Lanthanides and Actinides in Humic Acids of Soils and Paleosols of Forest-Steppe Conditions in the Southern Urals
Abstract
:1. Introduction
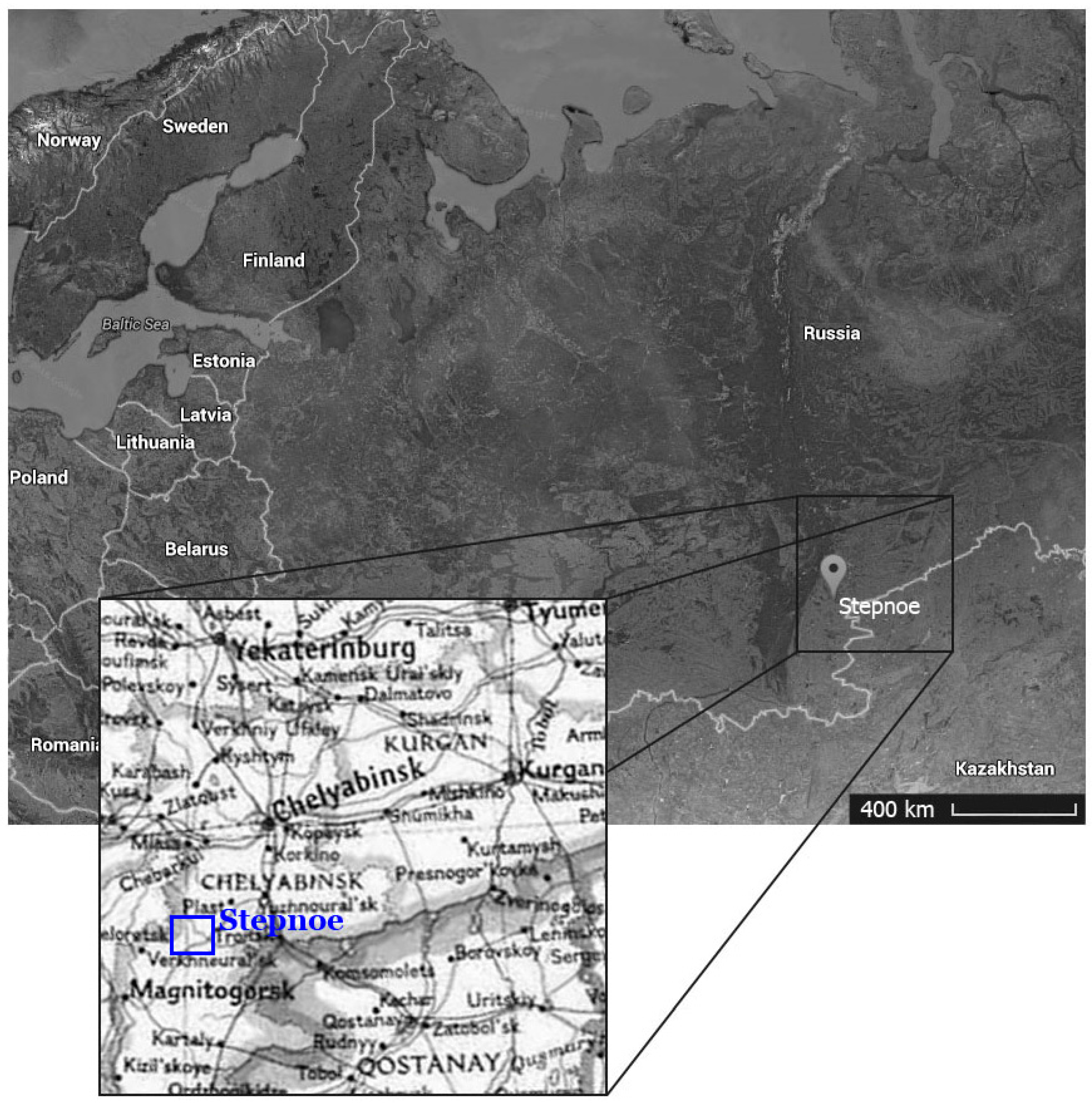
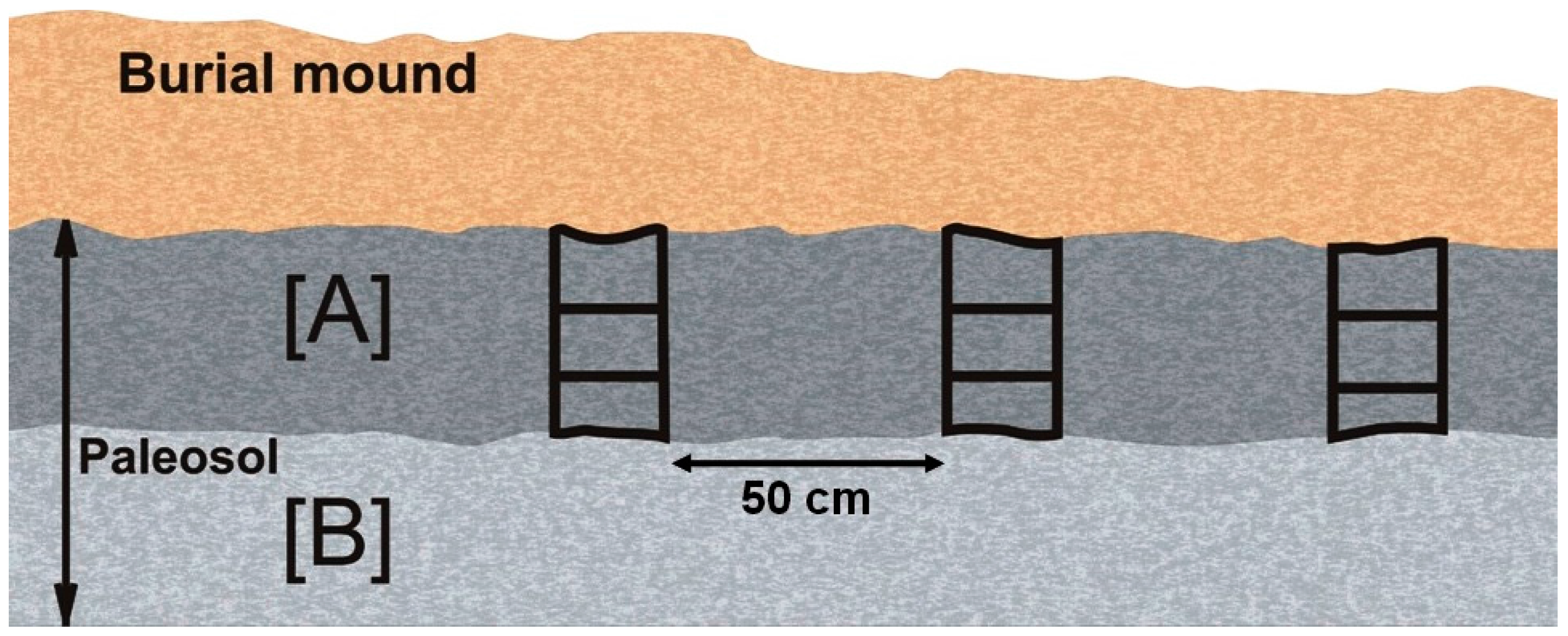
2. Materials and Method
3. Results and Discussion
- -
- the content of lanthanides and actinides in the soil mass marked above natural objects (recent background soils and paleosols buried under barrows and the spread on the territory of settlement 3.6–3.3 Ka);
- -
- the content of these elements in preparations of humic acids extracted from the abovementioned soils and paleosols;
- -
- the participation share of each element associated with humic acids in the total pool of recent background soils and paleosols.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dergacheva, M.I. Ecological functions of soil humus. Eurasian Soil Sci. 2001, 34 (Suppl. 1), 100–105. [Google Scholar]
- Bleam, W. Natural Organic Matter. In Soil and Environmental Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 7; pp. 333–384. [Google Scholar]
- Terbouche, A.; Ramdane-Terbouche, C.A.; Hauchard, D.; Djebbar, S. Evaluation of adsorption capacities of humic acids extracted from Algerian soil on polyaniline for application to remove pollutants such as Cd(II), Zn(II) and Ni(II) and characterization with cavity microelectrode. J. Environ. Sci. 2011, 23, 1095–1103. [Google Scholar] [CrossRef]
- Terbouche, A.; Djebbar, S.; Benali-Baitich, O.; Hauchard, D. Complexation Study of Humic Acids Extracted from Forest and Sahara Soils with Zinc(II) and Cadmium(II) by Differential Pulse Anodic Stripping Voltammetry (DPASV) and Conductimetric Methods. Water Air Soil Pollut. 2011, 216, 679–691. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; Taylor and Francis Group, LLC: Oxfordshire, UK, 2011; 505p. [Google Scholar]
- Motuzova, G.V.; Makarichev, I.P.; Dergham, H.M.; Stepanov, A.A.; Barsova, N.U. Soil Organic Matter and Its Interactions with Metals: Processes, Factors, Ecological Significance; Nova Science Publishers, Inc.: New York, NY, USA, 2012; 129p. [Google Scholar]
- Shaker, M.A.; Albishri, H.M. Dynamics and thermodynamics of toxic metals adsorption onto soil-extracted humic acid. Chemosphere 2014, 111, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.D. Microelements in Organic Matter of Soils; Izd Nauka: Novosibirsk, Russian, 1976; 105p. (In Russian) [Google Scholar]
- Minkina, T.M.; Motuzova, G.A.; Nazarenko, O.G. Interaction of heavy metals with organic matter of an ordinary chernozem. Eurasian Soil Sci. 2006, 39, 720–726. [Google Scholar] [CrossRef]
