Efficiency of Geographically Weighted Regression in Modeling Human Leptospirosis Based on Environmental Factors in Gilan Province, Iran
Abstract
:1. Introduction
2. Materials and Methods
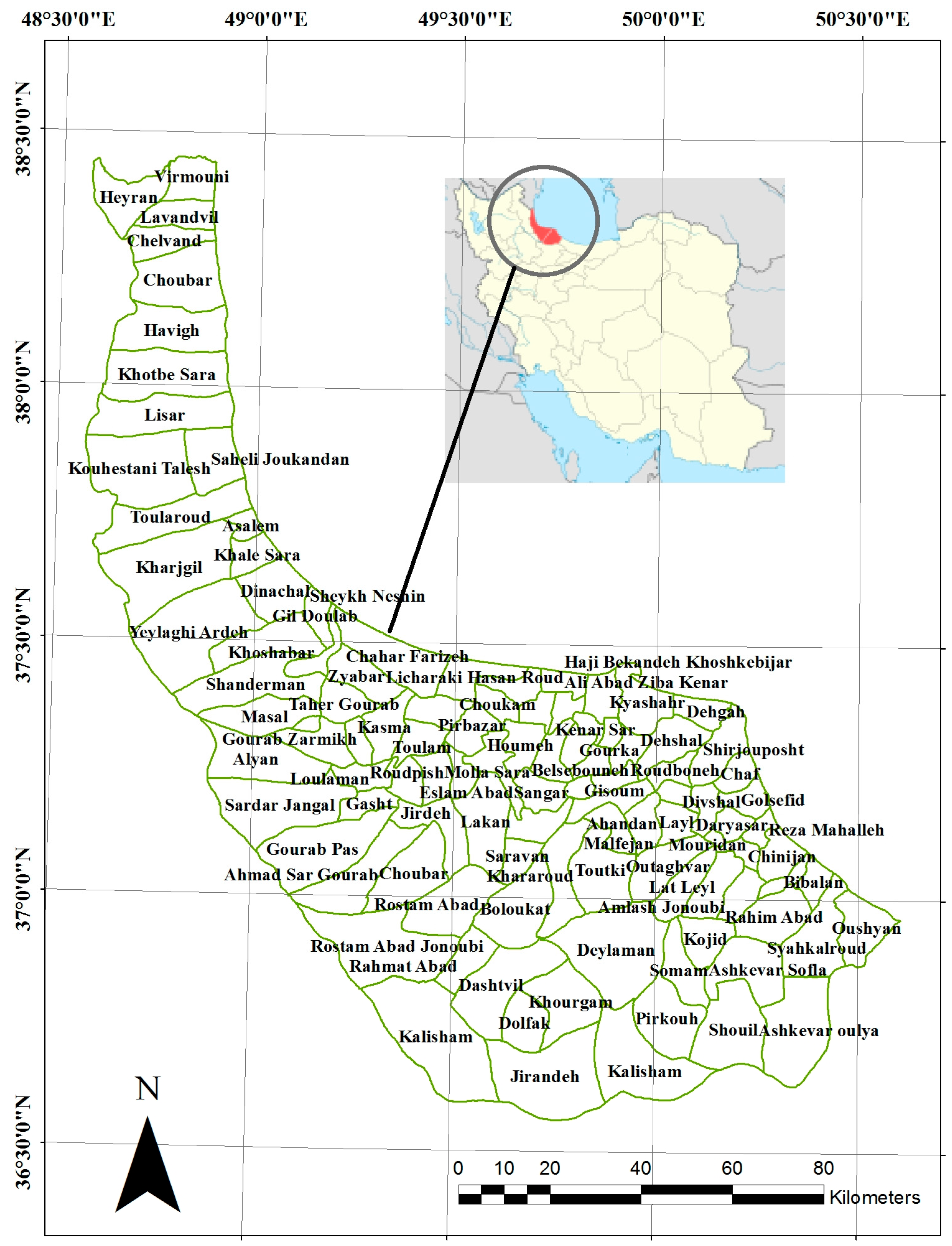
2.1. Study Area
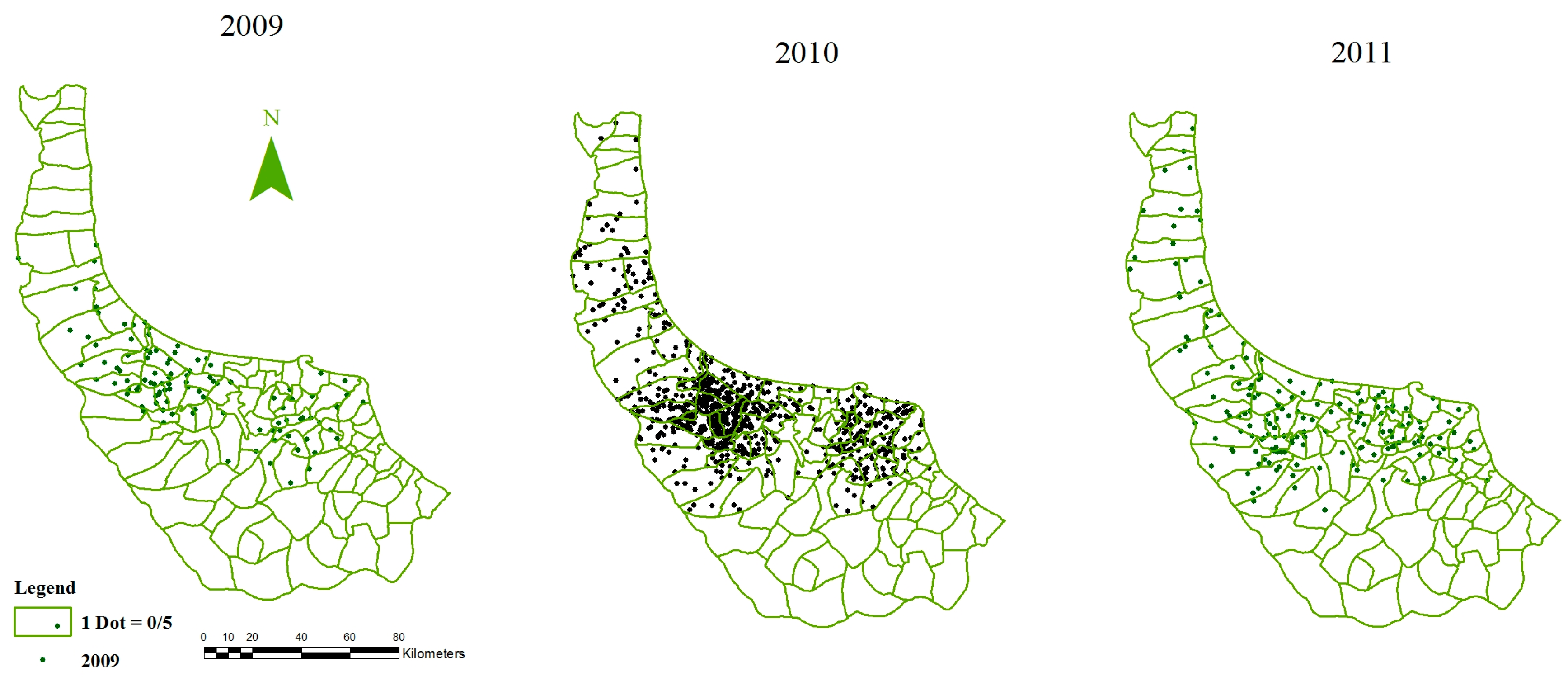
2.2. Data and Preprocessing
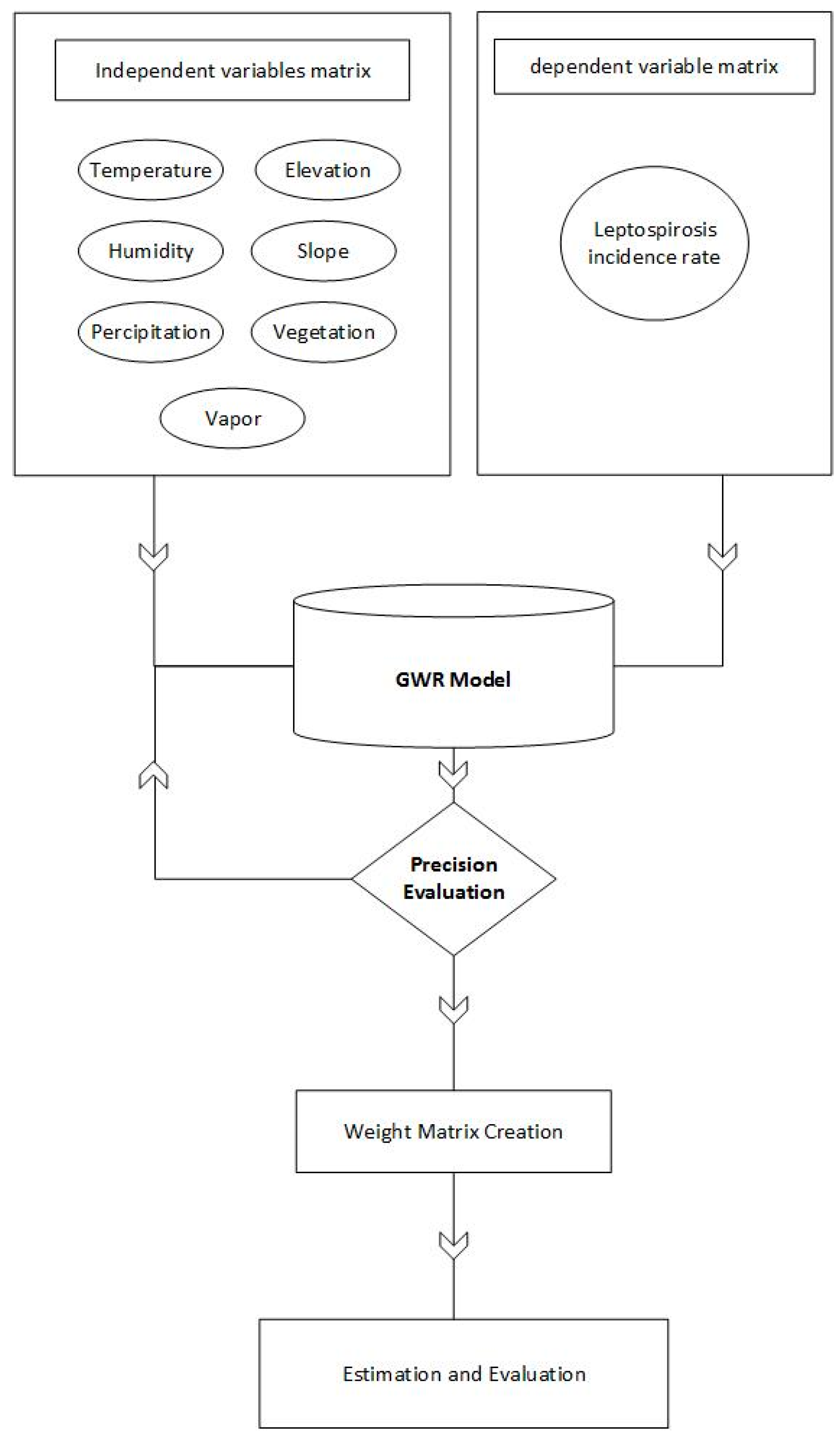
2.3. Structure of Study
2.4. GWR
- R2, which illustrates goodness of fit in model, were calculated for all models.
- MSE were calculated for evaluating the differences between observed and estimated values. p-values of coefficients were considered as significance levels more than 5 percent.
- Variance Inflation Factor (VIF) was considered less than 7.5 to ignore multicollinearity among independent variables.
- Jarque–Bera statistic greater than 0.1 was chosen to verify normality of residuals.
- Spatial autocorrelation more than 0.1 was selected to reject null hypothesis in residuals of Moran’s Index (positive Moran’s I = clustering trend, negative = random pattern).
