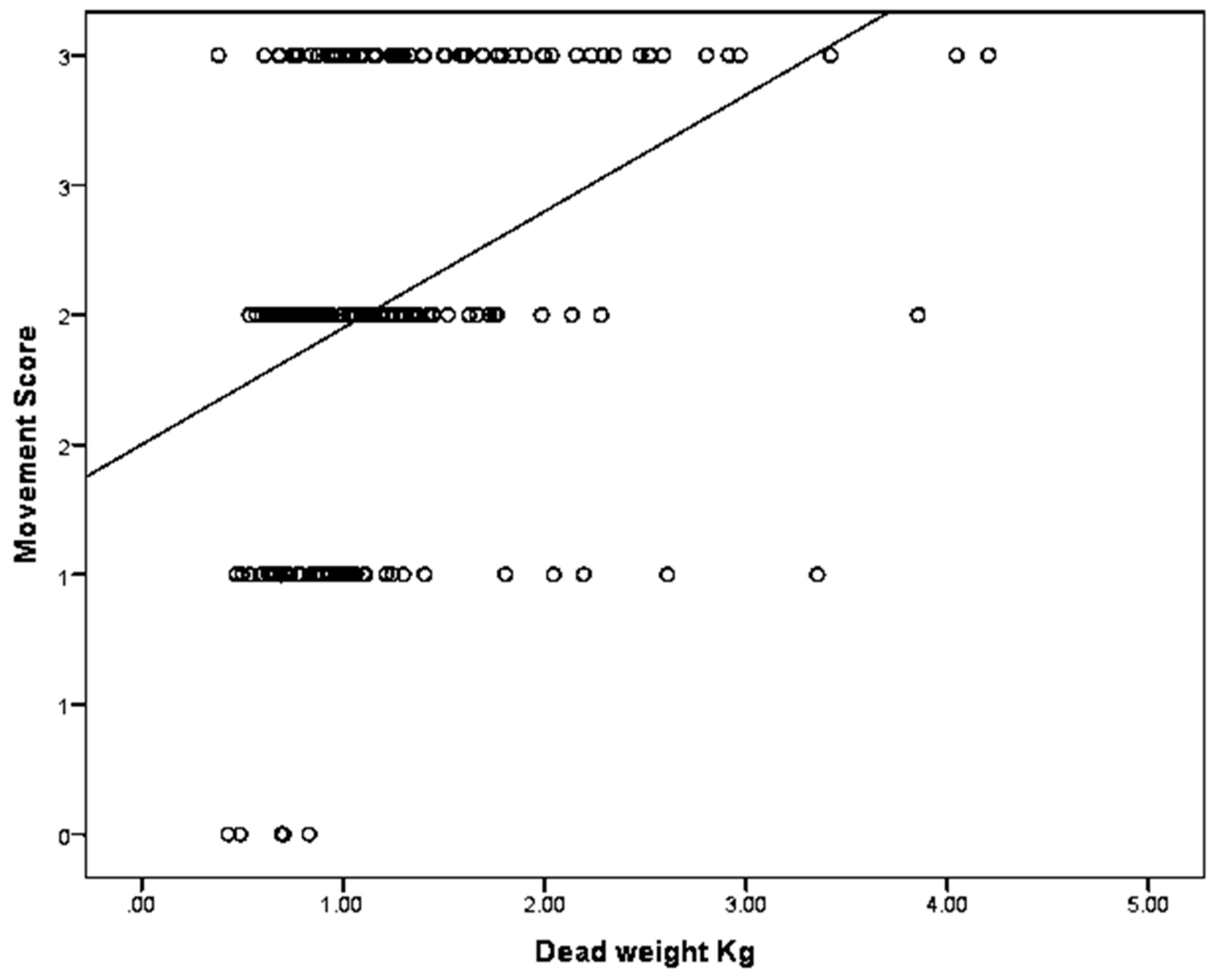
Figure 10.
The relationship between piglet dead weight and movement score, where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; Score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement. N = 202.
Figure 10.
The relationship between piglet dead weight and movement score, where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; Score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement. N = 202.
