Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
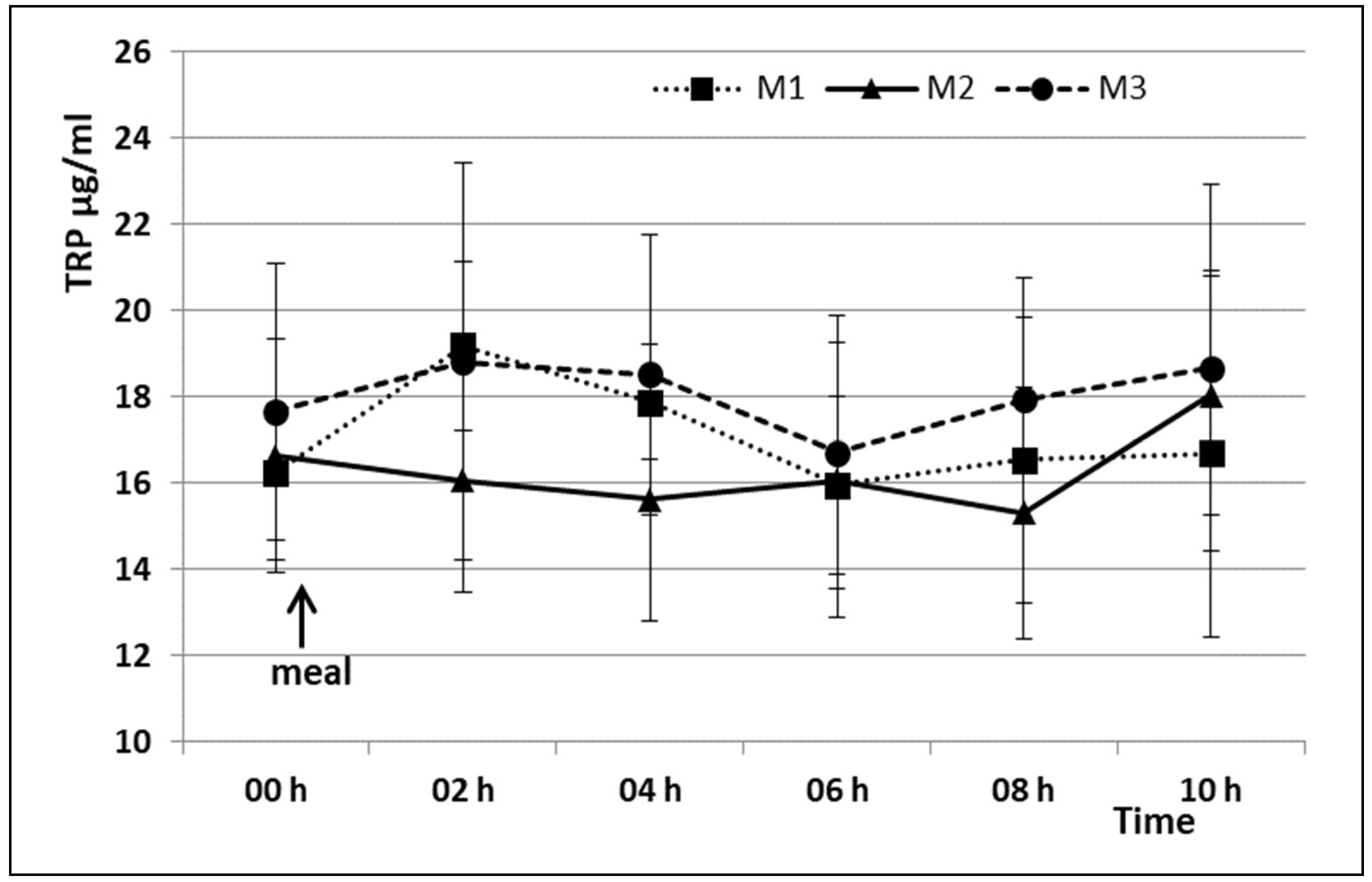
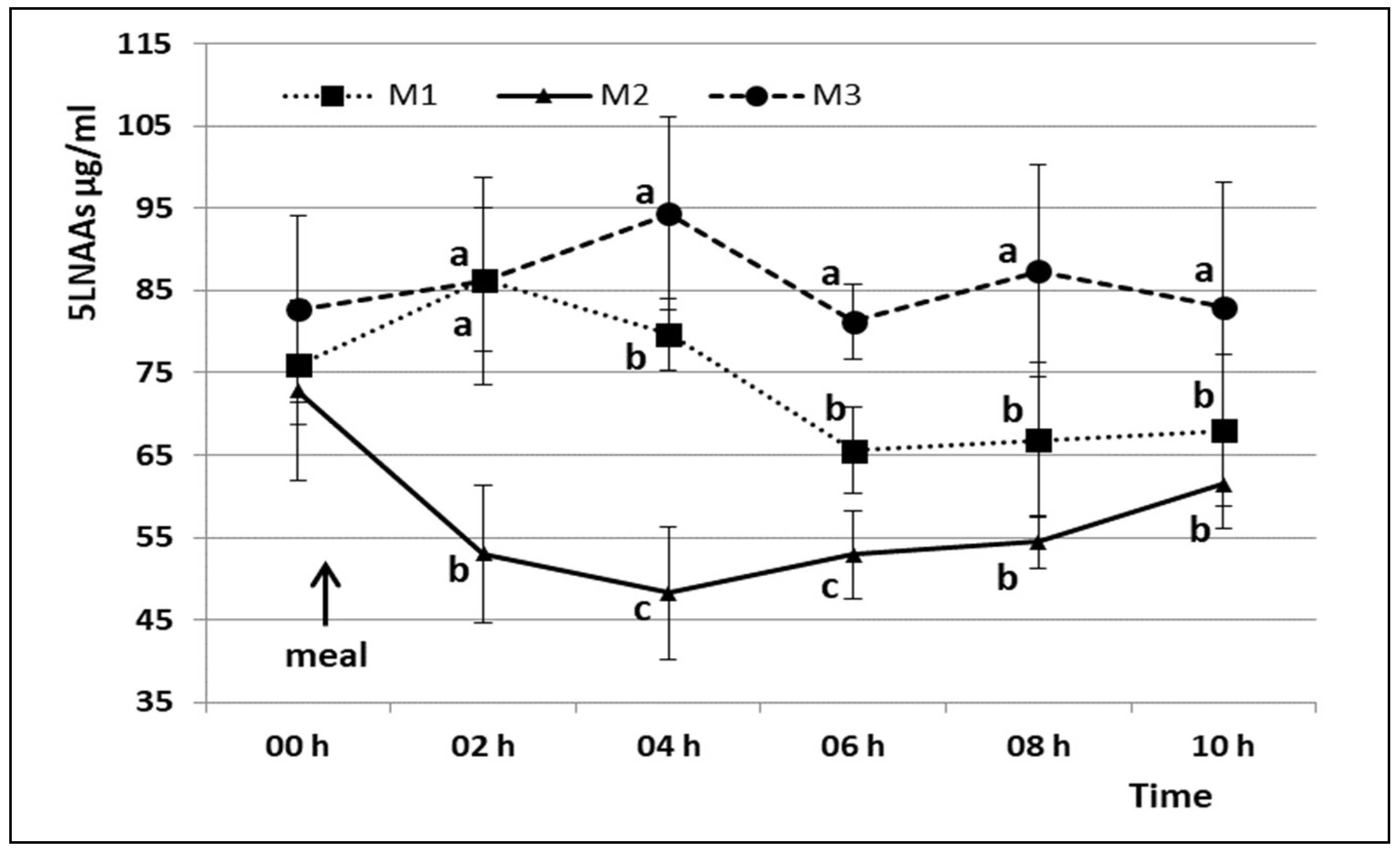
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Savolainen, P.; Zhang, Y.P.; Luo, J.; Lundeberg, J.; Leitner, T. Genetic evidence for an East Asian origin of domestic dogs. Science 2002, 298, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Mariti, C.; Ricci, E.; Carlone, B.; Moore, J.L.; Sighieri, C.; Gazzano, A. Dog attachment to man: A comparison between pet and working dog. J. Vet. Behav. Clin. Appl. Res. 2012, 8, 135–145. [Google Scholar] [CrossRef]
- Patronek, G.J.; Glickman, L.T.; Beck, A.M.; McCabe, G.P.; Ecker, C. Risk factors for relinquishment of dogs to an animal shelter. J. Am. Vet. Med. Assoc. 1996, 209, 572–581. [Google Scholar] [PubMed]
- Sachs, B.D.; Ni, J.R.; Caron, M.G. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. USA 2015, 112, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Rosado, B.; Garcıa-Belenguer, S.; Leon, M.; Chacon, G.; Villegas, A.; Palacio, J. Blood concentrations of serotonin, cortisol and dehydroepiandrosterone in aggressive dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Effect of fluoxetine on blood concentrations of serotonin, cortisol and dehydroepiandrosterone in canine aggression. J. Vet. Pharmacol. Ther. 2010, 34, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.C. Serotonin, a major player in the regulation of feeding and affect. Biol. Psychiatry 1998, 44, 795–797. [Google Scholar] [PubMed]
- Rodwell, V.W. Protein & amino acid metabolism: Conversion of amino acids to specialized products. In Review of Physiological Chemistry; Harper, H., Rodwell, V.W., Mayes, P.A., Eds.; Lange Medical Publication: Los Altos, CA, USA, 2015; pp. 430–439. [Google Scholar]
- Fernstrom, M.H.; Massoudi, M.S.; Fernstrom, J.D. Effect of 8-hydroxy-(di-n-propylamino)-tetralin on the tryptophan-induced increase in 5-hydroxytriptophan accumulation in rat brain. Life Sci. 1990, 47, 283–289. [Google Scholar] [CrossRef]
- Sharp, T.; Bramwell, S.R.; Grahame-Smith, D.G. Effect of acute administration of l-tryptophan on the release of 5-HT in rat hippocampus in relation to serotoninergic neuronal activity: An in vivo microdialysis study. Life Sci. 1992, 50, 1215–1223. [Google Scholar] [CrossRef]
