Diagnostic Utility of High Sensitivity Troponins for Echocardiographic Markers of Structural Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Computed tomography Coronary Angiography
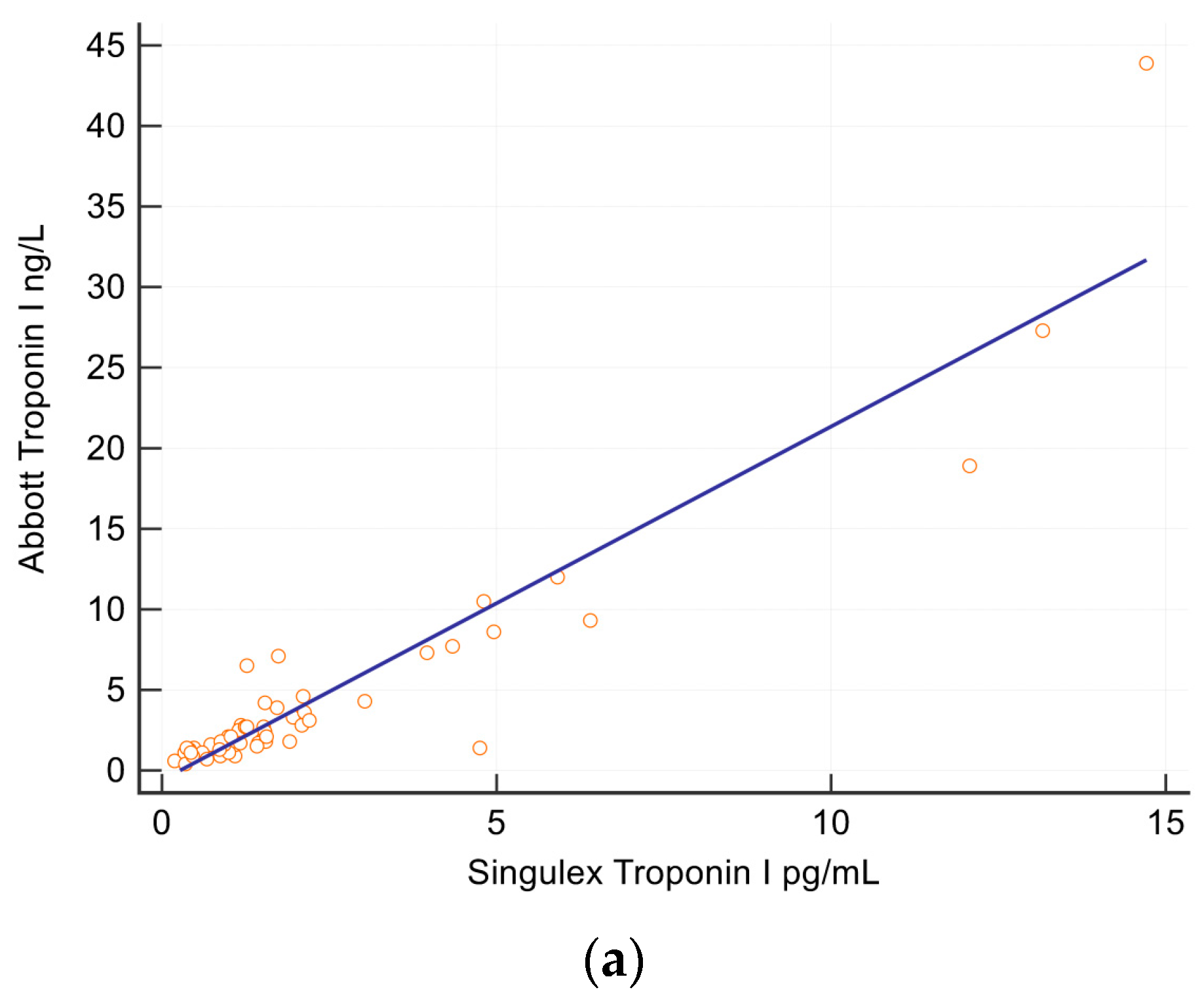
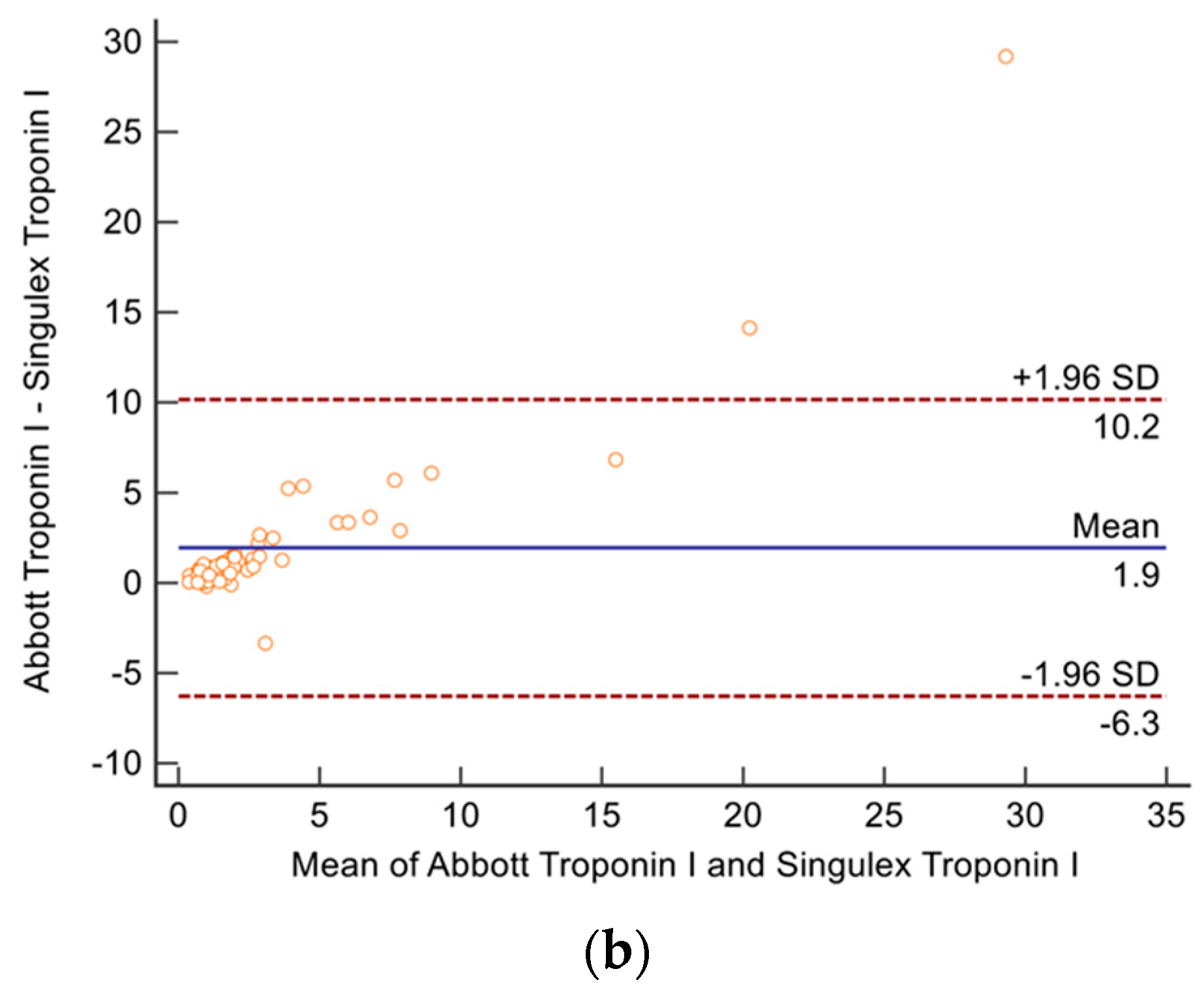
2.4. Troponin and NT-BNP Assays
2.5. Statistics
4. Results
4.1. Cohort Characteristics
4.2. Predictors of Structural Heart Disease
5. Discussion
Limitations
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Subcommittee, B.; Katus, H.A.; Apple, F.S.; Lindahl, B.; et al. Third universal definition of myocardial infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Salomaa, V.; Makarova, N.; Ojeda, F.; Wild, P.; Lackner, K.J.; Jørgensen, T.; Thorand, B.; Peters, A.; Petersmann, M.N.A.; et al. Troponin I and cardiovascular risk prediction in the general population: The BiomarCaRE consortium. Eur. Heart J. 2016, 37, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.; Theriault, S.; Aeschbacher, S.; Schoen, T.; Kunz, S.; von Rotz, M.; Estis, J.; Todd, J.; Risch, M.; Mueller, C.; et al. Factors independently associated with cardiac troponin I levels in young and healthy adults from the general population. Clin. Res. Cardiol. 2017, 106, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Lu, Q.A.; Todd, J.; Moecks, J.; Wians, F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: Implications for clinical practice. Clin. Chem. 2009, 55, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Akhigbe, P.; Wians, F. Long-term biological variation in cardiac troponin I. Clin. Biochem. 2012, 45, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.H.; Fraser, C.G.; Sandberg, S.; Goldschmidt, H. The index of individuality is often a misinterpreted quantity characteristic. Clin. Chem. Lab. Med. 1999, 37, 655–661. [Google Scholar] [PubMed]