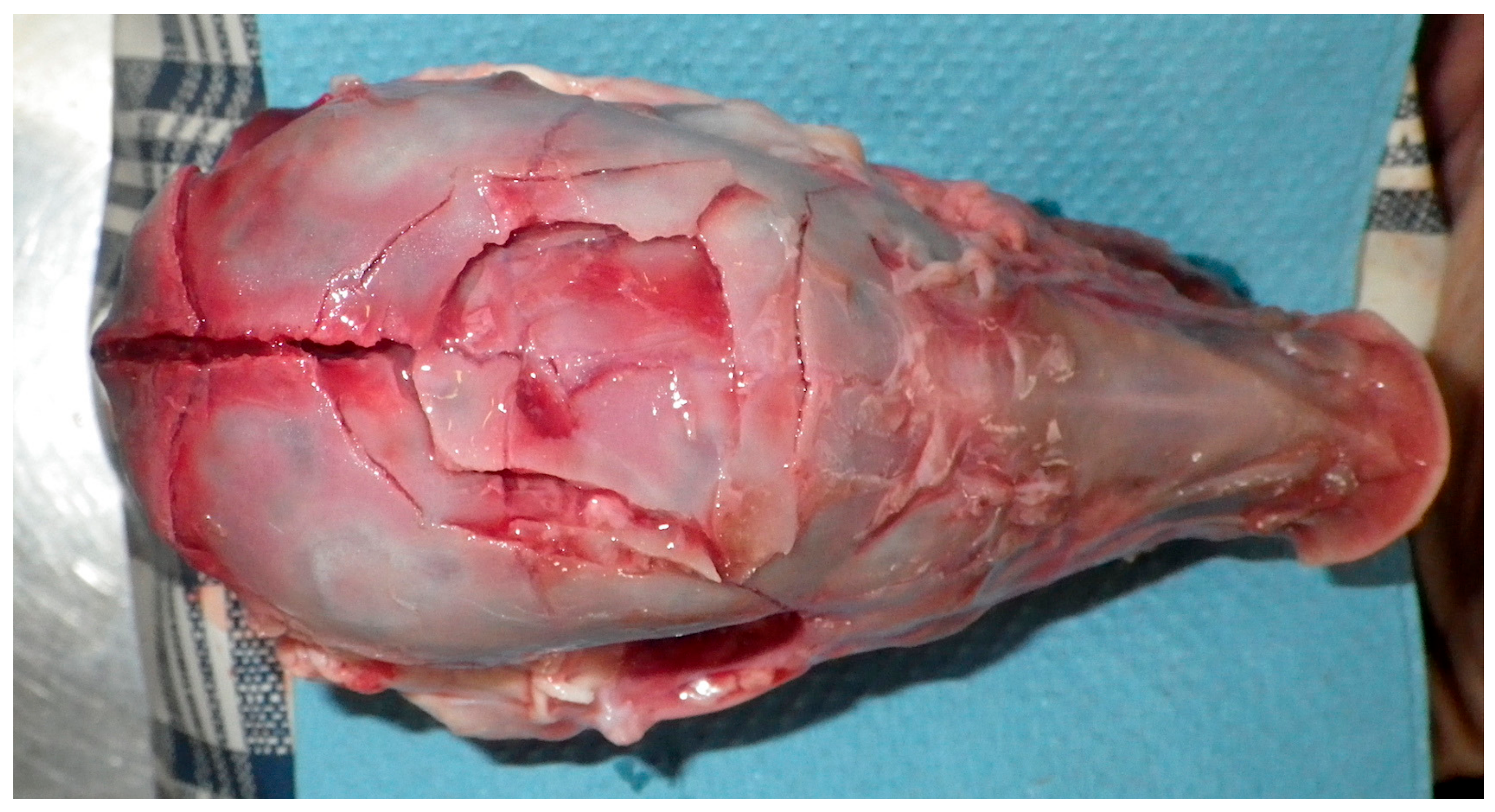
Figure 11.
Fracture pattern at impact point. Skin, periosteum and haematoma removed.
Figure 11.
Fracture pattern at impact point. Skin, periosteum and haematoma removed.