- McMenamy, R.H.; Oncley, J.L. The specific binding of l-tryptophan to serum albumin. J. Biol. Chem. 1958, 233, 1436–1447. [Google Scholar] [PubMed]
- Pardridge, W.M.; Fierer, G. Transport of tryptophan into brain from circulating albumin-bound pool in rats and rabbits. J. Neurochem. 1990, 54, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Okamoto, H.; Koh, S. Effects of various drugs on serum free and total tryptophan levels and brain tryptophan metabolism in rats. Jpn. J. Pharmacol. 1975, 25, 303–310. [Google Scholar] [CrossRef] [PubMed]
- McMenamy, R.H. The binding of indole analogues to defatted human serum albumin at different chloride concentrations. J. Biol. Chem. 1964, 239, 2835–2841. [Google Scholar] [PubMed]
- McArthur, J.N.; Dawkins, P.D.; Smith, M.J.H. The displacement of l-tryptophan and dipeptides from bovine serum albumin in vitro and from human plasma in vivo by antirheumatic drugs. J. Pharm. Pharmacol. 1971, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H.; Szabo, J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am. J. Phys. 1976, 230, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.M. Dependence of 5-HT and catecholamine synthesis on concentrations of precursor amino-acids in rat brain. Naunym Schmiedebergs Arch. Pharmacol. 1978, 303, 157–164. [Google Scholar] [CrossRef]
- Fernstrom, M.D.; Fernstrom, J.D. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after ingestion of sequential meals. Am. J. Clin. Nutr. 1995, 61, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Aromatic amino acids and monoamine synthesis in the central nervous system: Influence of the diet. J. Nutr. Biochem. 1990, 1, 508–517. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R.J. Elevation of plasma tryptophan by insulin in rat. Metabolism 1972, 21, 337–342. [Google Scholar] [CrossRef]
- Lotspeich, W.D. The role of insulin in the metabolism of amino acids. J. Biol. Chem. 1949, 179, 175–180. [Google Scholar] [PubMed]
