Inflammatory Biomarkers of Cardiometabolic Risk in Obese Egyptian Type 2 Diabetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Investigations
2.2. Statistical Analysis
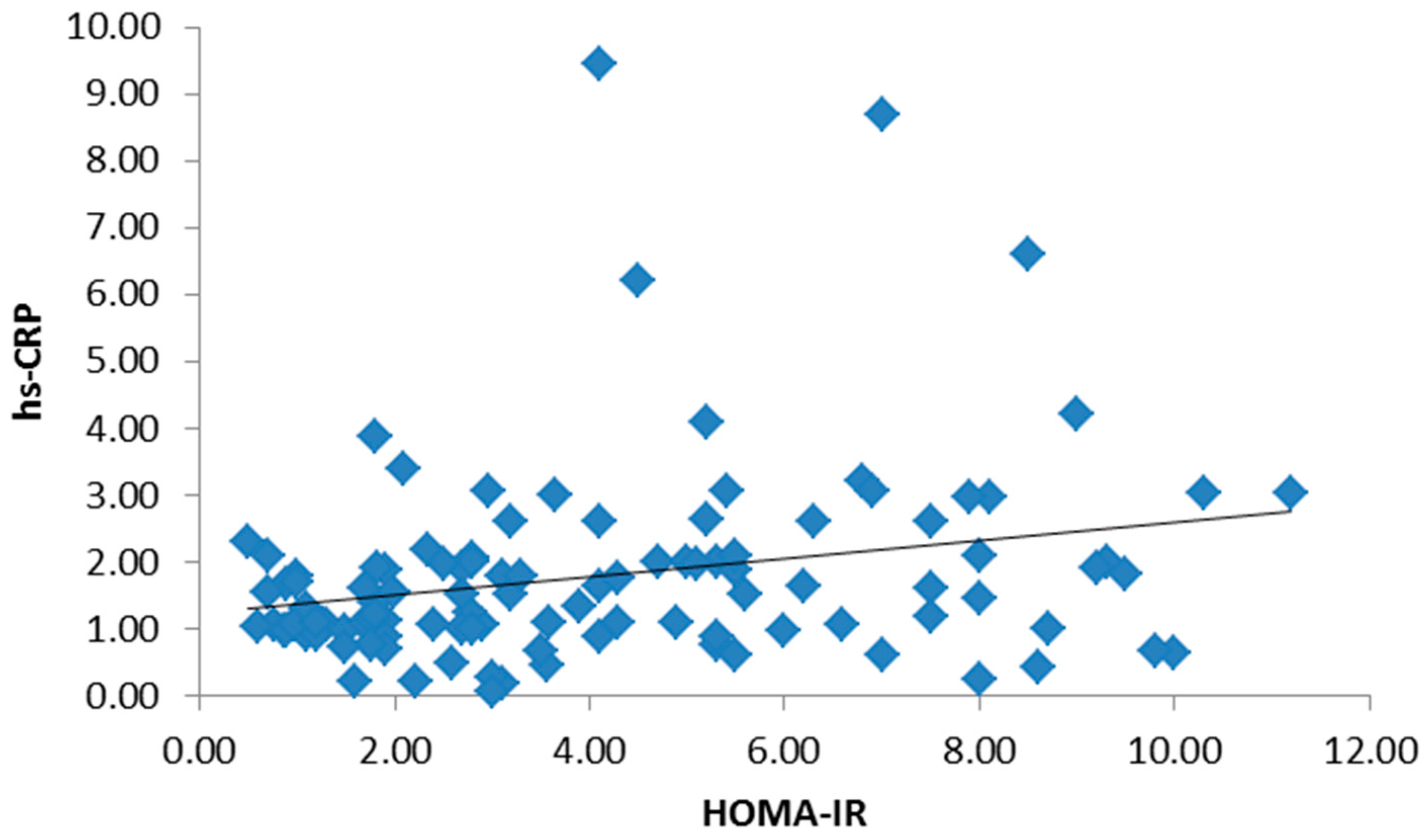
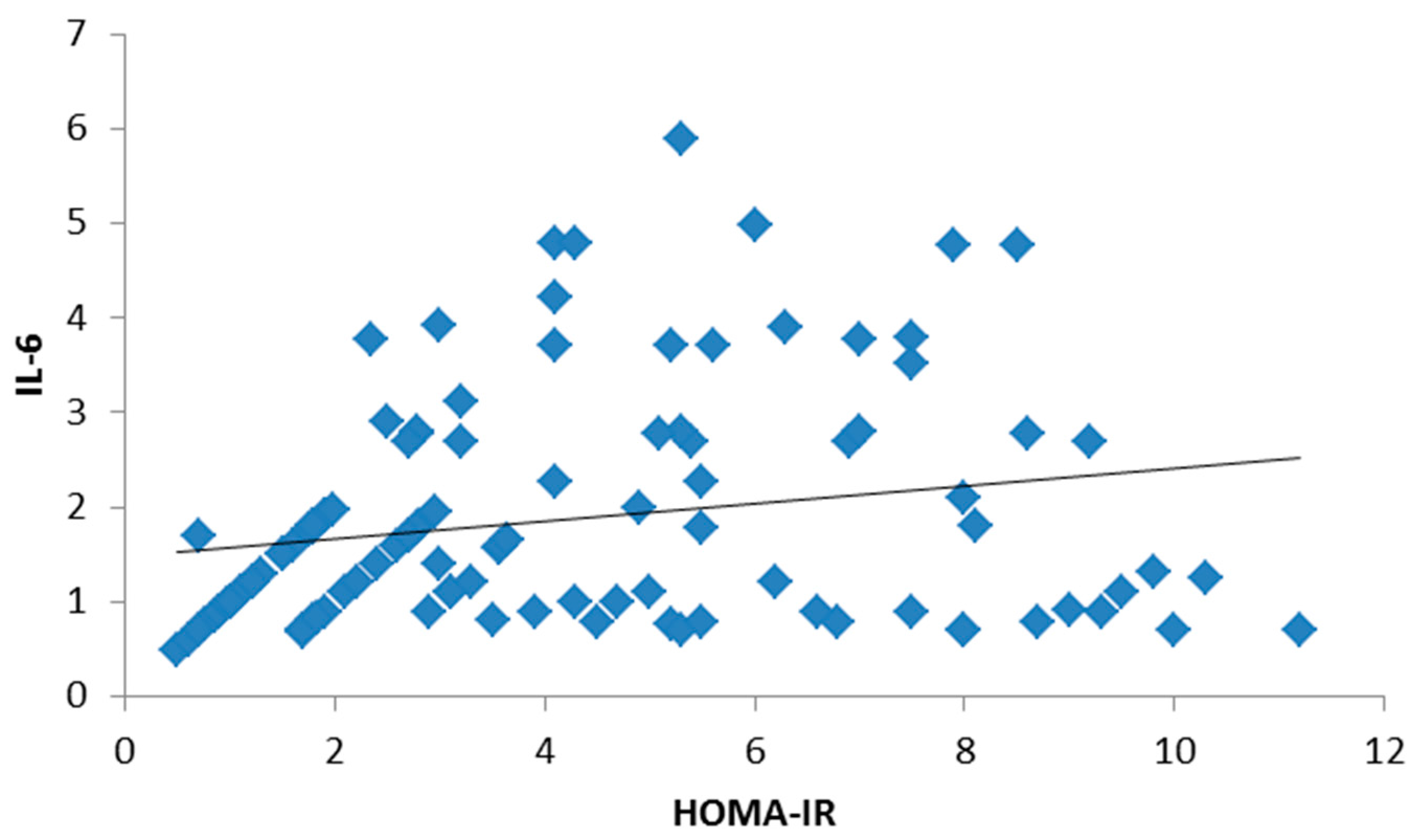