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holt, J.; Davis, S.; Leirs, H. A model of leptospirosis infection in an African rodent to determine risk to humans: Seasonal fluctuations and the impact of rodent control. Acta Trop. 2006, 99, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, W.S.; Pellegrini, D.C.; Sá, C.B.; Reis, R.B.; Ko, A.I.; Carvalho, M.S. Detection and modelling of case clusters for urban leptospirosis. Trop. Med. Int. Health 2008, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; McKenzie, M.; Pinnock, M.; McGrowder, D. Environmental risk factors associated with leptospirosis among butchers and their associates in Jamaica. Int. J. Occup. Environ. Med. 2010, 2, 47–57. [Google Scholar]
- World Health Organization (WHO). Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control; World Health Organization: Geneva, Switzerland, 2003; Available online: http://www.who.int/zoonoses/resources/Leptospirosis/en/ (accessed on 22 September 2017).
- Honarmand, H.; Eshraghi, S. Detection of Leptospires serogroups, which are common causes of human acute leptospirosis in Gilan, Northern Iran. Iran. J. Public Health 2011, 40, 107–114. [Google Scholar] [PubMed]
- Fonzar, U.J.V.; Langoni, H. Geographic analysis on the occurrence of human and canine leptospirosis in the city of Maringá, state of Paraná, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- McBride, A.; Athanazio, D.; Reis, M.; Ko, A. Leptospirosis. Curr. Opin. Infect. Dis. 2005, 18, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, H. A decade, the incidence of leptospirosis in Gilan. Iran. J. Infect. Dis. 2009, 47, 47–53. [Google Scholar]
- Liu, C.; Liu, Q.; Lin, H.; Xin, B.; Nie, J. Spatial analysis of dengue fever in Guangdong Province, China, 2001–2006. Asia Pac. J. Public Health 2014, 26, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mollalo, A.; Alimohammadi, A.; Shahrisvand, M.; Shirzadi, M.R.; Malek, M.R. Spatial and statistical analyses of the relations between vegetation cover and incidence of cutaneous leishmaniasis in an endemic province, northeast of Iran. Asian Pac. J. Trop. Dis. 2014, 4, 176–180. [Google Scholar] [CrossRef]
- Sadat, Y.K.; Karimipour, F.; Sadat, A.K. Investigating the Relation between Prevalence of Asthmatic Allergy with the Characteristics of the Environment Using Association Rule Mining. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, 40, 169–174. [Google Scholar] [CrossRef]
- Martinez, A.N.; Mobley, L.R.; Lorvick, J.; Novak, S.P.; Lopez, A.M.; Kral, A.H. Spatial analysis of HIV positive injection drug users in San Francisco, 1987 to 2005. Int. J. Environ. Res. Public Health 2014, 11, 3937–3955. [Google Scholar] [CrossRef] [PubMed]
- Guptill, S.C.; Moore, C.G. Investigating Vector-Borne and Zoonotic Diseases with Remote Sensing and GIS, in Essentials of Medical Geology; Springer: Berlin, Germany, 2013; pp. 647–663. [Google Scholar]
- Saksena, S.; Fox, J.; Epprecht, M.; Tran, C.C.; Castrence, M.; Nong, D.; Spencer, J.; Nguyen, L.; Finucane, M.; Vien, T.D.; et al. Role of Urbanization, Land-Use Diversity, and Livestock Intensification in Zoonotic Emerging Infectious Diseases. Available online: https://www.eastwestcenter.org/system/tdf/private/ephwp006.pdf?file=1&type=node&id=34816 (accessed on 22 September 2017).