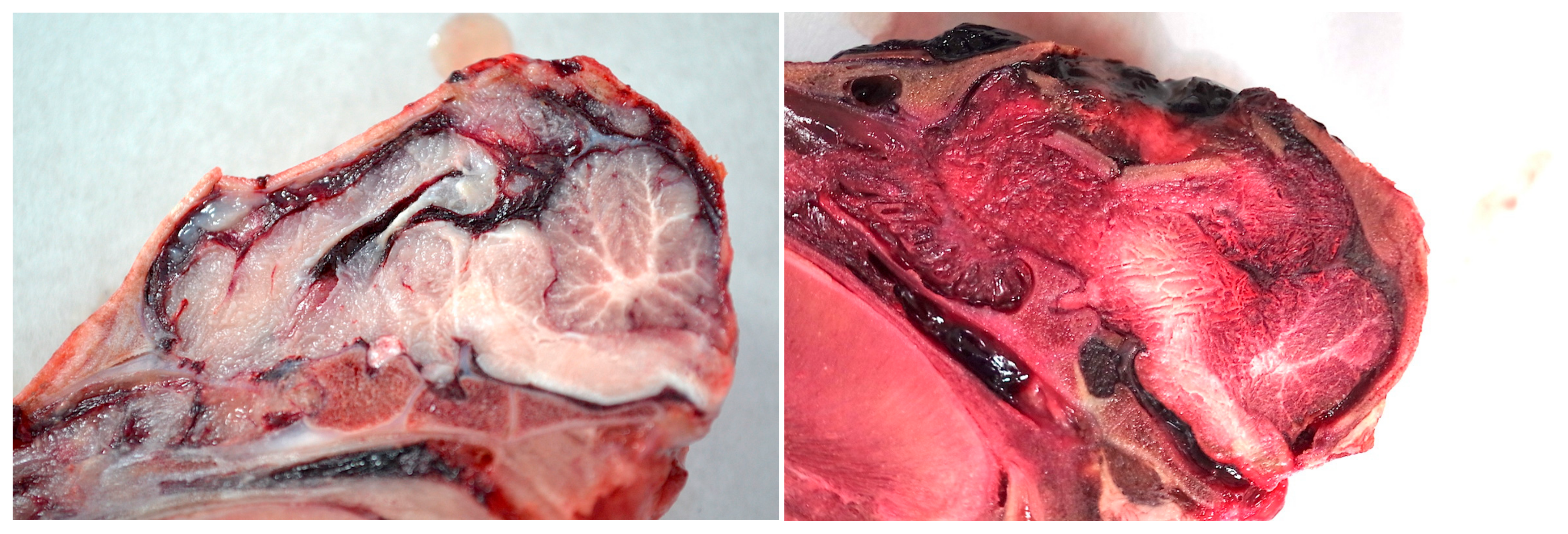
Figure 12.
Examples of post-shot trauma; medial sagittal section demonstrating bone fracture displacement and macroscopic brain damage (1 grain cartridge).
Figure 12.
Examples of post-shot trauma; medial sagittal section demonstrating bone fracture displacement and macroscopic brain damage (1 grain cartridge).
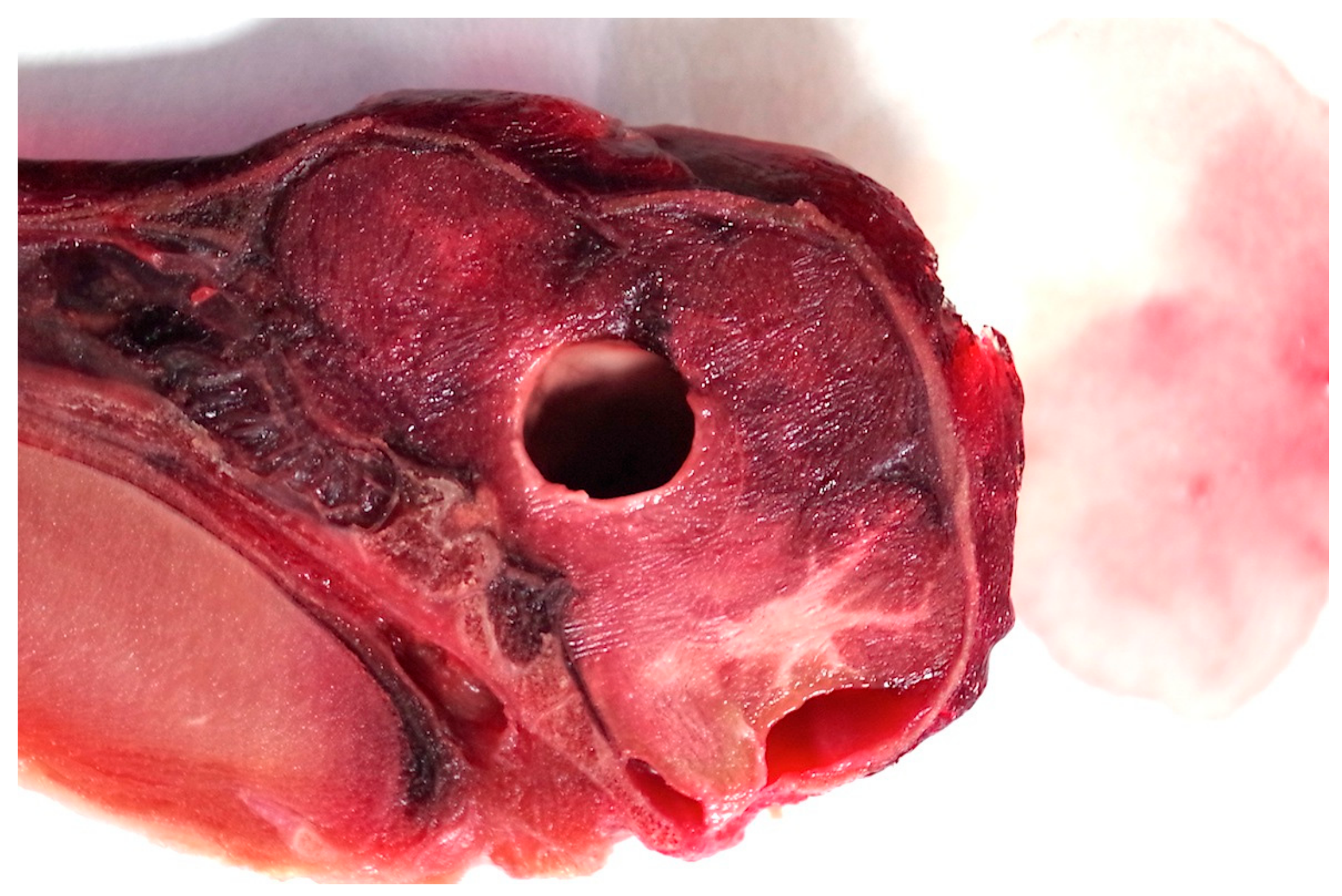
Figure 13.
Neopallial cyst (proencephaly) within the brain of piglet 171 (sagittal section).
Figure 13.
Neopallial cyst (proencephaly) within the brain of piglet 171 (sagittal section).
Table 1.
Subjective scoring system used to assess post-stun/kill movement based on the level of spinal reflex activity, ranging from 0 (no clonic activity post-stun) to 3 (severe uncontrolled physical movement).
Table 1.
Subjective scoring system used to assess post-stun/kill movement based on the level of spinal reflex activity, ranging from 0 (no clonic activity post-stun) to 3 (severe uncontrolled physical movement).
| Score | Descriptor | Description of intensity |
|---|
| 0 | No activity | Very little clonic activity. |