- Datta, R.; Quispe, M.A.; Sarkar, D. Greenhouse study on the phytoremediation potential of vetiver grass, Chrysopogon zizanioides L., in arsenic-contaminated soils. Bull. Environ. Contam. Toxicol. 2011, 86, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Clemente, R.; Pilar, M. Bernal Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 2006, 64, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Fan, Q.H.; Li, P.; Zheng, X.B.; Xu, J.Z.; Jin, Y.R.; Wu, W.S. The sorption of Eu(III) on calcareous soil: Effects of pH, ionic strength, temperature, foreign ions and humic acid. J. Radioanal. Nucl. Chem. 2011, 287, 231–237. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zhang, S.; Tian, Y.; Guo, W.; Wang, J. The influence of humic acids on the accumulation of lead (Pb) and cadmium (Cd) in tobacco leaves grown in different soils. J. Soil Sci. Plant Nutr. 2013, 13, 43–53. [Google Scholar]
- Vargas, C.; Pérez-Esteban, J.; Escolástico, C.; Masaguer, A.; Moliner, A. Phytoremediation of Cu and Zn by vetiver grass in mine soils amended with humic acids. Environ. Sci. Pollut. Res. 2016, 23, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.W.; Thomas, C.W. Worldwide fallout. In Transuranic Elements in the Environment; Hanson, W.C., Ed.; Tech. Inf. Center US Department of Energy: Washington, DC, USA, 1980; pp. 53–82. [Google Scholar]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhao, Y.; Wang, S.; Liu, M.; Duan, Z.; Chen, Y.; Li, F.; Xu, F.; Lu, T. Recent advances in synthesis and surface modification of lanthanide-doped upconversion nanoparticles for biomedical applications. Biotechnol. Adv. 2012, 30, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, C.; Prasad, P.N. Nanophotonics and Nanochemistry: Controlling the Excitation Dynamics for Frequency Up- and Down-Conversion in Lanthanide-Doped Nanoparticles. Acc. Chem. Res. 2013, 46, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhuab, H.; Chen, X. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 2015, 44, 1379–1415. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, R.; Alvarey-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelve (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Glasser, G.; Jones, R. Phosphate Rocks Fertilizer: Toxic Metals, radiaTion Hazards, Fluoride and Organic Growing. 2008. Available online: http://www.npwa.freeserve.co.uk/organic.html (accessed on 20 August 2009).