- Ferreira, M.; Ferreira, M. Influence of topographic and hydrographic factors on the spatial distribution of leptospirosis disease in são paulo county, brazil: An approach using geospatial techniques and gis analysis. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, 41, 197–201. [Google Scholar] [CrossRef]
- Mohammadinia, A.; Alimohammadi, A.; Habibi, R.; Shirzadi, M.R. Spatial and Statistical Analysis of Leptospirosis in Gilan Province, Iran. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 497–502. [Google Scholar] [CrossRef]
- Khoshdel, A.; Noori Fard, M.; Pezeshkan, R.; Salahi-Moghaddam, A. Mapping the Important Communicable Diseases of Iran. J. Health Dev. 2012, 1, 31–46. [Google Scholar]
- Ghaedamini Asadabadi, R.; Tofighi, S.; Ghaedamini, H.; Azizian, F.; Amerieon, A.; Shokri, M. A review of some infectious diseases distribution based on geographic information system (GIS) in the area of Chahar Mahal and Bakhtiari. J. Police Med. 2012, 1, 113–124. [Google Scholar]
- Aliyu, Y.; Shebe, M. Using GIS in the Management of health infrastructure within Kaduna Metropolis, Nigeria. Mediterr. J. Soc. Sci. 2013, 4, 125. [Google Scholar] [CrossRef]
- Charandabi, N.K.; Alesheikh, A. Risk Zoning of Cardiac Arrest in the Framework of the GIS and Metaheuristic Algorithms based on the Context Information. J. Geomat. Sci. Technol. 2015, 4, 109–122. [Google Scholar]
- Rai, P.K. Application of Multiple Linear Regression Model through GIS and Remote Sensing for Malaria Mapping in Varanasi District, India. Available online: http://www.hsj.gr/medicine/application-of-multiple-linear-regression-model-through-gis-and-remote-sensing-for-malaria-mapping-in-varanasi-district-india.pdf (accessed on 22 September 2017).
- Fleming, G.; Van der Merwe, M.; McFerren, G. Fuzzy expert systems and GIS for cholera health risk prediction in southern Africa. Environ. Model. Softw. 2007, 22, 442–448. [Google Scholar] [CrossRef]