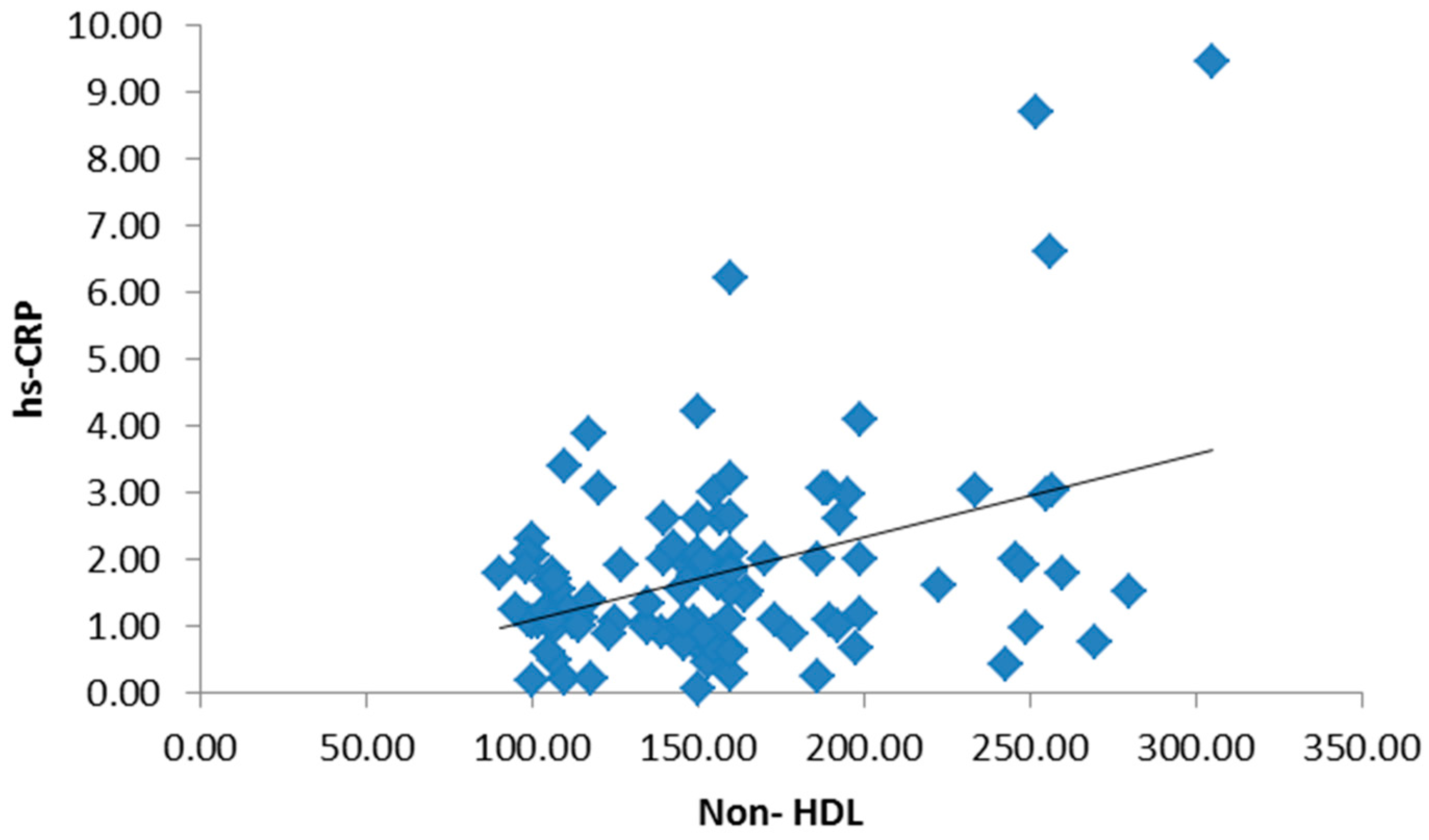
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/www.who.int/topics/obesity/en (accessed on 5 October 2017).
