1. Introduction
One of the essential features of Geological Disposal Facility (GDF) concepts for the disposal of high-level radioactive waste in the deep sub-surface is the protection of radioactive waste canisters from corrosion in order to avoid, or at least minimize, the release of radionuclides into the near-field environment and beyond. To achieve this most Waste Management Organisations (WMOs) have developed safety cases based on the cumulative properties of multi-barrier systems. Such systems usually comprise the wasteform itself, the waste canister material, the overpack container (e.g., copper or stainless steel) in which a canister may be housed, the backfill and buffer materials that surround and support the canister/container and separate them from the surrounding rock into which the waste deposition chamber has been excavated, and the surrounding geological barrier or “geosphere”. The components of the multi-barrier system that are introduced into the excavated subsurface geology are collectively referred to as the Engineered Barrier System (EBS).
The material constituting the backfill, or near-field buffer, in the EBS is required to be chemically and thermally inert with respect to the material the canister is made of and with the surrounding geosphere. It is also required to possess a very low porosity and hydraulic conductivity so that fluid transport is minimized. Good sorption properties are also favoured, so that if any radioactive material is released into the near-field its dispersion is limited not only by the negligible hydraulic gradient but also by chemical fixation. For many GDF concepts the preferred backfill/buffer material is bentonite, a montmorillonite clay of the smectite series. The bentonite buffer in the Swedish KBS concept [
1], for example, comprises a thick (1 m radius) cylindrical sleeve or ring around the waste canister/container. The compacted bentonite (density of 1.76 kg/m
3), arranged to form a sleeve comprised of stacked rings of brick segments, swells considerably when it comes into contact with groundwater. This bentonite swelling property is central to the safety case for the GDF as it seals voids in the EBS and fractures present in the adjacent host rock disturbed during the excavation process, thereby forming a continuous, low-permeability medium around the waste packages following water saturation. This acts to limit the corrosion rates of the waste packages and the transport of any radionuclides or contaminants potentially released from corroded containers and then dissolved in groundwaters [
2,
3,
4].
Monitoring of the GDF and its constituent parts is recognised internationally as being critical to decision-making during phased or staged disposal of radioactive waste [
2,
5,
6,
7,
8]. Monitoring, via continuous or periodic observation and measurement, of the key thermal, mechanical, chemical, and hydrological parameters of a GDF is not only required in order to evaluate the behaviour of the repository system and performance of the barriers in relation to their safety functions, but also to build and sustain public confidence in geological disposal [
4]. Indeed, monitoring is central to the European Community Seventh Framework Programme “Monitoring Developments for Safe Repository Operation and Staged Closure” (MoDeRn) Project [
9] and to MoDeRn 2020 [
10].
Whilst the need for pre-construction and operational monitoring of a GDF is fully accepted by all WMOs and national programmes in accord with the requirements of the International Atomic Energy Agency (IAEA) [
8,
11], the need for post-closure monitoring has until recently been questioned. One reason for this is that closure of a GDF would only occur once it is deemed, on the basis of all the pre- and operational monitoring coupled with forward modelling, to meet the standards required for post-closure safety. Another reason is that the maintenance of the monitoring systems cannot be guaranteed for durations beyond the accepted period of institutional control, perhaps a few hundred years. However, the European Commission (2004) has noted that there are likely to be societal pressures for monitoring for a period after repository closure in order to increase confidence in the evolution of the GDF system and our understanding of it. This recognition has led to the incorporation of ideas for post-closure monitoring into major projects concerned with GDF safety, such as MoDeRn [
9] and MoDeRn2020 [
8,
10].
The challenge for monitoring at any stage of GDF construction, operation and closure lies in collecting the key physical and chemical parameters, such as stress fields, groundwater flows, thermal gradients, bentonite swelling, and void-loss, in the vicinity of the waste with as little impact on the integrity of the multi-barrier EBS as possible, whilst providing data of reliable quality over prolonged timescales [
7,
8,
12]. These data must also be transmittable to the surface or at least distant analysis centres, so extensive research has been undertaken on wireless transmission networks and their coupling with remotely-sited sensing techniques such as microseismics, electric resistivity, acoustic emission, ultrasonic, gamma detection, and georadar. As all of these techniques require a power source in order to make measurements, their ability to monitor the parameters useful for the evaluation of EBS and near-field evolution beyond the local operational period and into the post-closure phase is limited. For example, electronic or electromagnet sensor devices that measure electrical resistivity, strain, and humidity or gas pressure can be emplaced within the bentonite buffer ring of the EBS and used to indirectly track bentonite re-saturation and swelling, but only for as long as their power sources last [
7,
12].
In order to extend the duration over which the EBS evolution, especially that of the bentonite buffer, can be monitored it is useful to consider alternative monitoring systems that do not require power at the sites of the sensors themselves. One approach to this is to embed in the EBS materials with diagnostic and measurable properties that change as the system evolves, thereby providing a proxy for its evolution, but which do not compromise the integrity of the EBS itself. This work describes and provides an initial evaluation of one such alternative approach to in-situ monitoring of any bentonite-based EBS using mineral or material magnets.
2. Materials and Methods
2.1. The Magnetic Monitoring Concept
Magnetic materials monitoring (MMM) focuses on utilising fluid-induced changes in the intrinsic magnetic properties of natural and synthetic materials to monitor the migration and recharge of fluids into the EBS of a bentonite-buffered GDF for high level waste.
The essential concept is to define suites of magnetic materials that react with fluids, or corrode, in predictable ways and in doing so change their magnetic properties in response to fluid ingress into the bentonite buffer situated around the waste packages. The time-resolved record of changes in magnetic properties then provides a passive, non-invasive monitor of how the EBS is re-saturating following back-filling, potentially both in terms of rates of fluid recharge and spatial variations in fluid ingress.
Magnetic monitoring is envisaged to be utilised initially to investigate fluid ingress on scales commensurate with single or multiple deposition sites as they are closed off (e.g., 5–10 ms lengthscales). Changes in magnetic signals are measured as time- and spatially-dependent deviations from the baseline values imposed by the magnetic materials embedded with the EBS measured near-site within the operational GDF. Such monitoring over the operational timescale of the GDF has the capacity to be complemented by longer-term magnetic monitoring from more remote magnetometer locations through upscaling of the system both spatially, with improvements in high-sensitivity magnetometers, and temporally through the use of successively less reactive magnets that corrode slowly and hence facilitate long-lived monitoring of the changes in magnetic signals beyond the operational phase of a GDF.