- DeNapoli, J.S.; Dodman, N.H.; Shuster, L.; Rand, W.M.; Gross, K.L. Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression and hyperactivity. J. Am. Vet. Med. Assoc. 2000, 217, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Mugford, R.A. The influence of nutrition on canine behaviour. J. Small Anim. Pract. 1987, 28, 1046–1055. [Google Scholar] [CrossRef]
- Dodman, N.H.; Reisner, I.R.; Shuster, L.; Rand, W.M.; Luescher, U.A.; Robinson, I.; Houpt, K.A. Effect of dietary protein content on behavior in dogs. J. Am. Vet. Med. Assoc. 1996, 208, 376–379. [Google Scholar] [PubMed]
- Gatta, D.; Pellegrini, O.; Casini, L.; Lubas, G.; Gazzano, A. Effect on dog faeces emission of two diets with different dietary protein sources and carbohydrates content and possible effect on behavior. Veterinaria 2013, 27, 7–12. [Google Scholar]
- Bosch, G.; Beerda, B.; Beynen, A.C.; van der Borg, J.; van der Poel, A.F.; Hendriks, W.R.H. Dietary TRP supplementation in privately owned mildly anxious dogs. Appl. Anim. Behav. Sci. 2009, 121, 97–205. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academy Press: Washington, DC, USA, 2006; p. 398. [Google Scholar]
- Wu, G. Determination of proline by reversed-phase high-performance liquid chromatography with automated pre-column o-phtaldialdhyde derivatization. J. Chromatogr. 1993, 641, 168–175. [Google Scholar] [CrossRef]
- Wu, G.; Davis, P.K.; Flynn, N.E.; Knabe, D.A.; Davidson, J.T. Endogenous synthesis of arginine plays and important role in maintaining arginine homeostasis in postweaning growing pigs. Nutr. Metabol. 1997, 2342–2349. [Google Scholar]
- Wu, G.; Meininger, C.J. Analysis of Citrulline, Arginine, and Methylarginines Using High Performance Liquid Chromatography in Methods in Enzymology; Elsevier: New York, NY, USA, 2008; Volume 440, pp. 177–189. [Google Scholar]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; Ruiz, J.L.; Manteca, X. Differences in serotonin serum concentration between aggressive English cocker spaniels and aggressive dogs of other breeds. J. Vet. Behav. Clin. Appl. Res. 2012, 8, 19–25. [Google Scholar] [CrossRef]