- Simón, L.; Afonin, A.; López-Díez, L.I.; González-Miguel, J.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A.; Kartashev, V.; Simón, F. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet. Parasitol. 2014, 200, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Karami, J.; Delfan, S.; Shamsoddini, A. Role of Time in Spatial Analysis of Diseases in Tehran. J. Geomat. Sci. Technol. 2016, 5, 227–238. [Google Scholar]
- Suepaul, S.; Carrington, C.V.; Campbell, M.; Borde, G.; Adesiyun, A.A. Seroepidemiology of leptospirosis in dogs and rats in Trinidad. Trop. Biomed. 2014, 31, 853–861. [Google Scholar] [PubMed]
- Benacer, D.; Thong, K.L.; Min, N.C.; Verasahib, K.B.; Galloway, R.L.; Hartskeerl, R.A.; Souris, M.; Zain, S.N.M. Epidemiology of human leptospirosis in Malaysia, 2004–2012. Acta Trop. 2016, 157, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, J.H.; Sykes, J.E.; Carpenter, T.E.; Foley, J. Spatial and temporal patterns of Leptospira infection in dogs from northern California: 67 cases (2001–2010). J. Am. Vet. Med. Assoc. 2013, 242, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Azimullah, A.; Aziah, B.; Fauziah, B. The rise of leptospirosis in Kelantan 2014: Characteristics, geographical pattern and associated factors. Int. J. Public Health Clin. Sci. 2016, 3, 52–62. [Google Scholar]
- García-Ramírez, L.M.; Giraldo-Pulgarin, J.Y.; Agudelo-Marín, N.; Holguin-Rivera, Y.A.; Gómez-Sierra, S.; Ortiz-Revelo, P.V.; Velásquez-Bonilla, N.J.; Caraballo-Arias, Y.; Mondragon-Cardona, A.; Lozada-Riascos, C.O.; et al. Geographical and occupational aspects of leptospirosis in the coffee-triangle region of Colombia, 2007–2011. Recent Pat. Anti-Infect. Drug Discov. 2015, 10, 42–50. [Google Scholar] [CrossRef]
- Shojaee, J.; Hosseini, A.; Abedi, G.; Bayatani, A.; Yazdani Cherati, J.; Kaveh, F.; Ramezankhani, R.; Rostami, F. Spatial Pattern and Distribution of Leptospirosis in Mazandaran Province Using Geographic Information System. J. Mazandaran Univ. Med. Sci. 2015, 25, 151–154. [Google Scholar]