- Aeschbacher, S.; Schoen, T.; Bossard, M.; van der Lely, S.; Glattli, K.; Todd, J.; Estis, J.; Risch, M.; Mueller, C.; Risch, L.; et al. Relationship between high-sensitivity cardiac troponin I and blood pressure among young and healthy adults. Am. J. Hypertens. 2015, 28, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; Kuznetsova, T.; Varo, N.; Thijs, L.; Delles, C.; Dominiczak, A.; Diez, J.; Staessen, J.A. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: A population-based study. Int. J. Cardiol. 2015, 185, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Patel, J.; MacLellan, W.R.; Fonarow, G.C. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003, 108, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Manzano-Fernandez, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdés, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Tanglay, Y.; Twerenbold, R.; Lee, G.; Wagener, M.; Honegger, U.; Puelacher, C.; Reichlin, T.; Mann, S.; Druey, S.; Hochgruber, T.; et al. Incremental value of a single high-sensitivity cardiac troponin I measurement to rule out myocardial ischemia. Am. J. Med. 2015, 128, 638–646. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, J.A.; Drazner, M.H.; Omland, T.; Ayers, C.R.; Khera, A.; Rohatgi, A.; Hashim, I.; Berry, J.D.; Das, S.R.; Morrow, D.A.; et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010, 304, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.T.; Nambi, V.; de Lemos, J.A.; Chambless, L.E.; Virani, S.S.; Boerwinkle, E.; Hoogeveen, R.C.; Liu, X.; Astor, B.C.; Mosley, T.H.; et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011, 123, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; Chen, Y.; Rawlings, A.; Hoogeveen, R.C.; Ballantyne, C.M.; Blumenthal, R.S.; Coresh, J.; Selvin, E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J. Am. Coll. Cardiol. 