2.2. Magnetic Monitoring as a System
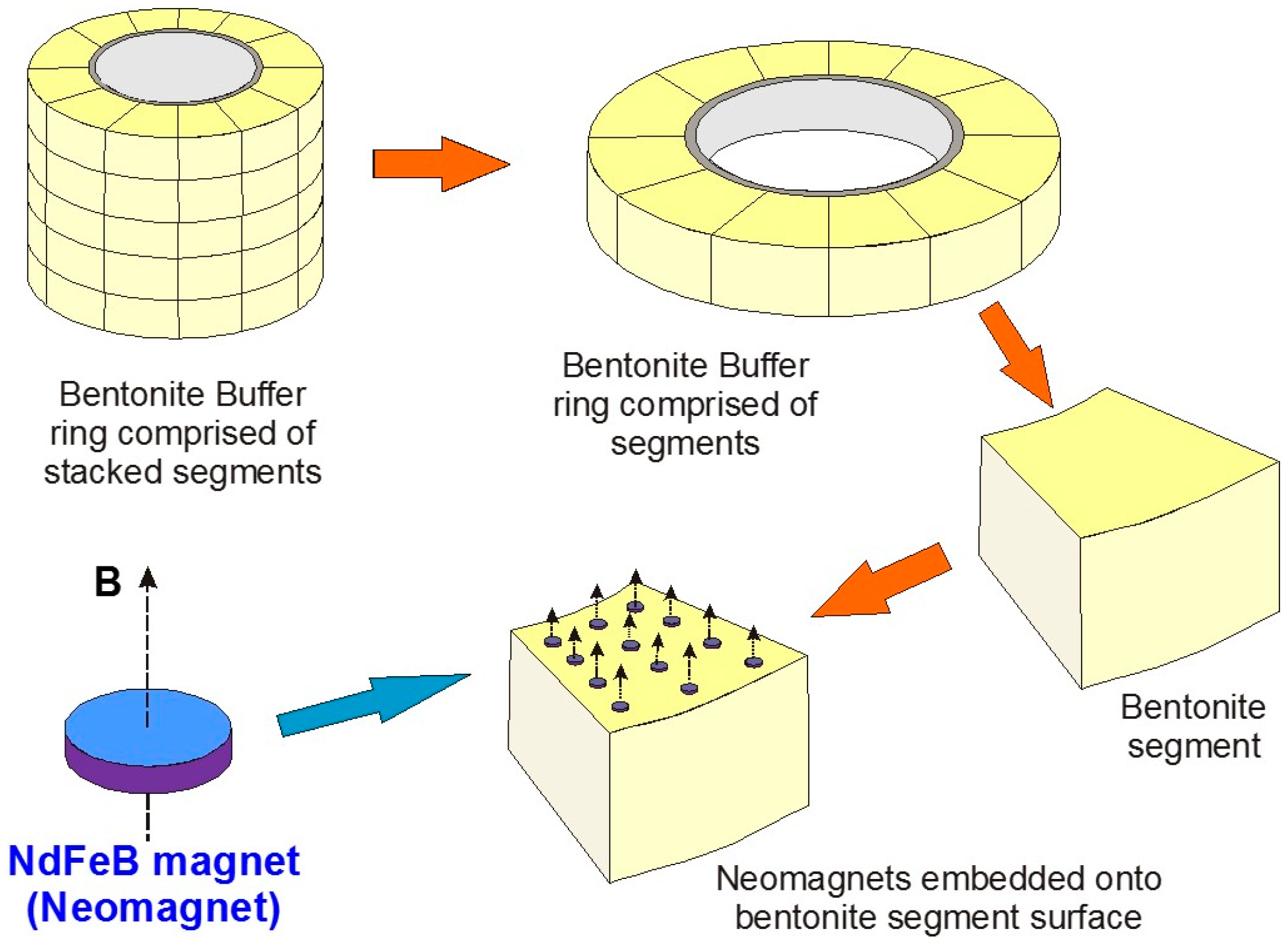
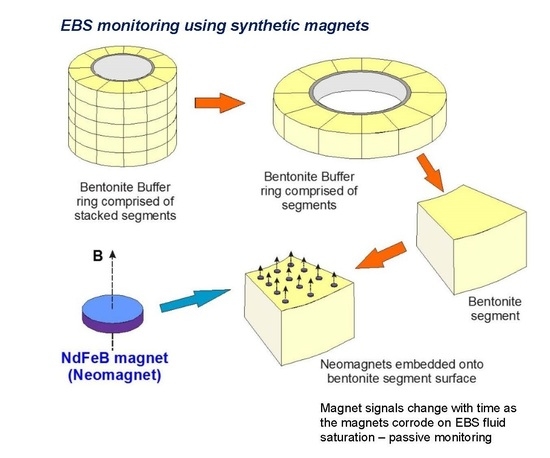
The principle of using magnets as a monitoring system is illustrated in
Figure 1, which depicts a bentonite buffer “sleeve” located around an individual HLW (High Level Waste) canister and overpack, simplified from the Swedish KBS-3V (vertical waste deposition site) GDF design concept [
1,
13]. Using neodymium (Nd) magnets (“Nd-Fe-B magnets”) as an example, arrays of small, oriented magnets can be embedded on pre-pressed bentonite segments that have appropriately-sized depressions in their upper surfaces to accommodate the magnets without increasing void space. The example in
Figure 1 has 12 such magnets embedded on one bentonite segment.
The magnets in this illustration are disc magnets with an axial magnetic field. If N45 grade Nd-Fe-B magnets are used the individual discs have residual flux density B fields (B
r) of 1.29–1.35 Tesla, or 12,900–13,500 Gauss. Whilst full field and magnetic flux density modelling of the array of disc magnets effectively disposed in annular sheets around the canister is not the objective of this paper and is beyond its scope, the flux density at a point situated above the axis of an axially-magnetised cylindrical magnet can be calculated analytically [
14,
15,
16] and used, with simplifying assumptions, to estimate the distance over which the magnetic signal produced by the system can be reliably measured. As modern ground-based and remote magnetometers can routinely measure with precisions of 0.1–1.0 nT (0.1–1 gamma), anomalies of the order of 10 nT are easily recorded and are assumed to provide a conservative lower limit of resolution for the method.