- Mardenta, R.N.; Nirmalawati, T.; Sulistyawati, S. Spatial Analysis of Leptospirosis Disease in Bantul Regency Yogyakarta. KEMAS Jurnal Kesehatan Masyarakat 2016, 12, 111–119. [Google Scholar]
- Zhao, J.; Liao, J.; Huang, X.; Zhao, J.; Wang, Y.; Ren, J.; Wang, X.; Ding, F. Mapping risk of leptospirosis in China using environmental and socioeconomic data. BMC Infect. Dis. 2016, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Gracie, R.; Barcellos, C.; Magalhães, M.; Souza-Santos, R.; Barrocas, P.R.G. Geographical scale effects on the analysis of leptospirosis determinants. Int. J. Environ. Res. Public Health 2014, 11, 10366–10383. [Google Scholar] [CrossRef] [PubMed]
- Habibi, R.; Alesheikh, A.A.; Mohammadinia, A.; Sharif, M. An Assessment of Spatial Pattern Characterization of Air Pollution: A Case Study of CO and PM2.5 in Tehran, Iran. ISPRS International. J. Geo-Inf. 2017, 6, 270. [Google Scholar]
- Saeidian, B.; Mesgari, M.S.; Ghodousi, M. Optimum allocation of water to the cultivation farms using Genetic Algorithm. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 631–638. [Google Scholar] [CrossRef]
- Saeidian, B.; Mesgari, M.S.; Ghodousi, M. Evaluation and comparison of Genetic Algorithm and Bees Algorithm for location–allocation of earthquake relief centers. Int. J. Disaster Risk Reduct. 2016, 15, 94–107. [Google Scholar] [CrossRef]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kala, A.K.; Tiwari, C.; Mikler, A.R.; Atkinson, S.F. A comparison of least squares regression and geographically weighted regression modeling of West Nile virus risk based on environmental parameters. PeerJ 2017, 5, e3070. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.K.; Alamdar, A.; Katsoyiannis, I.; Shen, H.; Ali, N.; Ali, S.M.; Bokhari, H.; Schäfer, R.B.; Eqani, S.A.M.A.S. Mapping human health risks from exposure to trace metal contamination of drinking water sources in Pakistan. Sci. Total Environ. 2015, 538, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically weighted regression: A method for exploring spatial nonstationarity. Geogr. Anal. 1996, 28, 281–298. [Google Scholar] [CrossRef]