- Colman, R.; Alecsander, B. The effects of lantanides and actinides on biood coagulation. J. Clin. Investig. 1964, 43, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Palasz, A.; Czekaj, P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim. Pol. 2000, 47, 1107–1114. [Google Scholar] [PubMed]
- Ali, M.; Kumar, A.; Kumar, M.; Pandey, B.N. The interaction of human serum albumin with selected lanthanide and actinide ions: Binding affinities, protein unfolding and conformational changes. Biochimie 2016, 123, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rovira, P.; Madejyn, E.; Madejyn, P.; Plaza, C. In situ remediation of metal-contaminated soils with organic amendments: Role of humic acids in copper bioavailability. Chemosphere 2010, 79, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Arancon, N.Q.; Edwards, C.A.; Atiyeh, R.; Metzger, J.D. Effects of vermicomposts produced from food waste on the growth and yields of greenhouse peppers. Bioresour. Technol. 2004, 93, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; De Nobili, M.; Aviad, T. Stimulatory effects of humic substances onplant growth. In Soil Organic Matter in Sustainable Agriculture; Magdoff, F., Weil, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Part 1: Fundamentals and Impact of Mineral-organic-Biota Interactions on the Formation, Transformation, Turnover, and Storage of Natural Nonliving Organic Matter (NOM); Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; Volume 2, pp. 305–340. [Google Scholar]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances asplant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Hernandez, O.; Calderín, A.; Huelva, R.; Martínez-Balmori, D.; Guridi, F.; Aguiar, N.; Olivares, F.; Pasqualoto, L.; Canellas, L.P. Humic substances from vermicompost enhance urban lettuce production. Agron. Sustain. Dev. 2015, 35, 225–232. [Google Scholar] [CrossRef]
- Sycheva, S.A. Soil-geomorphological aspects of the formation of the cultural layer of the ancient settlements. Pochvovedenie 1994, 3, 28–33. (In Russian) [Google Scholar]