| 1 | Mild activity | Some mild uncontrolled physical movement of limbs. |
| 2 | Moderate activity | Considerable uncontrolled physical movement of the limbs. |
| 3 | Severe | Severe uncontrolled physical convulsive movement, paddling of legs |
Table 2.
Results of the tests for differences in the remaining outcome variables between the two cartridge sizes, showing the U statistic, significance and mean (standard error, S.E.) within each group.
Table 2.
Results of the tests for differences in the remaining outcome variables between the two cartridge sizes, showing the U statistic, significance and mean (standard error, S.E.) within each group.
| Parameter | U | Exact p | 1-grain | 1.25-grain |
|---|
| Mean | S.E. | Mean | S.E. |
|---|
| Time to loss of movement (s) | 3760 | 0.446 | 101.88 | 4.49 | 86.95 | 4.21 |
| Total Haemorrhage Score | 2522.5 | 0.014 | 4.63 | 0.57 | 4.32 | 0.13 |
| Total Damage Score | 1543.5 | 0.000 | 7.22 | 0.14 | 8.68 | 0.26 |
| Plate or Shard % | 1215 | 0.000 | 15.2 | 0.97 | 27.44 | 1.78 |
| Presence of nose bleed | 3370.5 | 0.009 | 85.70% | 2.30% | 7.80% | 6.50% |
Table 3.