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; Available online: http://www.diabetesatlas.org/ (accessed on 5 October 2017).
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Kuusisto, J.; Lempiäinen, P.; Mykkänen, L.; Laakso, M. Insulin resistance syndrome predicts coronary heart disease events in elderly type 2 diabetic men. Diabetes Care 2001, 24, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P. The metabolic syndrome a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Erik, P.; Klein, S. Pathogenesis and Pathophysiology of the Cardiometabolic Syndrome. J. Clin. Hypertens. Greenwich 2009, 11, 761–765. [Google Scholar]
- David, C.; Yan, L.; Bikramjit, D. Metabolic syndrome: A marker of patients at high cardiovascular risk. Can. J. Cardiol. 2006, 22, 85–90. [Google Scholar]
- Olijhoek, J.K.; Van Der Graaf, Y.; Banga, J.D.; Algra, A.; Rabelink, T.J.; Visseren, F.L.J. The Metabolic Syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. Eur. Heart J. 2004, 25, 342–348. [Google Scholar] [CrossRef] [PubMed]
- DECODE Insulin Study Group. Plasma insulin and cardiovascular mortality in non-diabetic European men and women: A meta-analysis of data from eleven prospective studies. Diabetologia 2004, 47, 1245–1256. [Google Scholar]
- Buck, M.; Sowell, R.; Kaech, S.; Pearce, E. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Zcan, U.; Qiong Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–468. [Google Scholar]
- Ebron, K.; Andersen, C.J.; Aguilar, D.; Blesso, C.N.; Barona, J.; Dugan, C.E.; Jones, J.L.; Al-Sarraj, T.; Fernandez, M.L. A Larger Body Mass Index is Associated with Increased Atherogenic Dyslipidemia, Insulin Resistance, and Low-Grade Inflammation in Individuals with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Silvertown, J.D. Inflammation, C-reactive protein, and atherothrombosis. J. Periodontol. 2008, 79 (Suppl. 8), 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. A test in context: High-sensitivity C-reactive protein. J. Am. Coll. Cardiol. 2016, 67, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Wong, N.D.; Franklin, S.; Pio, J.; Fairchild, C.; Chen, R. Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care 2005, 28, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Ryu, O.H.; Lee, K.W.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, D.S.; Baik, S.H. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res. Clin. Pract. 2007, 75, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; Farzadfar, F. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Rasouli, N.; Philip, A. Adipocytokines and the Metabolic Complications of Obesity. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. 1), 64–73. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic review of metabolic syndrome biomarkers: A panel for early detection, management, and risk stratification in the West Virginian population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Wilson, M.L.; Summerbell, C.D.; Wilson, D.C. Arch-Obesity: Diagnosis, prevention, and treatment; evidence based answers to common questions. Arch. Dis. Child. 2002, 86, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Farha, M.; Behbehani, K.; Elkum, N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc. Diabetol. 2014, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.D.; Hu, L.T.; Zhao, G.Q.; Ma, Y.; Zhou, Z.Y.; Jiang, T. Epidemiological characteristics and risk factors of diabetic retinopathy in type 2 diabetes mellitus in Shandong Peninsula of China. Int. J. Ophthalomol. 2011, 4, 202–206. [Google Scholar]