- Propastin, P.A. Spatial non-stationarity and scale-dependency of prediction accuracy in the remote estimation of LAI over a tropical rainforest in Sulawesi, Indonesia. Remote Sens. Environ. 2009, 113, 2234–2242. [Google Scholar] [CrossRef]
- Akaike, H. Likelihood of a model and information criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- Bowman, A.W. An alternative method of cross-validation for the smoothing of density estimates. Biometrika 1984, 71, 353–360. [Google Scholar] [CrossRef]
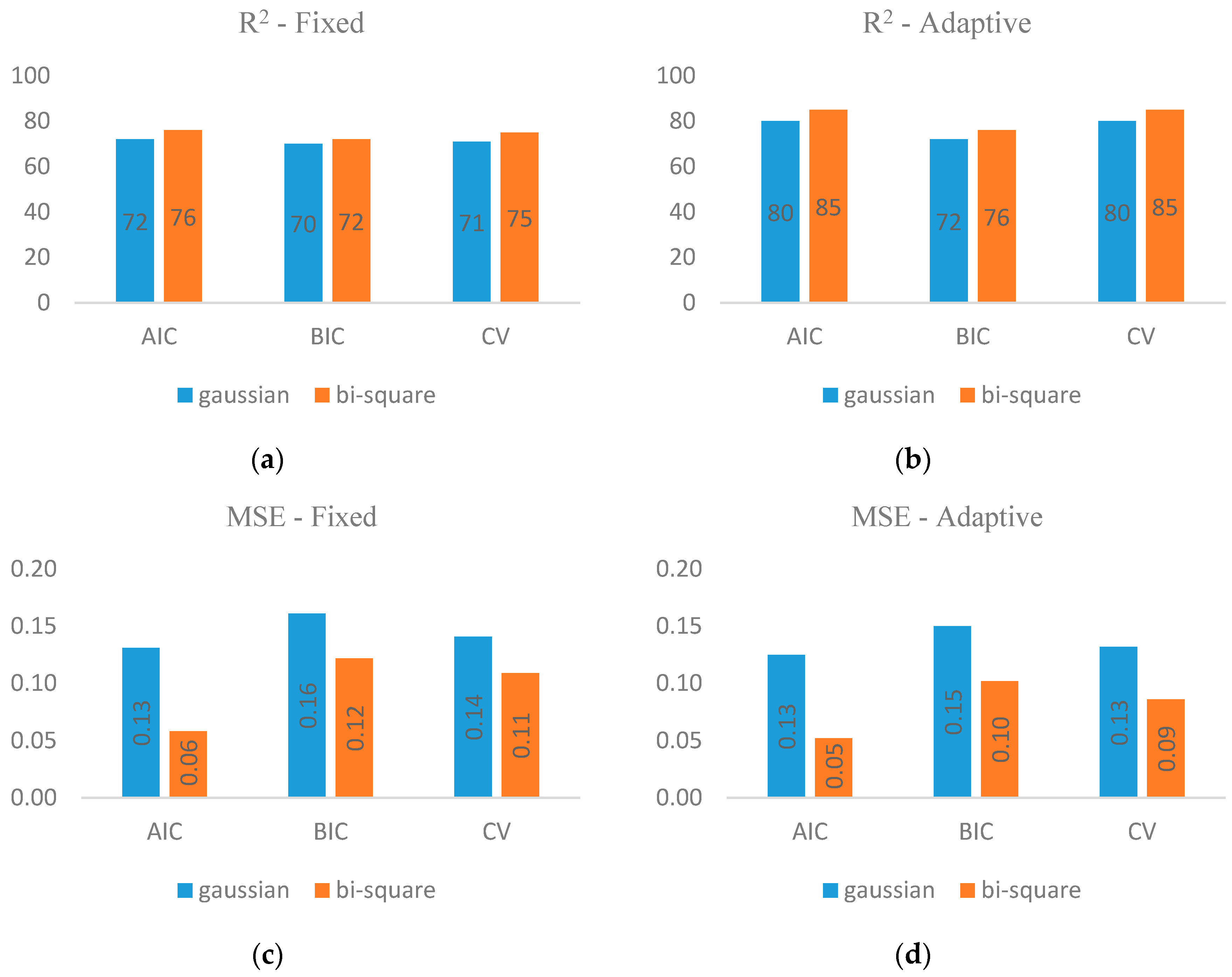
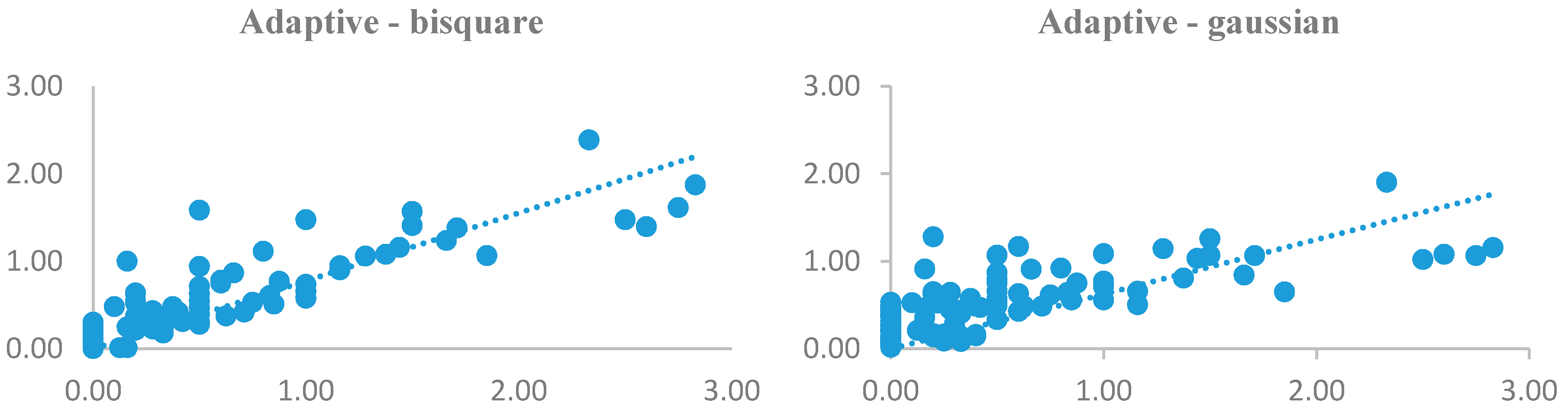

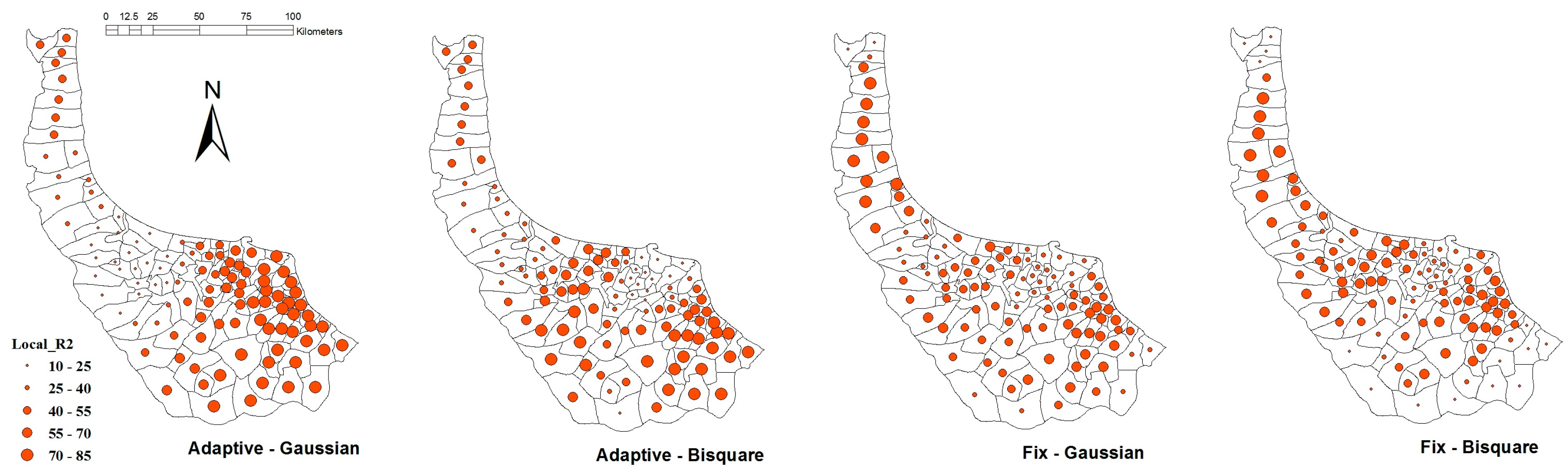
| Weighting Function | Bandwidth Criteria | Fixed Kernel | Adaptive | ||
|---|---|---|---|---|---|
| Bandwidth * | Bandwidth Criteria | Bandwidth ** | Bandwidth Criteria | ||
| Bisquare | AIC | 42,296 | 111 | 56 | 91 |
| BIC | 58,884 | 141 | 64 | 171 | |
| CV | 41,947 | 111 | 56 | 114 | |
| Gaussian | AIC | 20,973 | 114 | 56 | 104 |
| BIC | 28,375 | 190 | 68 | 177 | |
| CV | 20,973 | 130 | 56 | 125 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadinia, A.; Alimohammadi, A.; Saeidian, B. Efficiency of Geographically Weighted Regression in Modeling Human Leptospirosis Based on Environmental Factors in Gilan Province, Iran. Geosciences 2017, 7, 136. https://doi.org/10.3390/geosciences7040136
Mohammadinia A, Alimohammadi A, Saeidian B. Efficiency of Geographically Weighted Regression in Modeling Human Leptospirosis Based on Environmental Factors in Gilan Province, Iran. Geosciences. 2017; 7(4):136. https://doi.org/10.3390/geosciences7040136
Chicago/Turabian StyleMohammadinia, Ali, Abbas Alimohammadi, and Bahram Saeidian. 2017. "Efficiency of Geographically Weighted Regression in Modeling Human Leptospirosis Based on Environmental Factors in Gilan Province, Iran" Geosciences 7, no. 4: 136. https://doi.org/10.3390/geosciences7040136