- Sycheva, S.A.; Leonova, N.B.; Aleksandrovsky, A.L. A Guide to the Study of Paleoecology of Cultural Layers of Ancient Settlements; MSU: Moscow, Russian, 2000; 88p. (In Russian) [Google Scholar]
- AST. National Soil Atlas of the Russian Federation; AST: Moscow, Russian, 2011; 632p. (In Russian) [Google Scholar]
- Climate-Data. Plast. Available online: http://en.climate-data.org/location/32588/ (accessed on 7 December 2017).
- Kulikov, P.V. Vascular Plants of the Chelyabinsk Region; UrB RAS: Yekaterinburg, Russian, 2010; 969p. (In Russian) [Google Scholar]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; 181p. [Google Scholar]
- Kupriyanova, E.V.; Zdanovich, D.G. Ancient of the Forest-Steppe Trans-Urals; Burial Stepnoe: Chelyabinsk, Russia, 2008; 196p. (In Russian) [Google Scholar]
- Zdanovich, G.B.; Batanina, I.M. Arkaim—The Country of Cities; Arkaim: Chelyabinsk, Russia, 2007; 260p. (In Russian) [Google Scholar]
- Dergacheva, M.I.; Nekrasova, O.A.; Okoneshnikova, M.V.; Vasil’eva, D.I.; Gavrilov, D.A.; Ochur, K.O.; Ondar, E.E. Ratio of Elements in Humic Acids as a Source of Informationon the Environment of Soil Formation. Contemp. Probl. Ecol. 2012, 5, 497–504. [Google Scholar] [CrossRef]
- Dergacheva, M.I.; Nekrasova, O.A.; Vasil’eva, D.I.; Fadeeva, V.P. Humic acid elemental composition of different formation condition virgin chernozems. Bull. Orenbg. State Univ. 2012, 10, 90–96. (In Russian) [Google Scholar]
- Dergacheva, M.I. Archaeological Pedology; Siberian Branch of Russian Academy of Science: Novosibirsk, Russian, 1997; 228p. (In Russian) [Google Scholar]
- Dergacheva, M.I. Pedohumic method in paleoenvironmental reconstructions: An example from Middle Siberia. Quat. Int. 2003, 106–107, 73–78. [Google Scholar] [CrossRef]
- Dergacheva, M.; Fedeneva, I.; Bazhina, N.; Nekrasova, O.; Zenin, V. Shestakovo site of Western Siberia (Russia): Pedogenic features, humic substances and paleoenvironment reconstructions for last 20–25 ka. Quat. Int. 2015. [Google Scholar] [CrossRef]
- Tyurin, I.V. Soil Organic Matter and Its Role in Fertility; Akademii Nauk SSSR: Moskow, Russian, 1965; 108p. (In Russian) [Google Scholar]