Parameter estimates from the general linear model (GLM) testing for an effect of dead weight (kg), brain haemorrhage score, macroscopic brain damage score, and nose/skin haemorrhaging/laceration on time to loss of movement.
Table 3.
Parameter estimates from the general linear model (GLM) testing for an effect of dead weight (kg), brain haemorrhage score, macroscopic brain damage score, and nose/skin haemorrhaging/laceration on time to loss of movement.
| Parameter | B | S.E. | t | Sig. |
|---|
| Intercept | 134.031 | 27.562 | 4.863 | 0.000 |
| Dead weight (kg) | −1.935 | 5.628 | −0.344 | 0.731 |
| Total brain haemorrhage score | 4.930 | 4.919 | 1.002 | 0.317 |
| Total brain damage score | −3.270 | 2.023 | −1.616 | 0.108 |
| Nose/skin haemorrhage/laceration | −36.856 | 9.906 | −3.721 | 0.000 |
| Shard-plate displacement score | −0.412 | 0.280 | −1.473 | 0.142 |
Table 4.
Parameter estimates from the GLM testing for an effect of dead weight (kg), brain haemorrhage score, macroscopic brain damage score, and nose/skin haemorrhage/laceration on movement score.
Table 4.
Parameter estimates from the GLM testing for an effect of dead weight (kg), brain haemorrhage score, macroscopic brain damage score, and nose/skin haemorrhage/laceration on movement score.
| Parameter | B | S.E. | t | Sig. |
|---|
| Intercept | 1.491 | 0.408 | 3.651 | 0.000 |
| Dead weight (kg) | 0.385 | 0.083 | 4.622 | 0.000 |
| Nose/skin haemorrhage/laceration | 0.488 | 0.147 | 3.325 | 0.001 |
| Total brain haemorrhage score | 0.024 | 0.073 | 0.324 | 0.746 |
| Total brain damage score | −0.050 | 0.030 | −1.656 | 0.099 |
| Shard-plate displacement Score | −0.003 | 0.004 | −0.752 | 0.453 |
Table 5.
The number of piglets that demonstrated a nose bleed or skin laceration for each movement score, where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement.
Table 5.
The number of piglets that demonstrated a nose bleed or skin laceration for each movement score, where Score 0 = very little movement; Score 1 = some mild uncontrolled physical movement of limbs; score 2 = considerable uncontrolled physical movement of limbs; and Score 3 = gross uncontrolled physical movement.
| Nose bleed or Skin laceration | Movement Score |
|---|
| 0 | 1 | 2 | 3 |
|---|
| Yes | 1 | 26 | 77 | 60 |
| No | 4 | 20 | 8 | 6 |
Table 6.
The percentage of piglets with nose bleed and broken skin by cartridge size. Actual numbers are in brackets. There was a highly significant association between grain size and condition (chi-square = 70.03, df = 3, exact
p < 0.001). Analysis of the effect of haemorrhages from the nasal passages and skin lacerations is given in
Table 4 and
Table 5.
Table 6.
The percentage of piglets with nose bleed and broken skin by cartridge size. Actual numbers are in brackets. There was a highly significant association between grain size and condition (chi-square = 70.03, df = 3, exact
p < 0.001). Analysis of the effect of haemorrhages from the nasal passages and skin lacerations is given in
Table 4 and
Table 5.
| Cartridge Size | None | Nose Bleed (Nb) | Skin Broken (Sb) | Nb and Sb |
|---|
| 1-grain | 14.3% (21) | 63.9% (94) | 2.7% (4) | 19.0% (28) |
| 1.25-grain | 30.9% (17) | 20.0% (11) | 41.8% (23) | 7.3% (4) |
| Total | 18.8% (38) | 52.0% (105) | 13.4% (27) | 15.8% (32) |