The flux density at a distance z axially above a magnetic rod or cylinder of radius
R and thickness
D, with characteristic residual flux density B
r+, is given by the following equation [
14]:
Based on (1), a single N45 Nd-Fe-B magnet disc with radius of 20 mm and thickness 5 mm would produce an axial flux density of 10 nT at a distance of ca. 5 m immediately above the magnet, increasing to 100 nT at 2.4 m and 1000 nT at ca. 1 m. Whilst large and easily measurable as anomalies relative to the Earth’s field (ca. 50,000 nT), these flux densities are only present immediately above the single magnet, and decrease significantly laterally over only millimetres. In order to “spread out” the magnetic flux anomaly laterally or enlarge the effective axial region, a larger radius disc could be used or several disc magnets could be deployed and arranged to form a sheet or much larger radius disc-shaped array (
Figure 1). At a very simplistic level, if it is assumed that the average B
r for an array of disc magnets is proportional to the area of discs divided by the area of bentonite surface, then a 1.5 m radius planar array containing 100 discs each of 20 mm radius would result in an easily measurable flux density 1000 nT as far as 5 m axially above the array. The same calculation performed assuming a 0.5 m diameter magnet-free core inside a 1 m radius bentonite buffer ring leads to an estimate of 875 nT at 5 m above the magnet array plane in the canister/bentonite buffer deposition site.