- Ponomareva, V.V.; Plotnikova, T.A. The methodology and the results of fractionation of chernozem humus. J. Soil Sci. 1968, 11, 104–117. (In Russian) [Google Scholar]
- Taylor, S.R. Abundance of chemical elements in the continental crust—A new table. Geochim. Cosmochim. Acta. 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Glazovskaya, M.A.; Kasimov, N.S.; Teplitskaya, T.A. Landscape-Geochemical Bases of Background Monitoring of the Environment; Nauka: Moscow, Russian, 1989; 264p. (In Russian) [Google Scholar]
- Nekrasova, O.A.; Dergacheva, M.I. The content of trace elements in chernozem ordinary and their humic acids. Bull. Tomsk State Univ. Biol. 2011, 4, 7–16. (In Russian) [Google Scholar]
| Characteristics | Objects of Extracting HA | ||
|---|---|---|---|
| Recent Background Soils | Paleosols under Barrows | Paleosols of Settlement | |
| Thickness, cm | 13–15 | 20–23 | 10–12 |
| pH H2O | 7.1–7.2 | 7.2–7.3 | 6.7–7.3 |
| χ 10−6 SGSE/g | 2.3–3.5 | 2.6–3.3 | 2.4–4.4 |
| TOC, % | 2.0–4.5 | 0.4–0.7 | 0.4–1.1 |
| HA, % | 49 | 51 | 55 |
| CHA:CFA | 2.5 | 2.6 | 2.6 |
| C in HA, wt % | 50.5 | 52.4 | 53.5 |
| H:C in HA | 0.79–1.01 | 0.87–1.03 | 0.68–0.83 |
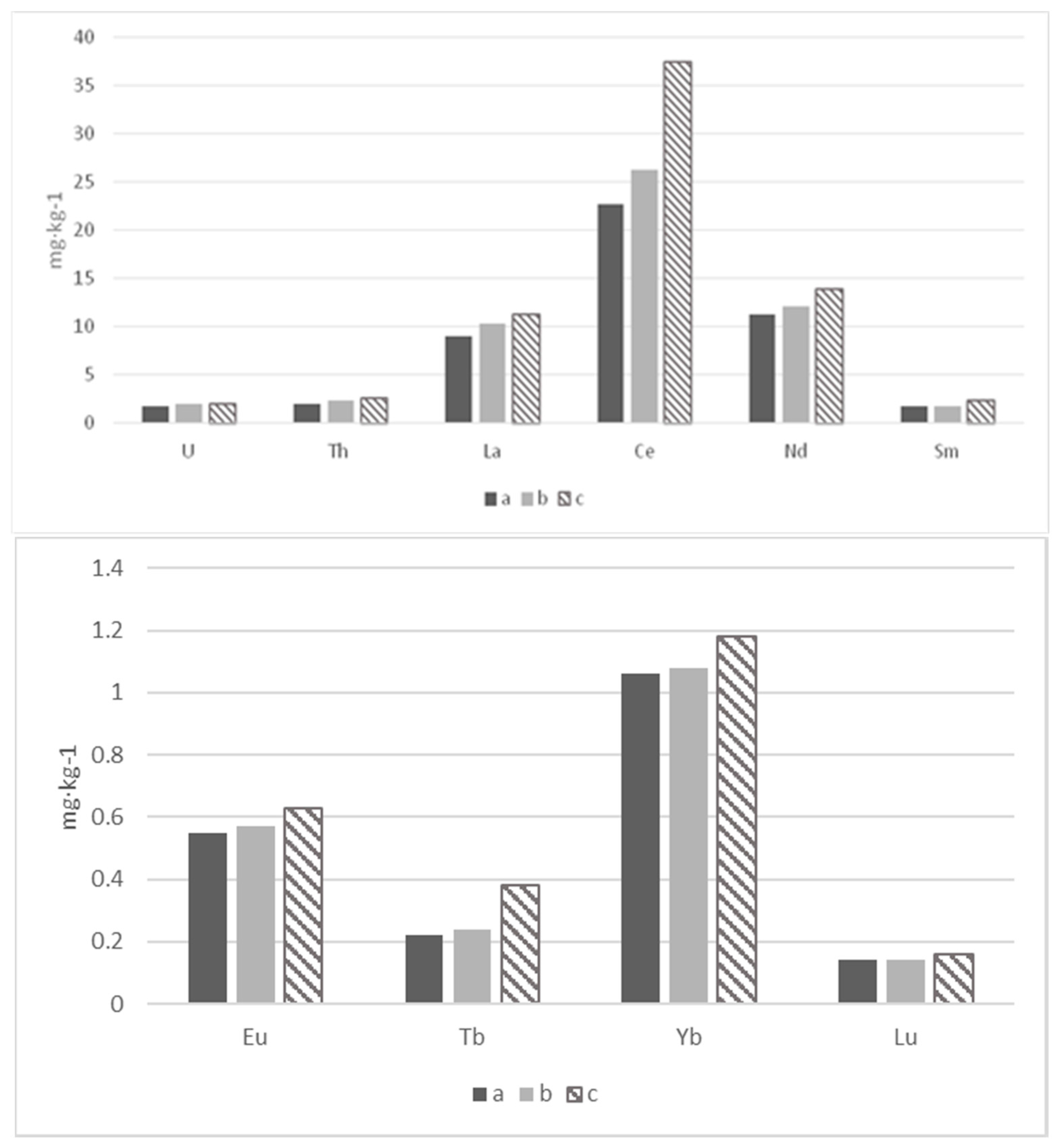
| Section | Depth (cm) | La | Ce | Sm | Eu | Tb | Yb | Lu | U | Th |
|---|---|---|---|---|---|---|---|---|---|---|
| Paleosols under barrows | ||||||||||
| 3 | 0–7 | 11.2 | 27.9 | 2.13 | 0.61 | 0.18 | 1.22 | 0.17 | 2.43 | 2.35 |
| 7–14 | 9.4 | 25.4 | 1.93 | 0.60 | 0.29 | 1.05 | 0.14 | 1.77 | 2.01 | |
| 14–21 | 8.8 | 20.3 | 1.73 | 0.52 | 0.18 | 1.04 | 0.14 | 1.53 | 1.95 | |
| 4 | 0–7 | 7.7 | 50.5 | 1.39 | 0.47 | 0.17 | 1.01 | 0.13 | 1.11 | 1.55 |
| 7–14 | 10.3 | 24.9 | 2.03 | 0.48 | 0.23 | 1.13 | 0.15 | 2.24 | 2.07 | |
| 14–23 | 7.1 | 22.9 | 2.76 | 0.66 | 0.26 | 1.06 | 0.13 | 2.01 | 1.67 | |
| 6 | 0–7 | 9.8 | 22.9 | 1.84 | 0.51 | 0.24 | 1.11 | 0.15 | 2.07 | 2.30 |
| 7–14 | 10.3 | 24.6 | 1.81 | 0.58 | 0.27 | 1.19 | 0.16 | 1.75 | 2.31 | |
| 14–21 | 7.7 | 18.1 | 1.52 | 0.48 | 0.24 | 0.99 | 0.12 | 1.03 | 1.64 | |
| 8 | 0–7 | 10.6 | 27.8 | 1.89 | 0.54 | 0.25 | 1.12 | 0.15 | 2.29 | 2.39 |
| 7–14 | 7.7 | 17.6 | 1.35 | 0.54 | 0.22 | 0.91 | 0.12 | 1.69 | 1.72 | |