2016, 68, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ford, I.; Shah, A.S.; Zhang, R.; McAllister, D.A.; Strachan, F.E.; Caslake, M.; Newby, D.E.; Packard, C.J.; Mills, N.L. High-Sensitivity Cardiac Troponin, Statin Therapy, and Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2016, 68, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Zeller, T.; Glynn, R.J.; Ridker, P.M.; Blankenberg, S. High-sensitivity cardiac troponin I and B-type natriuretic Peptide as predictors of vascular events in primary prevention: Impact of statin therapy. Circulation 2015, 131, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Ametepe, E.S.; Yam, Y.; Dwivedi, G.; Chow, B.J. Cardiac CT assessment of left ventricular mass in mid-diastasis and its prognostic value. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 95–102. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, J.A.; Ayers, C.R.; Levine, B.; deFilippi, C.R.; Wang, T.J.; Hundley, W.G.; Berry, J.D.; Seliger, S.L.; McGuire, D.K.; Ouyang, P.; et al. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation 2017, 135, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Xanthakis, V.; Larson, M.G.; Wollert, K.C.; Aragam, J.; Cheng, S.; Ho, J.; Coglianese, E.; Levy, D.; Colucci, W.S.; Felker, G.M.; et al. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: Implications for screening. J. Am. Heart Assoc. 2013, 2, e000399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koycheva, R.Y.; Cholakov, V.; Andreev, J.; Penev, M.; Iliev, R.; Nancheva, K.; Tsoneva, V. Cardiac Biomarkers and Left Ventricular Hypertrophy in Asymptomatic Hemodialysis Patients. Open Access Maced. J. Med. Sci. 2016, 4, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Tan, X.; Cao, X.; Zou, J. Assessed