- Dipace, V. Tryptophan effect on dog behavior: A review. Dog Behavior. 2015, 1, 23–31. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Drew, M.D.; Huang, Q.; Silver, T.I.; Weber, L.P. Postprandial impairment of flow-mediated dilation and elevated methylglyoxal after simple but not complex carbohydrate consumption in dogs. Nutr. Res. 2012, 32, 278–284. [Google Scholar] [CrossRef] [PubMed]
| Beef Meat | Puffed Rice | D1 | D2 | |
|---|---|---|---|---|
| Dry Matter | 33.20 | 94.40 | 44.56 | 92.00 |
| Crude protein | 59.34 | 6.57 | 27.74 | 28.26 |
| Crude fat | 39.15 | 1.91 | 16.78 | 16.30 |
| NSC | - | 73.83 | 46.40 | 46.64 |
| Crude fiber | - | 1.38 | 0.87 | 2.50 |
| Ash | 1.51 | 16.31 | 8.21 | 6.30 |
| ME (kcal/kg) | 5405 | 2976 | 4021 | 4007 |
| M1 | M2 | M3 | ||||
|---|---|---|---|---|---|---|
| 8:00 AM | 8:00 PM | 8:00 AM | 8:00 PM | 8:00 AM | 8:00 PM | |
| Puffed Rice (g) | 125 | 125 | 250 | - | - | - |
| Beef Meat (g) | 225 | 225 | - | 450 | - | - |
| Olive Oil (g) | 3 | - | 3 | - | - | - |
| Dry food (g) | - | - | - | - | 260 | 260 |
| Time | M1 | M2 | M3 | p |
|---|---|---|---|---|
| t0 | 0.217 ± 0.037 | 0.229 ± 0.031 | 0.213 ± 0.025 | n.s. |
| t2 | 0.224 ± 0.030 b | 0.306 ± 0.049 a | 0.217 ± 0.035 b | 0.001 |
| t4 | 0.225 ± 0.026 b | 0.327 ± 0.058 a | 0.197 ± 0.036 b | 0.001 |
| t6 | 0.244 ± 0.030 b | 0.303 ± 0.049 a | 0.205 ± 0.030 b | 0.015 |
| t8 | 0.246 ± 0.020 ab | 0.280 ± 0.039 a | 0.206 ± 0.025 b | 0.001 |
| t10 | 0.243 ± 0.029 a | 0.294 ± 0.041 a | 0.224 ± 0.017 b | 0.001 |
| t24 | 0.210 ± 0.024 a | 0.155 ± 0.027 b | 0.189 ± 0.020 a | 0.041 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzano, A.; Ogi, A.; Torracca, B.; Mariti, C.; Casini, L. Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level. Animals 2018, 8, 63. https://doi.org/10.3390/ani8050063
Gazzano A, Ogi A, Torracca B, Mariti C, Casini L. Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level. Animals. 2018; 8(5):63. https://doi.org/10.3390/ani8050063
Chicago/Turabian StyleGazzano, Angelo, Asahi Ogi, Beatrice Torracca, Chiara Mariti, and Lucia Casini. 2018. "Plasma Tryptophan/Large Neutral Amino Acids Ratio in Domestic Dogs Is Affected by a Single Meal with High Carbohydrates Level" Animals 8, no. 5: 63. https://doi.org/10.3390/ani8050063