| 14–22 | 7.1 | 16.9 | 1.25 | 0.55 | 0.16 | 0.89 | 0.11 | 1.30 | 1.70 | |
| Average (n = 12) | 9.0 ± 1.5 | 22.7 ± 4.0 | 1.80 ± 0.41 | 0.55 ± 0.06 | 0.22 ± 0.04 | 1.06 ± 0.10 | 0.14 ± 0.02 | 1.77 ± 0.44 | 1.97 ± 0.30 | |
| Paleosols of settlement | ||||||||||
| G3-1 | 0–10 | 9.4 | 23.0 | 1.76 | 0.54 | 0.17 | 1.04 | 0.14 | 2.32 | 2.18 |
| G3-2 | 0–12 | 8.4 | 21.6 | 1.60 | 0.52 | 0.27 | 1.06 | 0.12 | 2.27 | 2.89 |
| G4-1 | 0–11 | 9.8 | 25.3 | 1.67 | 0.49 | 0.18 | 1.04 | 0.14 | 2.37 | 2.23 |
| G4-2 | 0–11 | 8.5 | 29.8 | 2.07 | 0.60 | 0.33 | 1.10 | 0.15 | 2.05 | 2.26 |
| B6-1 | 0–10 | 8.8 | 20.8 | 1.48 | 0.52 | 0.23 | 1.00 | 0.13 | 1.11 | 2.07 |
| B6-2 | 0–12 | 16.9 | 37.3 | 2.18 | 0.73 | 0.28 | 1.22 | 0.15 | 1.67 | 2.40 |
| Average (n = 6) | 10.3 ± 3.3 | 26.3 ± 6.3 | 1.79 ± 0.27 | 0.57 ± 0.09 | 0.24 ± 0.06 | 1.08 ± 0.08 | 0.14 ± 0.01 | 1.97 ± 0.45 | 2.34 ± 0.27 | |
| Recent background soils | ||||||||||
| 1-03 | 0–2 | 13.6 | 28.9 | 2.27 | 0.62 | 0.32 | 1.18 | 0.15 | 1.80 | 3.07 |
| 2–10 | 11.7 | 68.4 | 2.99 | 0.71 | 0.27 | 1.45 | 0.16 | 1.77 | 2.42 | |
| 10–15 | 9.7 | 24.9 | 1.58 | 0.51 | 0.20 | 1.09 | 0.15 | 2.18 | 2.43 | |
| 3-03 | 2–7 | 10.4 | 29.1 | 2.14 | 0.55 | 0.49 | 1.24 | 0.17 | 1.90 | 2.50 |
| 7–12 | 12.6 | 30. 9 | 1.96 | 0.61 | 0.48 | 1.12 | 0.15 | 1.98 | 2.54 | |
| 8-03 | 2–10 | 10.0 | 42.7 | 3.51 | 0.78 | 0.49 | 0.97 | 0.15 | 2.10 | 2.85 |
| Average (n = 6) | 11.3 ± 1.6 | 37.5 ± 16.3 | 2.41 ± 0.71 | 0.63 ± 0.10 | 0.38 ± 0.13 | 1.18 ± 0.16 | 0.16 ± 0.01 | 1.96 ± 0.16 | 2.64 ± 0.26 | |
| Earth’s crust | ||||||||||
| 30 | 60 | 6 | 1.2 | 0.9 | 3 | 0.5 | 2.7 | 9.6 | ||
| Biosphere | ||||||||||
| 12.0 | 32.0 | 4.5 | 0.6 | 0.6 | 1.9 | 0.5 | 1.9 | 7.6 | ||
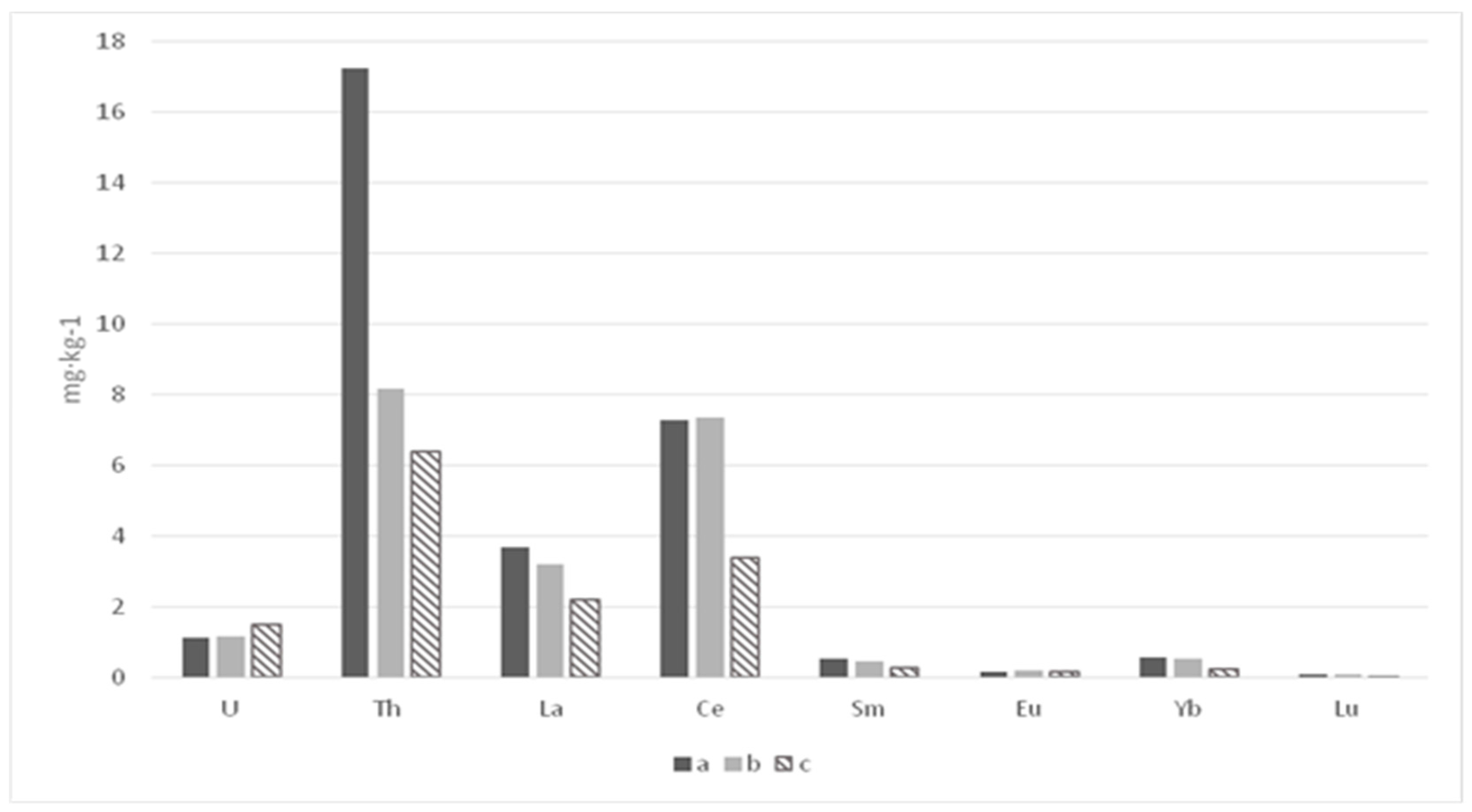
| Section | Depth (cm) | La | Ce | Sm | Eu | Tb * | Yb | Lu | U | Th |
|---|---|---|---|---|---|---|---|---|---|---|
| Recent background soils | ||||||||||
| 1-03 | 0–2 | 2.0 | 3.5 | 0.26 | 0.12 | <0.01 | 0.25 | 0.04 | 1.30 | 5.2 |
| 2–10 | 2.1 | 4.8 | 0.32 | 0.14 | <0.01 | 0.28 | 0.05 | 1.27 | 6.9 | |
| 10–15 | 2.1 | 3.9 | 0.40 | 0.15 | <0.01 | 0.28 | 0.05 | 1.31 | 7.9 | |