value of high-sensitivity cardiac troponin T for cardiovascular disease among CKD patients. Ren. Fail. 2016, 38, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Ucar, H.; Gur, M.; Kivrak, A.; Koyunsever, N.Y.; Seker, T.; Akilli, R.E.; Türkoğlu, C.; Kaypakli, O.; Sahin, D.Y.; Elbasan, Z.; et al. High-sensitivity cardiac troponin T levels in newly diagnosed hypertensive patients with different left ventricle geometry. Blood Press. 2014, 23, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Anegawa, T.; Kai, H.; Adachi, H.; Hirai, Y.; Enomoto, M.; Fukami, A.; Otsuka, M.; Kajimoto, H.; Yasuoka, S.; Iwamoto, Y.; et al. High-sensitive troponin T is associated with atrial fibrillation in a general population. Int. J. Cardiol. 2012, 156, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Murphy, S.A.; Brown, K.; Jarolim, P.; Mercuri, M.; Antman, E.M.; Morrow, D.A. Cardiovascular Biomarker Score and Clinical Outcomes in Patients With Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Oldgren, J.; Siegbahn, A.; Wallentin, L. Application of Biomarkers for Risk Stratification in Patients with Atrial Fibrillation. Clin. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rumayor, A.A.; de Lemos, J.A.; Rohatgi, A.K.; Ayers, C.R.; Powell-Wiley, T.M.; Lakoski, S.G.; Berry, J.D.; Khera, A.; Das, S.R. Addition of highly sensitive troponin T and N-terminal pro-B-type natriuretic peptide to electrocardiography for detection of left ventricular hypertrophy: Results from the Dallas Heart Study. Hypertension 2013, 61, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L. Troponin and the J-Curve of Diastolic Blood Pressure: When Lower Is Not Better. J. Am. Coll. Cardiol. 2016, 68, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD |
|---|---|
| Age | 58 (11) |