- Landsberg, L.; Molitch, M. Diabetes and hypertension: Pathogenesis, prevention and treatment. Clin. Exp. Hypertens. 2004, 26, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.Y.; Chao, C. Diabetes and Hypertension: Is There a Common Metabolic Pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar]
- Richard, N.R. Obesity-Related Hypertension. Ochsner J. 2009, 9, 133–136. [Google Scholar]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Friedman, S.L.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Villa-Jimenez, O.; Lazo-del Vallin, S.; Diago, M.; Adams, L.A.; Romero-Gomez, M.; et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2017, 45, 332–344. [Google Scholar] [PubMed]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Francque, S. A fatty liver leads to decreased kidney function? J. Hepatol. 2017, in press. [Google Scholar]
- Amartey, N.A.A.; Nsiah, K.; Mensah, F.O. Plasma Levels of Uric Acid, Urea and Creatinine in Diabetics Who Visit the Clinical Analysis Laboratory (CAn-Lab) at Kwame Nkrumah University of Science and Technology, Kumasi. Ghana J. Clin. Diagn. Res. 2015, 9, BC05. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.R.; Pankaja, S.N.; Gouri, M.B. Albumin/Creatinine Ratio, As Predictor of Microalbuminuria, a Risk Factor for Nephropathy in Type 2 Diabetes Mellitus Patients. Int. J. Health Sci. Res. 2011, 1, 34–40. [Google Scholar]
- Haque, N.; Debnath, B.C.; Ibrahim, M.; Sirajuddin, K.; Majumder, M.; Hossain, M.S. Association of HbA1c with Urinary ACR & eGFR in Type-2 Diabetes Mellitus. Pulse 2011, 5, 6–11. [Google Scholar]
- Nada, A.M. Red cell distribution width in type 2 diabetic patients. Diabetes Metab. Syndr. Obes. Target. Ther. 2015, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khaleel, M. Comparative Study of Serum Lipid Profile of Obese and Non-Obese Students (Male) of Aljouf University. IJBAR 2016, 7, 35–37. [Google Scholar] [CrossRef]
- Songa, R.; Siddhartha, K.; Sudhakar, K. Lipid profile in type2 Diabetes mellitus with obesity. Bull. Pharm. Med. Sci. 2016, 1, 132–137. [Google Scholar]
- Yadav, N.K.; Thanpari, C.; Shrewastwa, M.K.; Mittal, R.K. Comparison of lipid profile in type-2 obese diabetics and obese non-diabetic individuals. A hospital based study from Western Nepal. Kathmandu Univ. Med. J. 2012, 10, 44–47. [Google Scholar] [CrossRef]
- Robins, S.J.; Rubins, H.B.; Faas, F.H.; Schaefer, E.J.; Elam, M.B.; Anderson, J.W.; Collins, D. Insulin resistance and cardiovascular events with low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care 2003, 26, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Resnick, H.E.; Jones, K.; Ruotolo, G.; Jain, A.K.; Henderson, J.; Lu, W.; Howard, B.V. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: The Strong Heart Study. Diabetes Care 2003, 26, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.K.; Meigs, J.B.; Sullivan, L.M.; D'Agostino, R.B.; Wilson, P.W. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005, 54, 3252–3257. [Google Scholar] [CrossRef] [PubMed]