| 3-03 | 2–7 | 2.2 | 3.0 | 0.26 | 0.13 | <0.01 | 0.26 | 0.03 | 1.31 | 5.9 |
| 7–12 | 2.7 | 3.0 | 0.20 | 0.20 | <0.01 | 0.18 | 0.05 | 1.36 | 8.7 | |
| 8-03 | 0–2 | 1.8 | 2.2 | 0.18 | 0.11 | <0.01 | 0.21 | 0.03 | 2.10 | 3.7 |
| Average (n = 6) | 2.2 ± 0.3 | 3.4 ± 0.9 | 0.27 ± 0.07 | 0.14 ± 0.03 | - | 0.24 ± 0.04 | 0.04 ± 0.001 | 1.48 ± 0.28 | 6.4 ± 1.7 | |
| Paleosols under barrows | ||||||||||
| 3 | 0–7 | 3.2 | 7.4 | 0.51 | 0.12 | <0.01 | 0.62 | 0.09 | 0.55 | 9.4 |
| 7–14 | 8.2 | 16.0 | 1.09 | 0.23 | <0.01 | 1.03 | 0.17 | 1.51 | 21.0 | |
| 14–21 | 4.4 | 12.3 | 0.65 | 0.14 | 0.08 | 0.97 | 0.12 | 1.42 | 12.6 | |
| 4 | 0–7 | 4.1 | 8.8 | 0.63 | 0.15 | <0.01 | 0.60 | 0.09 | 0.85 | 20.7 |
| 7–14 | 4.0 | 8.8 | 0.50 | 0.16 | <0.01 | 0.64 | 0.10 | 0.98 | 14.2 | |
| 14–23 | 3.0 | 6.3 | 0.43 | 0.15 | <0.01 | 0.52 | 0.09 | 0.74 | 10.9 | |
| 6 | 0–7 | 2.4 | 4.8 | 0.40 | 0.13 | <0.01 | 0.31 | 0.05 | 0.91 | 19.3 |
| 7–14 | 3.3 | 6.9 | 0.46 | 0.14 | 0.03 | 0.56 | 0.08 | 1.05 | 20.4 | |
| 14–21 | 2.9 | 6.2 | 0.44 | 0.12 | 0.07 | 0.44 | 0.07 | 1.09 | 19.3 | |
| 7 | 0–7 | 3.0 | 5.3 | 0.46 | 0.12 | 0.09 | 0.39 | 0.07 | 1.41 | 23.3 |
| 7–14 | 3.4 | 6.3 | 0.47 | 0.12 | 0.05 | 0.44 | 0.06 | 1.37 | 17.5 | |
| 8 | 0–7 | 3.6 | 6.5 | 0.41 | 0.12 | <0.01 | 0.42 | 0.07 | 1.19 | 17.3 |
| 7–14 | 3.5 | 7.2 | 0.44 | 0.14 | <0.01 | 0.52 | 0.07 | 1.15 | 18.3 | |
| 14–22 | 3.5 | 4.1 | 0.47 | 0.13 | <0.01 | 0.53 | 0.10 | 1.10 | 21.9 | |
| 10 | 0–7 | 3.5 | 6.9 | 0.46 | 0.14 | <0.01 | 0.39 | 0.05 | 1.48 | 14.3 |
| 7–14 | 2.9 | 3.2 | 0.45 | 0.13 | <0.01 | 0.41 | 0.06 | 1.04 | 16.9 | |
| 14–21 | 3.3 | 6.6 | 0.50 | 0.09 | <0.01 | 0.54 | 0.07 | 1.00 | 16.0 | |
| Average (n = 17) | 3.7 ± 1.3 | 7.3 ± 3.0 | 0.52 ± 0.16 | 0.14 ± 0.03 | - | 0.55 ± 0.19 | 0.08 ± 0.03 | 1.11 ± 0.27 | 17.3 ± 3.93 | |
| Paleosols of settlement | ||||||||||
| G3-1 | 0–10 | 1.2 | 3.6 | 0.16 | 0.18 | <0.01 | 0.16 | 0.04 | 1.64 | 4.5 |
| G3-2 | 0–12 | 2.6 | 5.6 | 0.38 | 0.11 | <0.01 | 0.39 | 0.07 | 0.92 | 7.6 |
| G4-1 | 0–11 | 3.0 | 6.7 | 0.09 | 0.16 | 0.05 | 0.45 | 0.08 | 0.78 | 12.7 |
| G4-2 | 0–11 | 2.2 | 4.5 | 0.29 | 0.14 | <0.01 | 0.33 | 0.06 | 1.36 | 2.1 |