Whilst the magnitudes of the axial flux densities estimated here are subject to order-of-magnitude uncertainties arising from the gross simplifications and assumptions used, they nevertheless indicate that an optimized design of permanent magnets embedded on planes within the bentonite buffer ring is capable of producing an initial magnetic field that can be measured remotely from within the operational GDF. Temporal changes (decreases) in the magnetic flux density caused by fluid-activated corrosion of the neo magnets during the progressive saturation of the bentonite EBS could be measured and used to monitor saturation provided the time-dependent corrosion behaviour of the magnets in contact with bentonite and realistic fluids is understood.
2.3. Experimental Program and Design
In this paper we present a distillation of the results of simple batch experiments involving magnets, bentonite, and fluids. The degradation of magnetic properties and signatures caused by fluid-induced corrosion of the different magnets are quantified to allow an evaluation of the sensitivity of magnetic monitoring with fluid ingress and saturation. We also summarise the effects of magnet corrosion on the bentonite matrix in order to understand if such corrosion might have a negative impact on the swelling properties of the bentonite buffer in a GDF [
17].
Three types of synthetic permanent magnets (Grade N42 Nd-Fe-B, Grade 5 AlNiCo, and ceramic Ba
0.6Fe
2O
3–Sr
0.6Fe
2O
3 F30 ferrite), embedded in powdered MX80 Na-montmorillonite, have been reacted with three sets of solutions (
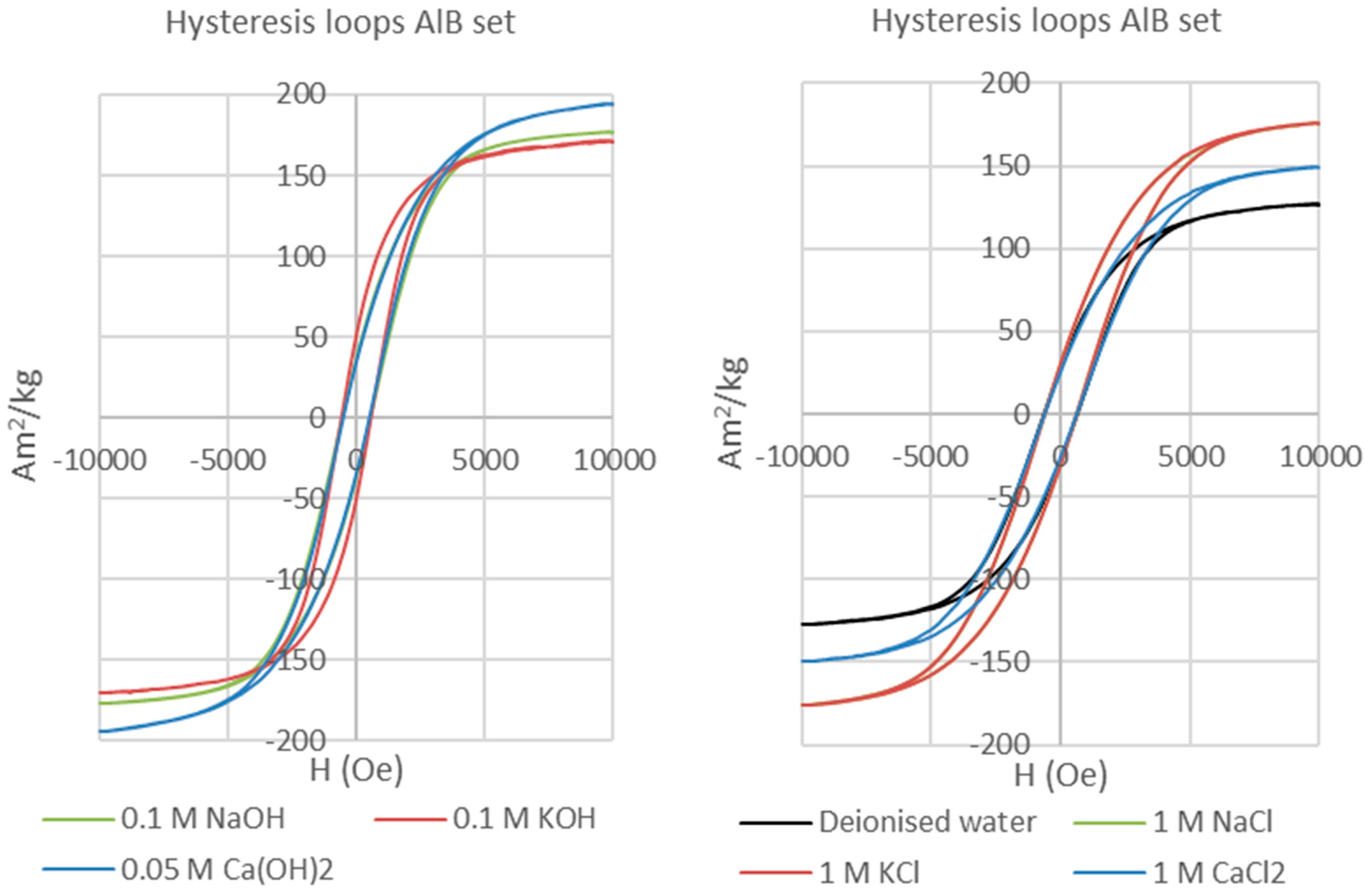
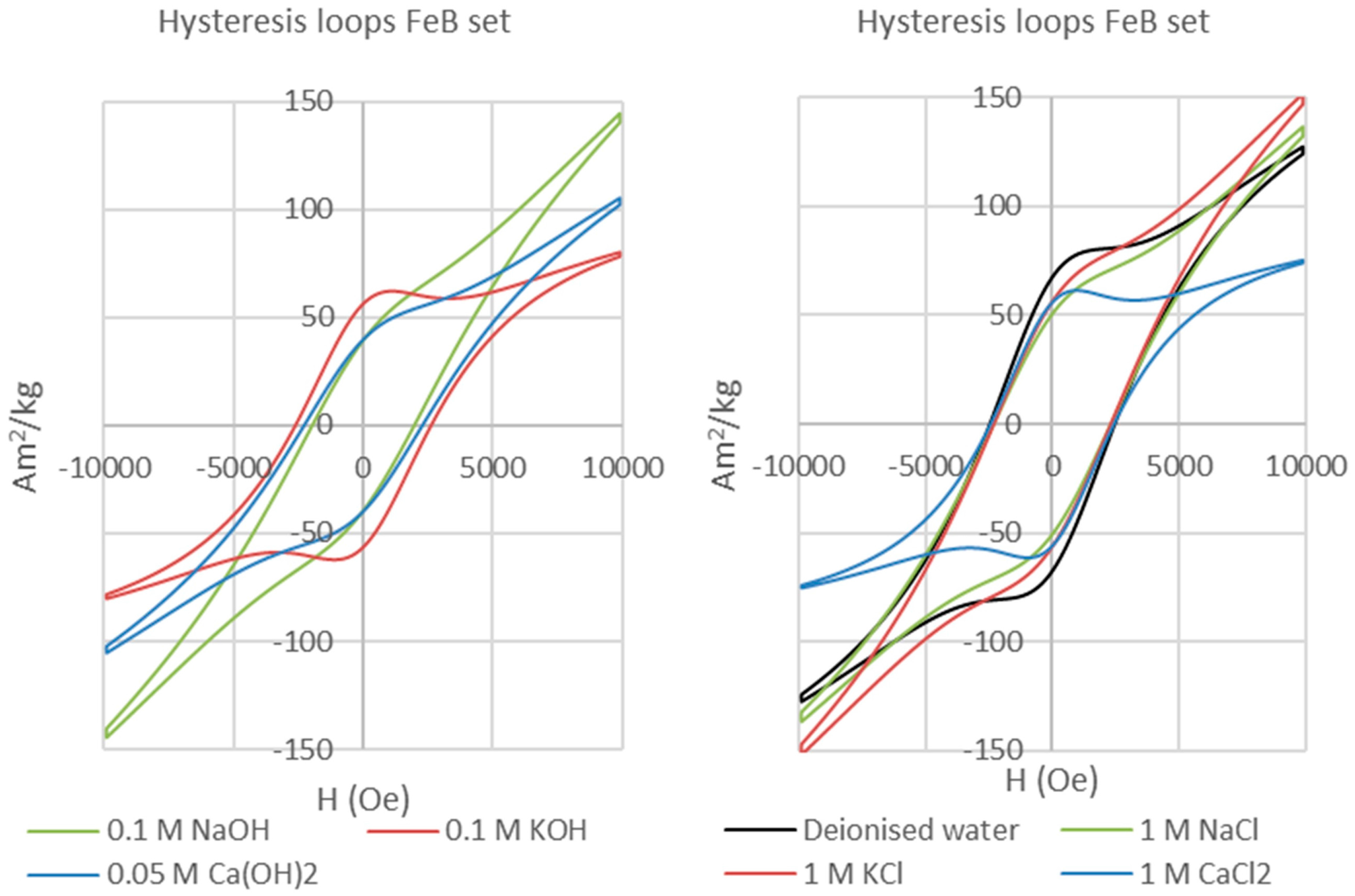
Table 1), generating seven experiments for each magnet type in the presence of bentonite. We also have performed a set of experiments identical in terms of the magnets and solutions used but without bentonite (i.e., magnet-solution only experiments) in order to assess magnet corrosion in the absence of bentonite. The results of those experiments are not directly relevant to the behaviour of the bentonite-bearing EBS and hence will be reported elsewhere.
The experiments were run at 70 °C for 155 days each, and performed in tightly sealed 50 mL serum bottles with initial magnet:bentonite volume ratios of 1:19 and solids:solution ratios of 1:3.
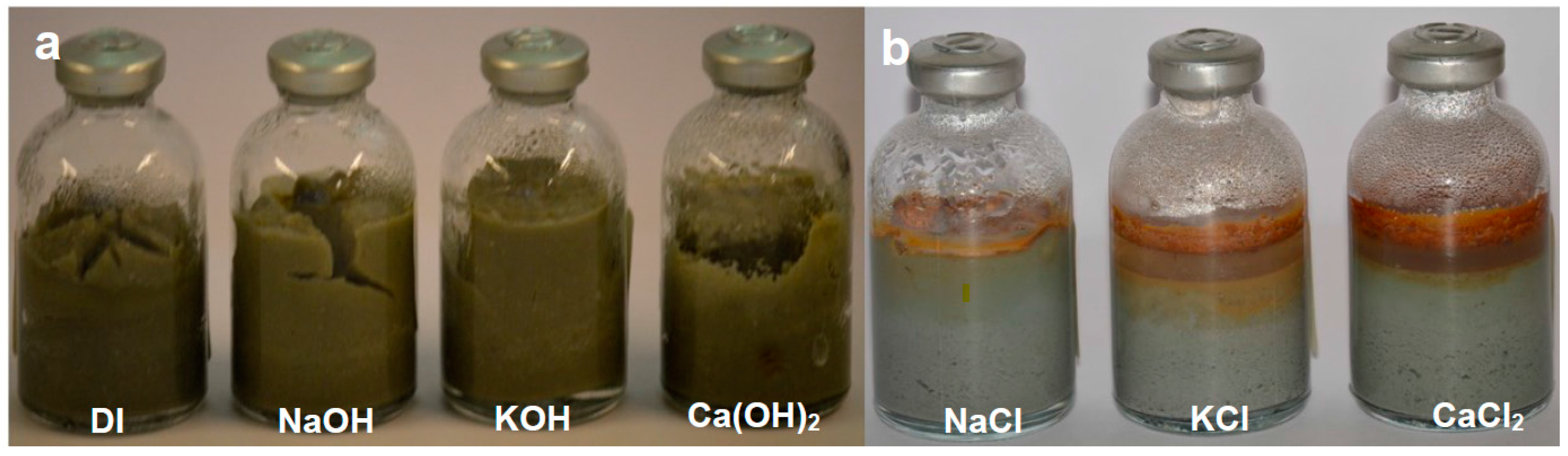
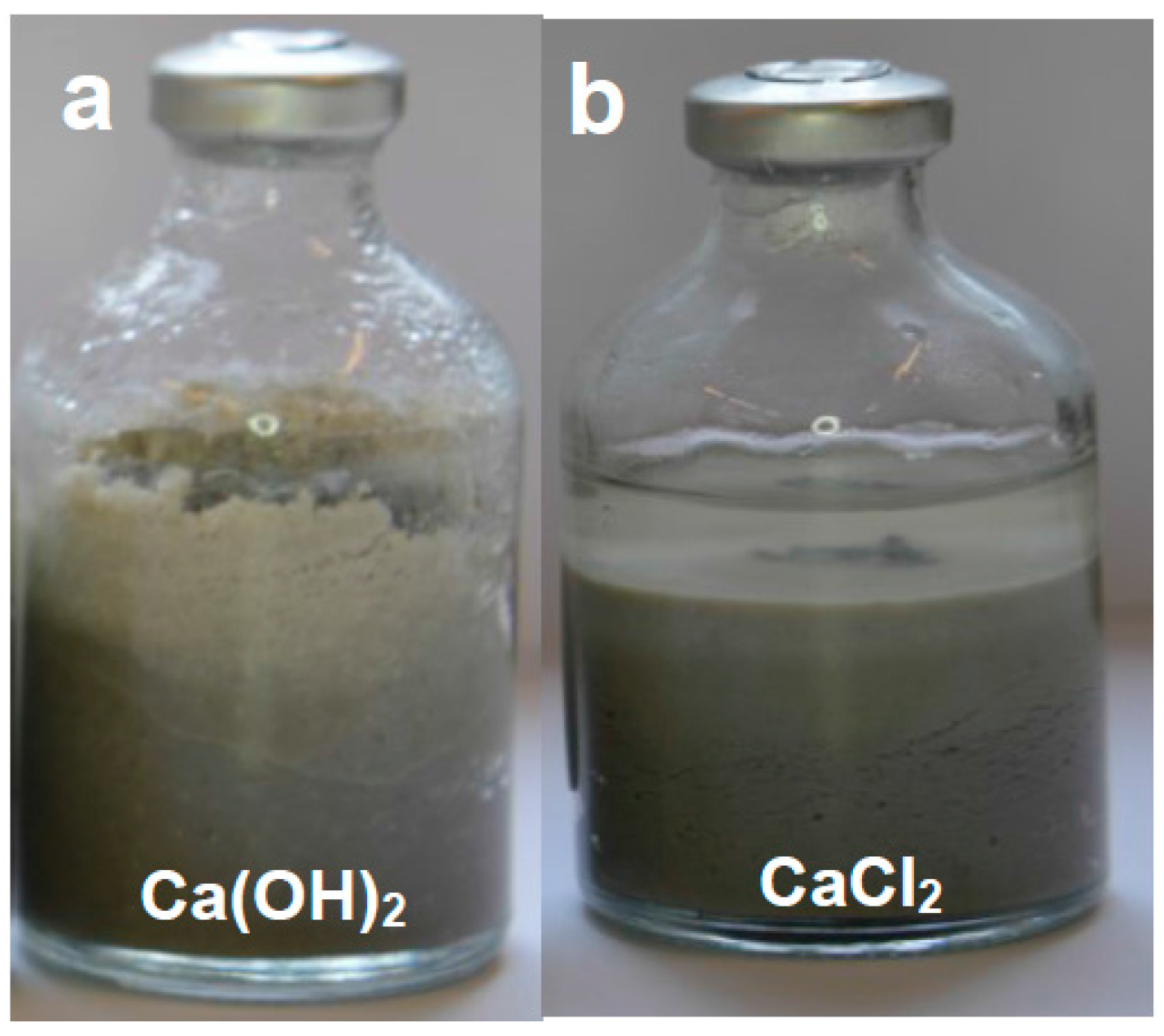
A temperature of 70 °C was chosen as it is regarded to be the average expected temperature affecting the EBS, taking into account the thermal gradient between the waste canister and the geosphere. The durations of the experiments were selected to allow measurable reaction to take place and enable comparisons to be made between them and with subsequent confined fluid flow experiments. The changes in magnet character (colour, state of aggregation) and bentonite swelling and colour was monitored for each experiment using photographs taken at monthly intervals prior to final extraction.