- Banerji, M.; Lam, M.; Rochelle Chaiken, R. Insulin Resistance and the Metabolic Syndrome. In Principles of Diabetes Mellitus; Poretsky, L., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Meerarani, P.; Badimon, J.J.; Zias, E.; Fuster, V.; Moreno, P.R. Metabolic syndrome and diabetic atherothrombosis: Implications in vascular complications. Curr. Mol. Med. 2006, 6, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.; Timm, A.; Damasceno, N. Influence of obesity and cardiometabolic makers on lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in adolescents: The healthy young cross-sectional study. Lipids Health Dis. 2013, 12, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.P.; Du, Y.M.; Zhong, L.N.; Dong, Z.Q.; Wang, X.; Mao, Y.J.; Lu, Q.H. Plasma Lipoprotein- associated Phospholipase A2 in Patients with Metabolic Syndrome and Carotid Atherosclerosis. Lipids Health Dis. 2011, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Madhu, S.V.; Suneja, S. Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL and glycaemic status in early stages of type-2 diabetes mellitus. Indian J. Med. Res. 2015, 141, 107–114. [Google Scholar] [PubMed]
- Persson, M.; Nilsson, J.A.; Nelson, J.J.; Hedblad, B.; Berglund, G. The epidemiology of Lp-PLA, Distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis 2007, 190, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.L.; Biggs, M.L.; Kizer, J.R.; Cushman, M.; Hokanson, J.E.; Furberg, C.D.; Mukamal, K.J. Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) and Future Risk of Type 2 Diabetes: Results from the Cardiovascular Health Study. J. Clin. Endocrinol. Metab. 2012, 97, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, L.; Wilensky, R.L.; Mohler, E.R.; Zelewski, A.; Macphee, C.; Shi, Y. Advanced glycation endproducts upregulate expression of lipoprotein-associated phospholipase A2 in peripheral blood mononuclear cells [abstract 736-P]. In Proceedings of the 65th Annual Scientific Sessions of the American Diabetes Association, San Diego, CA, USA, 10–14 June 2005. [Google Scholar]
- Rajeev, G.; Abul Faiz, F.; Siddiqui, S.; Singhai, M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-Obese Diabetics: Pre- and Postinsulin Effects. N. Am. J. Med. Sci. 2012, 4, 180–184. [Google Scholar]
- An, P.; Wang, H.; Wu, Q.; Guo, X.; Wu, A.; Zhang, Z.; Zhang, D.; Xu, X.; Mao, Q.; Shen, X.; Zhang, L. Elevated serum transaminase activities were associated with increased serum levels of iron regulatory hormone hepcidin and hyperferritinemia risk. Sci. Rep. 2015, 5, 13106. [Google Scholar] [CrossRef] [PubMed]
- Scott, M. Metabolic Syndrome: Connecting and Reconciling Cardiovascular and Diabetes Worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar]
- White, M.F. The insulin signalling system and the IRS proteins. Diabetologia 1997, 40 (Suppl. 2), S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Martínez, R.G.; Alonso, K.R.; Novik, A.V. Metabolic Syndrome. Clinical and pathophysiological basis for a rational therapeutical approach. Rev. Méd. Chile 2009, 137, 685–694. [Google Scholar]
- Phosat, C.; Panprathip, P.; Chumpathat, N.; Prangthip, P.; Chantratita, N.; Soonthornworasiri, N.; Puduang, S.; Kwanbunjan, K. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: A cross-sectional study. BMC Endocr. Disord. 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Cezard, G.; Gill, J.; Wallia, S.; Douglas, A.; Sheikh, A.; Wild, S.H.; Tuomilehto, J.; McKnight, J.; Murray, G.; et al. Associations between weight change and biomarkers of cardiometabolic risk in South Asians: Secondary analyses of the PODOSA Trial. Int. J. Obes. 2016, 40, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Candales, A.; Burgos, P.; Hernandez-Suarez, D.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, 341. [Google Scholar]