| G6-1 | 0–10 | 0.7 | 3.3 | 0.10 | 0.16 | <0.01 | 0.12 | 0.02 | 1.97 | 1.5 |
| G6-2 | 0–12 | 2.2 | 4.7 | 0.33 | <0.05 | <0.01 | 0.11 | <0.01 | 0.40 | 3.4 |
| G6-3 | 0–11 | 3.6 | 9.5 | 0.58 | 0.14 | <0.01 | 0.54 | 0.06 | 0.76 | 7.5 |
| B6-1 | 0–10 | 4.5 | 10.7 | 0.62 | 0.16 | <0.01 | 0.90 | 0.10 | 0.94 | 15.1 |
| B6-2 | 0–12 | 6.3 | 11.9 | 0.95 | 0.25 | <0.01 | 0.99 | 0.16 | 1.14 | 15.8 |
| B6-3 | 0–11 | 5.6 | 12.9 | 0.79 | 0.19 | <0.01 | 1.08 | 0.14 | 1.51 | 11.6 |
| Average (n = 10) | 3.2 ± 1.8 | 7.4 ± 3.6 | 0.43 ± 0.30 | 0.17 ± 0.05 | - | 0.51 ± 0.36 | 0.08 ± 0.04 | 1.14 ± 0.48 | 8.18 ± 5.34 | |
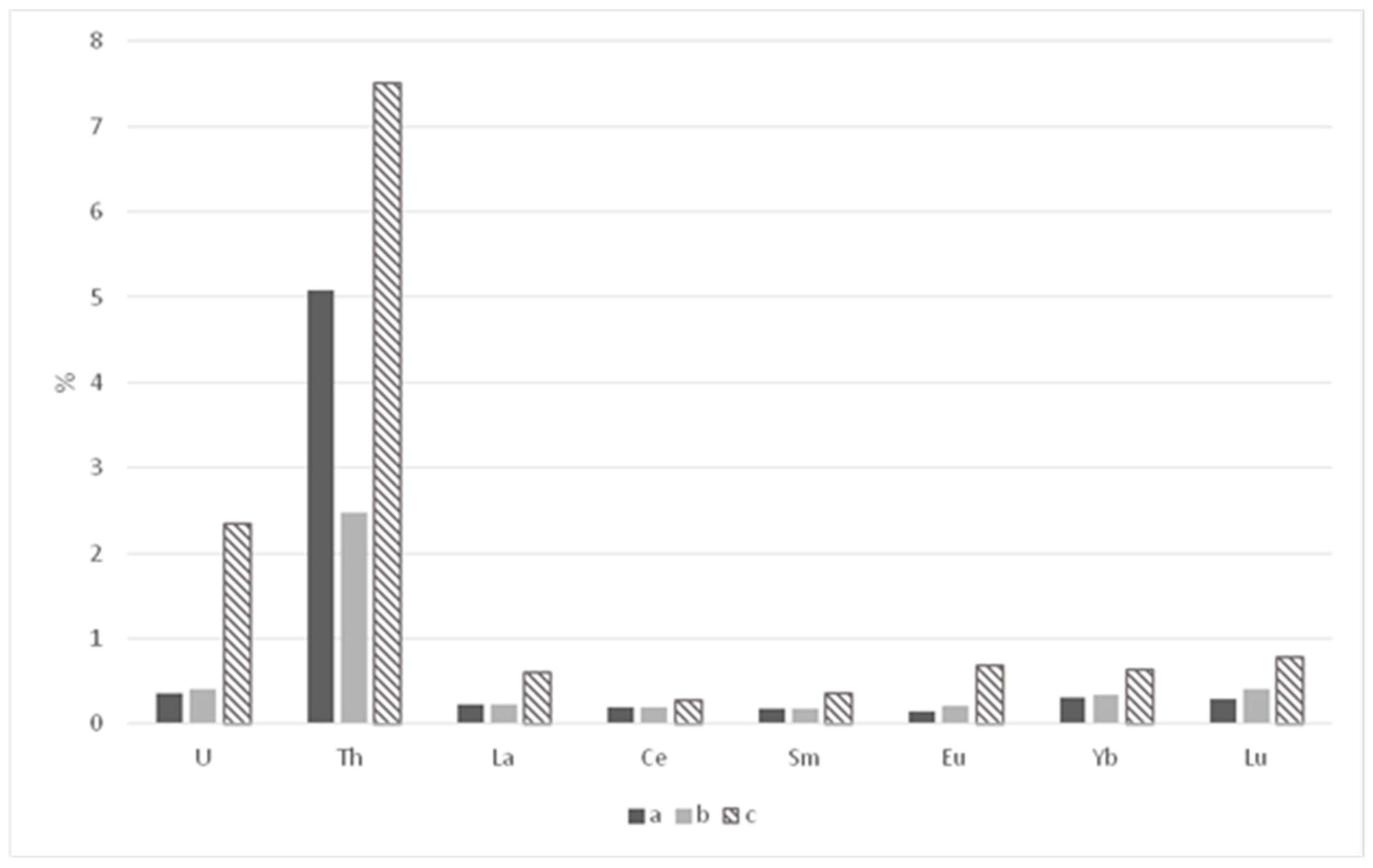
| Parameters | Elements | |||||||
|---|---|---|---|---|---|---|---|---|
| La | Ce | Sm | Eu | Yb | Lu | U | Th | |
| Paleosols under barrows | ||||||||
| Mass, mg/kg−1 | 0.0210 | 0.0420 | 0.0030 | 0.0008 | 0.0032 | 0.0005 | 0.0064 | 0.1000 |
| Share, % | 0.23 | 0.19 | 0.17 | 0.15 | 0.30 | 0.29 | 0.36 | 5.08 |
| Paleosols of settlement | ||||||||
| Mass, mg/kg−1 | 0.0227 | 0.0522 | 0.0030 | 0.0012 | 0.0036 | 0.0005 | 0.0080 | 0.0580 |
| Share, % | 0.22 | 0.20 | 0.17 | 0.21 | 0.34 | 0.41 | 0.41 | 2.48 |
| Recent background soils | ||||||||
| Mass, mg/kg−1 | 0.0682 | 0.1054 | 0.0084 | 0.0043 | 0.0074 | 0.0012 | 0.0459 | 0.1984 |
| Share, % | 0.60 | 0.28 | 0.35 | 0.69 | 0.63 | 0.78 | 2.34 | 7.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dergacheva, M.; Nekrasova, O.; Rikhvanov, L.; Zdanovich, D. Lanthanides and Actinides in Humic Acids of Soils and Paleosols of Forest-Steppe Conditions in the Southern Urals. Geosciences 2018, 8, 97. https://doi.org/10.3390/geosciences8030097
Dergacheva M, Nekrasova O, Rikhvanov L, Zdanovich D. Lanthanides and Actinides in Humic Acids of Soils and Paleosols of Forest-Steppe Conditions in the Southern Urals. Geosciences. 2018; 8(3):97. https://doi.org/10.3390/geosciences8030097
Chicago/Turabian StyleDergacheva, Maria, Olga Nekrasova, Leonid Rikhvanov, and Dmitry Zdanovich. 2018. "Lanthanides and Actinides in Humic Acids of Soils and Paleosols of Forest-Steppe Conditions in the Southern Urals" Geosciences 8, no. 3: 97. https://doi.org/10.3390/geosciences8030097