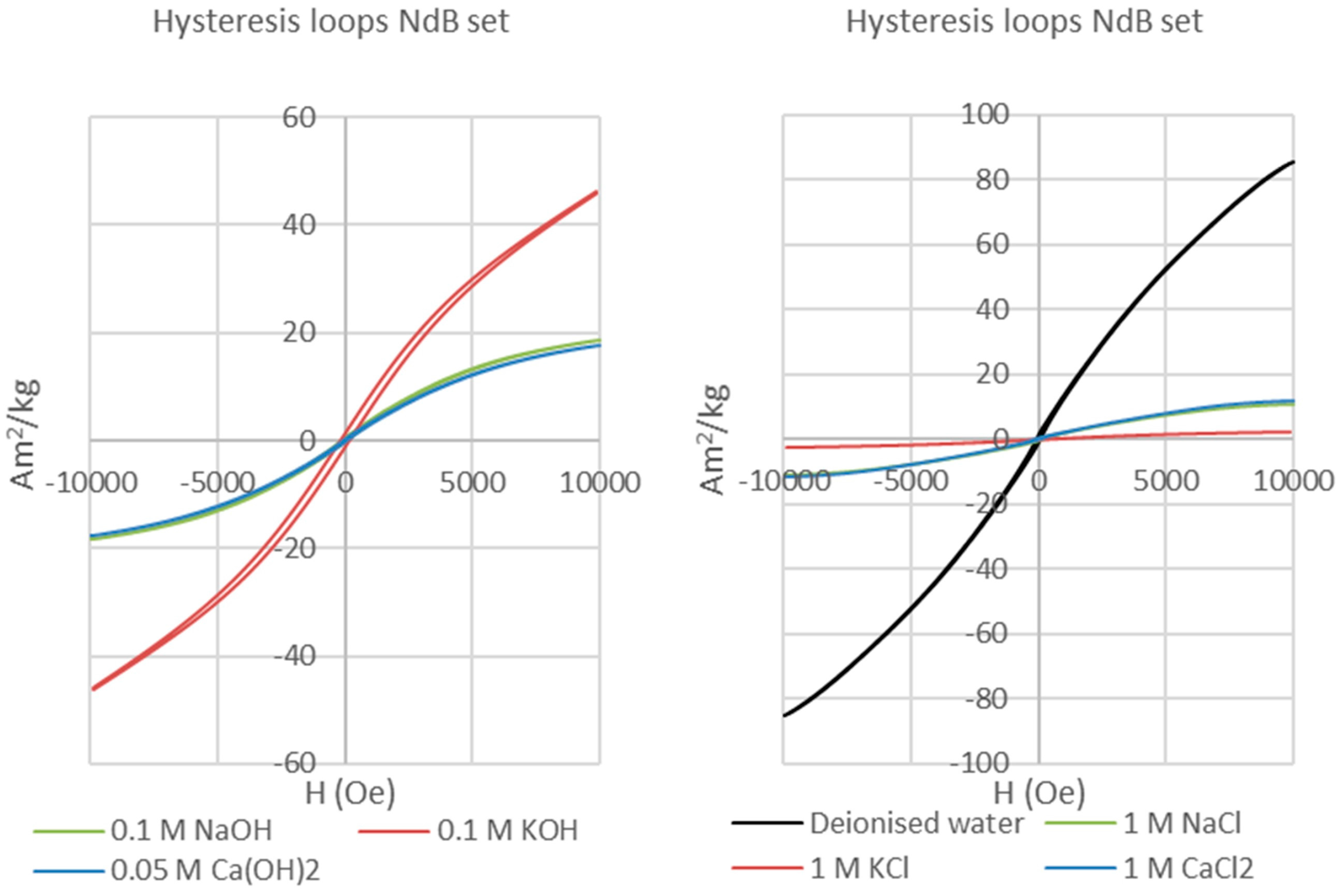
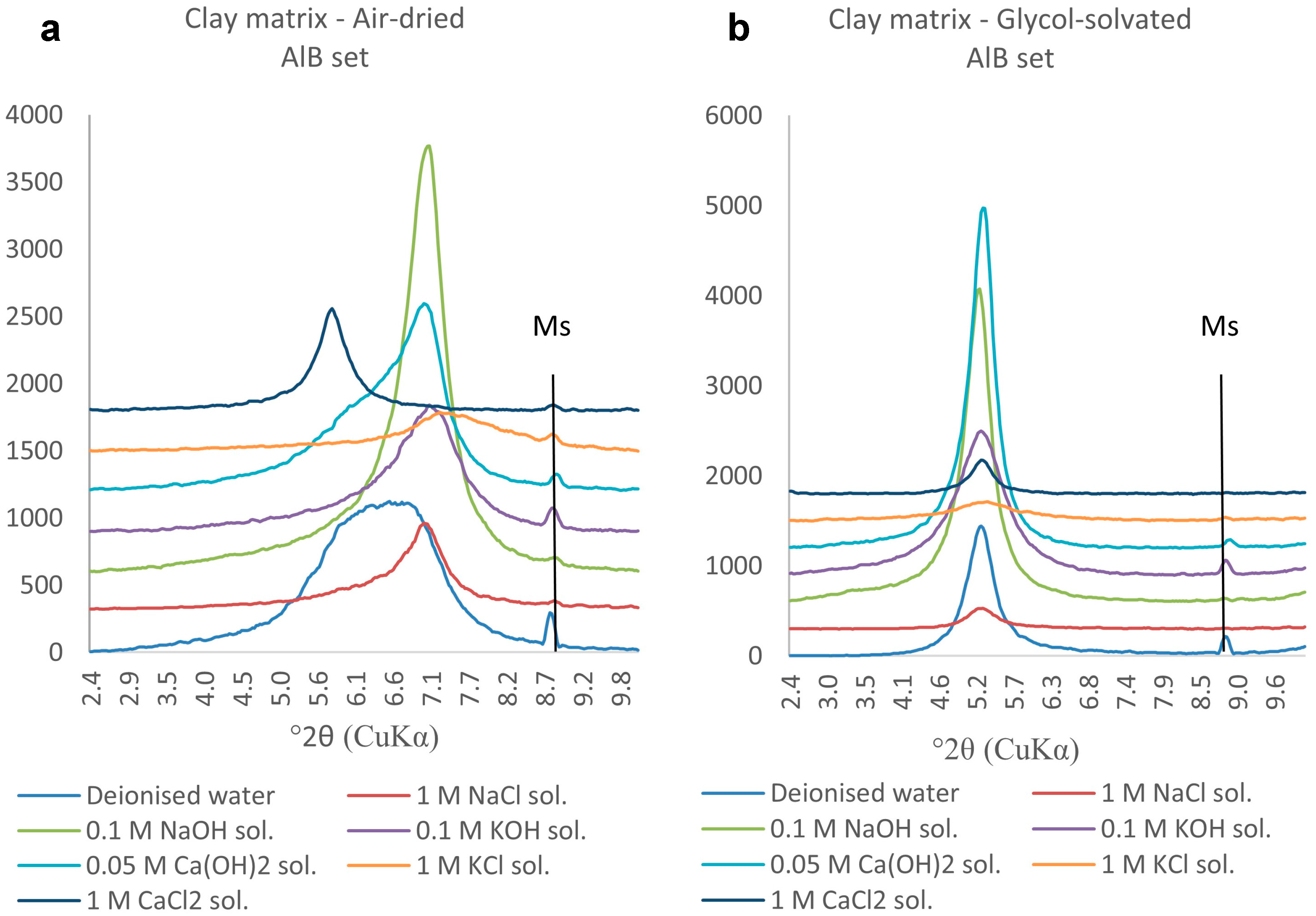
The bentonite clay matrix and the corroded permanent magnets were magnetically separated after each experiment. The clay matrix was analysed on oriented mounts with a Bruker D8 Advance diffractometer with Sol-X Energy dispersive detector (2–70° 2θ range, 0.025 step size, and 1.5 s step time). The magnetic analyses (hysteresis and backfield) were performed with a Princeton Measurements dual-head AGM/VSM (alternating gradient field magnetometer (AGM) / vibrating sample magnetometer (VSM); Princeton Measurements Corporation, Princeton, USA). Hysteresis (M
s—saturation magnetization, M
rs—remanence, and H
c—coercivity) and Backfield (H
cr—coercivity of remanence) parameters were calculated using the free software RockMagAnalyzer version 1.0 (Ludwig Maximilians Universität, München, Germany). Both hysteresis and backfield curves were fitted using log-Gaussian functions. Weights, hysteresis, and backfield values for the magnets are presented in
Appendix A. The thermomagnetic analyses were performed with a Princeton Measurements dual-head AGM/VSM with high-T furnace applied (maximum temperature 790 °C) and in Argon atmosphere to prevent the oxidation of samples upon heating.