- Ridker, P.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Moving beyond JUPITER: Will inhibiting inflammation reduce vascular event rates? Curr. Atheroscler. Rep. 2013, 15, 295. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Thompson, P.L. Colchicine for secondary prevention of cardiovascular disease. Curr. Atheroscler. Rep. 2014, 16, 391. [Google Scholar] [CrossRef] [PubMed]

| Variable | Group I (n = 55) | Group II (n = 55) | Group III (n = 55) | F | p-Value |
|---|---|---|---|---|---|
| WC (cm) | 80.04 ± 9.68 | 114.13 ± 14.18 | 111.1 ± 12.53 | 30.3 | <0.001 |
| BMI (kg/m2) | 22.35 ± 2.19 | 35.6 ± 6.12 | 34.9 ± 7.33 | 29.1 | <0.001 |
| Variable | Group I (n = 55) | Group II (n = 55) | Group III (n = 55) | F | p-Value |
|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 172.18 ± 21.87 | 231.78 ± 57.78 | 224.78 ± 42.96 | 11.76 | <0.01 |
| TG (mg/dL) | 122.8 ± 24.27 | 219.25 ± 89.09 | 221.25 ± 99.58 | 4.85 | <0.01 |
| HDL-C (mg/dL) | 51.75 ± 11.73 | 33.56 ± 8.16 | 31.52 ± 7.26 | 20.18 | <0.01 |
| Non-HDL-C (mg/dL) | 120.43 ± 12.73 | 198.23 ± 31.08 | 193.26 ± 37.66 | 20.18 | <0.01 |
| LDL-C (mg/dL) | 87.75 ± 12.73 | 140.12 ± 35.61 | 143.72 ± 37.66 | 20.18 | <0.01 |
| Lp-PLA2 (nmol/min/mL) | 17.95 ± 6.73 | 22.72 ± 6.01 | 24.72 ± 5.06 | 20.18 | <0.01 |
| Apolipoprotein B (g/L) | 0.96 ± 0.31 | 1.11 ± 0.34 | 1.15 ± 0.29 | 20.18 | <0.01 |
| Variable | Group I (n = 55) | Group II (n = 55) | Group III (n = 55) | F | p-Value |
|---|---|---|---|---|---|
| AST (U/L) | 37.51 ± 14.27 | 49.99 ± 17.9 | 54.71 ± 14 | 6.38 | <0.01 |
| ALT (U/L) | 32.58 ± 13.53 | 47.49 ± 10.16 | 45.38 ± 11.66 | 5.32 | <0.01 |
| GGT (IU/L) | 31.17 ± 12.22 | 43.17 ± 11.37 | 41.18 ± 9.47 | 5.37 | <0.01 |
| Creatinine (mg/dL) | 0.92 ± 0.29 | 0.99 ± 0.54 | 2.28 ± 1.54 | 10.33 | <0.01 |
| Urea (mg/dL) | 28.73 ± 4.74 | 27.17 ± 6.54 | 34.27 ± 5.91 | 9.8 | <0.01 |
| Albumin (g/dL) | 4.21 ± 0.34 | 4.12 ± 0.58 | 3.34 ± 0.48 | 7.4 | <0.01 |
| ACR (mg/mmol) | 10.95 ± 4.73 | 11.18 ± 7.07 | 37.81 ± 24.01 | 8.11 | <0.01 |
| Variable | Group I (n = 55) | Group II (n = 55) | Group III (n = 55) | F | p-Value |
|---|---|---|---|---|---|
| hs-CRP (mg/L) | 1.45 ± 0.73 | 1.62 ± 0.99 | 2.32 ± 1.11 | 7.23 | <0.01 |
| IL-6 (pg/mL) | 1.65 ± 1.01 | 1.73 ± 1.14 | 2.53 ± 1.34 | 4.85 | <0.01 |
| TNF-α (pg/mL) | 1.82 ± 0.81 | 1.87 ± 1.09 | 2.17 ± 0.89 | 20.18 | <0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, L.A.A.; Shora, H.A.; El-Deen, I.M.; El-Sayed, E.-S.A.E.-S. Inflammatory Biomarkers of Cardiometabolic Risk in Obese Egyptian Type 2 Diabetics. Med. Sci. 2017, 5, 25. https://doi.org/10.3390/medsci5040025
Barakat LAA, Shora HA, El-Deen IM, El-Sayed E-SAE-S. Inflammatory Biomarkers of Cardiometabolic Risk in Obese Egyptian Type 2 Diabetics. Medical Sciences. 2017; 5(4):25. https://doi.org/10.3390/medsci5040025
Chicago/Turabian StyleBarakat, Lamiaa A. A., Hassan A. Shora, Ibrahim M. El-Deen, and El-Sayed Abd El-Sameeh El-Sayed. 2017. "Inflammatory Biomarkers of Cardiometabolic Risk in Obese Egyptian Type 2 Diabetics" Medical Sciences 5, no. 4: 25. https://doi.org/10.3390/medsci5040025