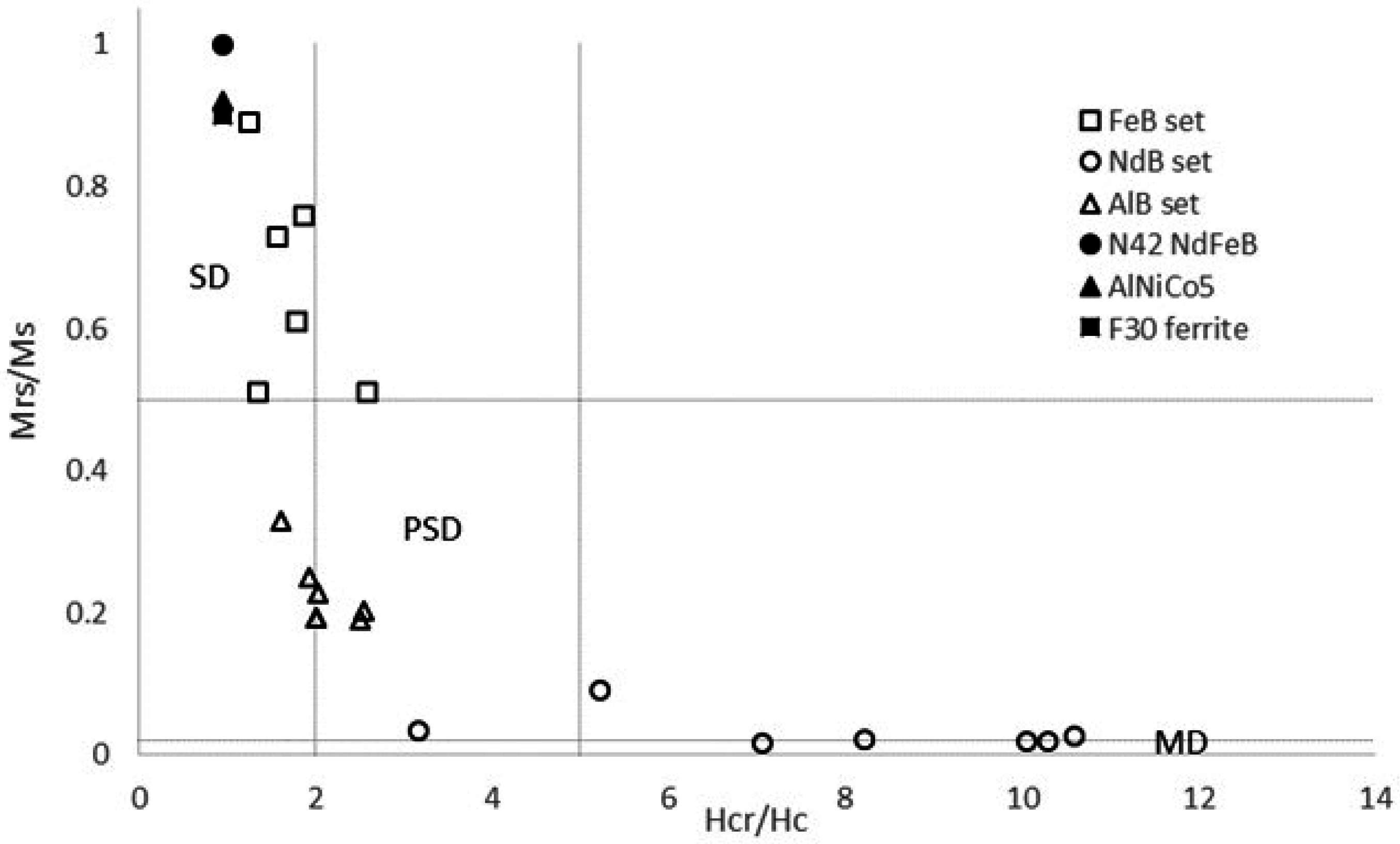
Following the studies of Day et al. [
18] and Dunlop [
19,
20] on the grain size and compositional dependencies of the magnetic hysteresis properties of titanomagnetites and magnetites, a graphical approach using a plot of M
rs/M
s against H
cr/H
c was adopted in this work to analyse and classify the magnet behaviour and evolution.
4. Discussion
The analyses of magnetic hysteresis and backfield properties on the reacted/corroded magnets allow the synthetic magnets to be classified according to their material-dependent reactivity:
Non-reactive permanent magnets. Grade F30 hexagonal ferrite did not exhibit corrosion-related precipitates or traces of oxidation. Consistent with this, their magnetic analysis (
Figure 9) showed that all treated ferrites retained the single-domain properties of the initial material.
Weakly reactive permanent magnets. Grade 5 AlNiCo showed intense corrosion effects and associated precipitation of Fe/Al (oxyhydr) oxides when reacted with saline solutions, but the variations of the hysteresis and backfield properties were consistent in all treated samples and consistent with an evolution to pseudo-single domain magnetic state.
Highly reactive permanent magnets. Grade N42 Nd-Fe-B showed intense corrosion, marked by disaggregation of the fragments and precipitation of Fe and Nd (oxyhydr) oxides when reacted with saline solutions and deionized water, and oxidation and precipitation of Fe (oxyhydr) oxides when reacted in alkaline solutions. Magnetic hysteresis analysis demonstrated a clear transition from a single-domain starting material to superparamagnetic behaviour for the treated magnets.
These distinctive corrosion responses of the different magnets under different aqueous solution compositions and in the presence of bentonite indicate their considerable potential for magnetic monitoring of the re-saturation of bentonite-based EBS. The faster corrosion/alteration rates and marked decreases in magnetic coercivity observed for the Nd-Fe-B neo magnets indicate that these may be suitable for operational monitoring of fluid recharge and saturation in a GDF, whereas the less reactive AlNiCo magnets offer potential for longer-term monitoring, including post-closure monitoring. Ferrite magnets, which appear to be unreactive based on our experiments over durations of 155 days but could show reaction over longer timescales or in the presence of extreme fluids, may be useful in providing a baseline magnetic signal that can be registered by magnetometer arrays.
In the case of Nd-Fe-B neo magnets, comparison of the results of these experiments in the presence of bentonite with those for magnet-solution interactions alone [
17] demonstrates that bentonite has a dramatic effect on magnet corrosion in the presence of saline water under static conditions (i.e., no or negligible fluid flow). In the absence of fluid flow, the presence of bentonite facilitates the development of two electric double layers with opposite polarity (one generated by the clay and the other by the corrosion on the magnet surface). This in turn causes enhanced corrosion of the magnets compared with the corrosion of the magnets in water alone [
17]. This important electrical buffering effect provided by bentonite implies that experiments on neo magnet corrosion in humid environments or in the presence of fluids alone are unlikely to be relevant to magnet corrosion in an EBS once saturation commences.
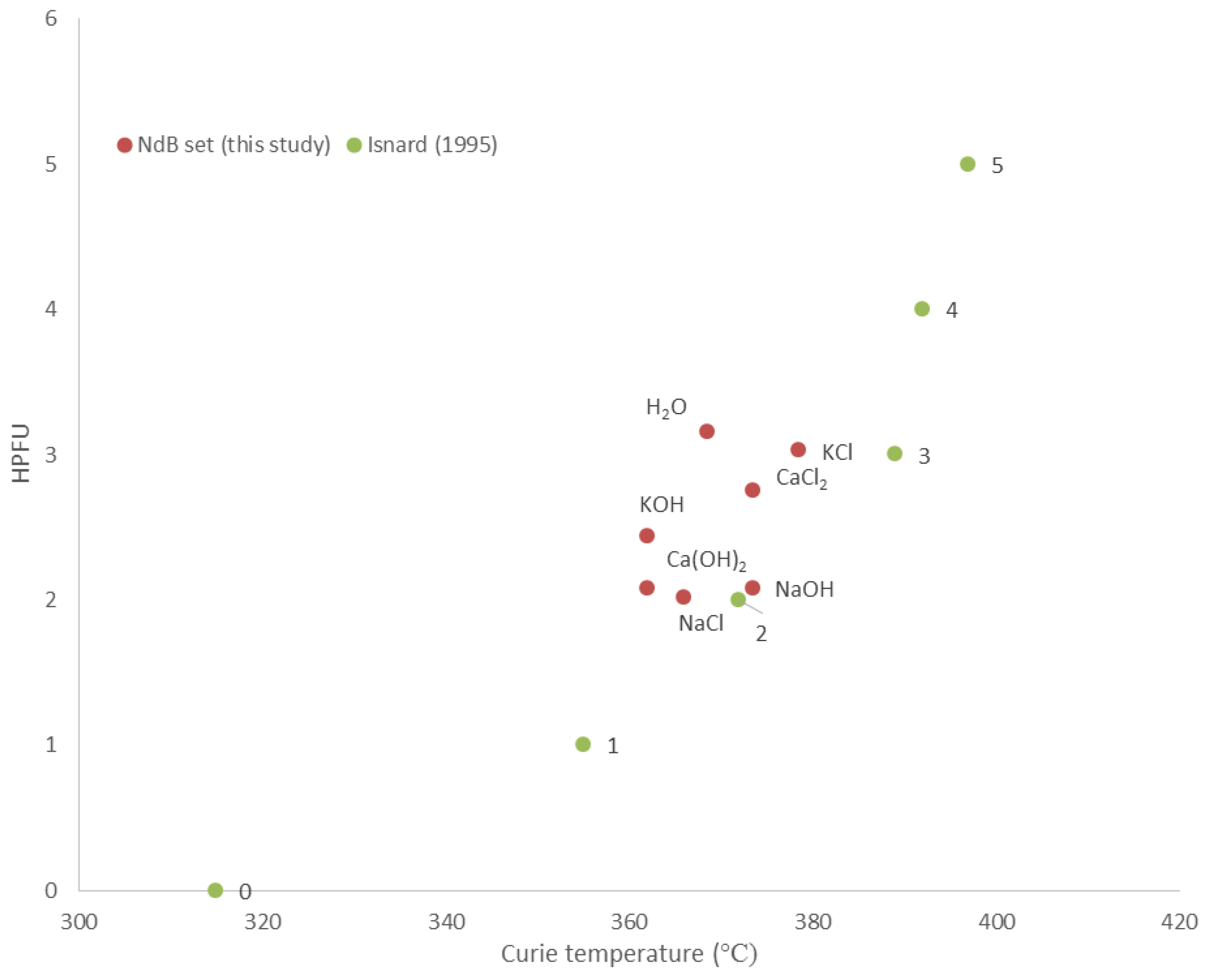
Conversely, the presence of magnets, whether reactive (neo magnets) or not (ferrites), is demonstrated to have a negligible effect on the swelling properties of bentonite. The insertion of reactive ferromagnets does not compromise the integrity of the EBS. Furthermore, the formation of Nd-Fe-B hydrides with an average hydrogenation state of 2–3 HPFU as the corrosion products of neomagnets places strong limits on the potentially deleterious evolution of H
2 gas with corrosion where neo magnets are used as the monitoring materials. This means that magnets can be embedded in the bentonite, for example, on bentonite block surfaces (
Figure 1), to form bespoke magnet arrays designed to optimise magnetic signal and sensitivity to fluid ingress. The design of such arrays could in principle be tuned to the geometry of the EBS (e.g., vertical vs horizontal or inclined deposition chambers) and the distance-sensitivity requirements of the magnetometer measuring system.
5. Conclusions
Neo magnets and AlNiCo magnets are corroded by saline and/or alkaline fluids in the presence of bentonite. Over time this leads to measurable degradation in their magnetic properties, such as their intrinsic magnetic field, that can serve as a proxy for saturation of the surrounding bentonite. Bentonite in these magnet-fluid-bentonite experiments at 70 °C shows negligible replacement by non-swelling clays and so retains the swelling property that is central to its role in ensuring the safety of the engineered barrier. Together, these results confirm the potential for the use of high-field synthetic magnets in pre-designed arrays to monitor EBS evolution and hence the safety case for deep geological disposal.
These results represent a positive starting point for Magnetic Materials Monitoring. Incorporation of this system into future GDF designs requires further development and testing of the method in two stages. Firstly, modelling of the initial signals produced by arrays of multiple magnets configured within the bentonite buffer is required in order to optimise array design for measurement of those signals at distances of metres to tens of metres. This modelling would also inform magnet array design for optimising sensitivity to change in signal with time as corrosion ensues and single domain ferromagnets transform to pseudo-single domain and multi-domain superparamagnetic materials. Secondly, the modelled multiple magnet system needs to be tested in up-scaled experiments, preferably within a URL (Underground Research Laboratory). These “proof of concept” tests would involve emplacement of the preferred magnet array in the bentonite buffer surrounding a heat source representing a spent fuel or high-level waste canister, set in the surrounding rock constituting the geosphere and engineered disturbed zone. This model deposition chamber, sealed off and artificially hydrated over time, would be instrumented with magnetometers to measure the evolution of magnetic signal with time, thereby monitoring EBS fluid saturation, and then sampled to test the modelling and magnetic analysis predictions.