| Number (%) | |
| Male | 29 (37) |
| Hypertension | 40 (51) |
| Hypercholesterolemia | 37 (47) |
| Diabetes | 9 (15) |
| Smoking History | 28 (36) |
| Family History of Cardiovascular Disease | 26 (33) |
| Abdominal Obesity | 27 (35) |
| Post-menopausal | 32 (41) |
| Imaging | Number (%) |
| LVHEcho | 29 (38%) |
| LAEEcho | 29 (38%) |
| Composite SHDEcho | 34 (45%) |
| LV Systolic Dysfunction | 8 (11%) |
| Valvular Disease | 8 (11%) |
| Coronary Artery Disease (CTCA) | 32 (52%), 7 were obstructive |
| CT LV mass > 81.7 g/m2 | 15 (25%) |
| Median (IQR) | |
| IVSd | 0.93 (0.79–1.1) |
| Echo LV mass | 140 (105–179) |
| CT LV mass | 135 (113–175) |
| Ejection fraction | 58 (52–58) |
| Biomarkers | Median (IQR) |
| Singulex hs-Tn (ng/L) | 1.2 (0.7–2.0) |
| Abbott hs-Tn (ng/L) | 1.8 (1.1–3.3) |
| Roche hs-Tn (ng/L) | 6.8 (3.7–10.3) |
| NT-proBNP (ng/L) | 10 (4.7–22.9) |
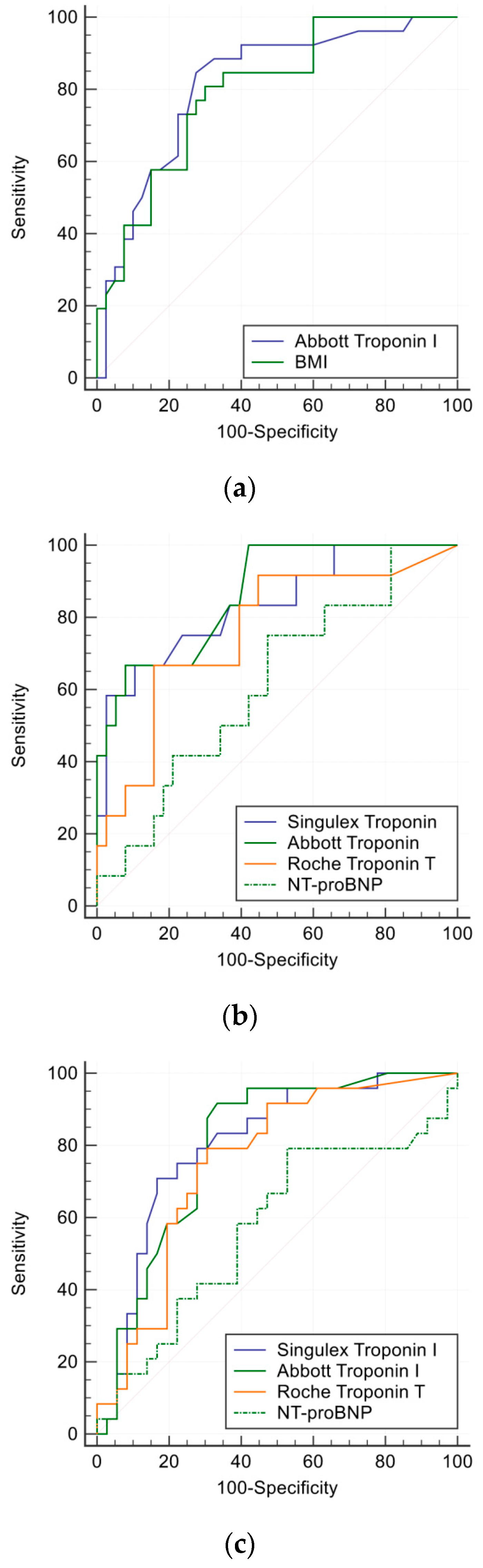
| C-Statistic (95% CI) | Optimal Cut point (troponins ng/L, BNP pg/L) | Sensitivity | Specificity | |
|---|---|---|---|---|
| LV systolic dysfunction | ||||
| NT pro-BNP | 0.97 (0.9 to 0.99) | >29 | 100% | 92% |
| Singulex troponin I | 0.82 (0.71 to 0.91) | >1.7 | 83% | 80% |
| Abbott troponin I | 0.77 (0.66 to 0.86) | >12 | 50% | 100% |
| LVHEcho | ||||
| Singulex TnI | 0.84 (0.72–0.92) | >1.5 | 70% | 88% |
| Abbott TnI | 0.84 (0.73–0.92) | >1.7 | 85% | 75% |
| Roche TnT | 0.75 (0.63–0.85) | >6.5 | 77% | 68% |
| NT-proBNP | 0.62 (0.49–0.74) | >6.3 | 79% | 53% |
| LAEEcho | ||||
| Singulex TnI | 0.74 (0.6–0.85) | >1.2 | 71% | 71% |
| Abbott TnI | 0.78 (0.66–0.88) | >1.4 | 90% | 53% |
| Roche TnT | 0.55 (0.42–0.67) | >8.2 | 48% | 69% |
| NT-proBNP | 0.68 (0.62–0.85) | >18 | 52% | 88% |
| LVHCT | ||||
| Singulex TnI | 0.85 (0.72–0.93) | >2.1 | 62% | 97% |
| Abbott TnI | 0.87 (0.75–0.94) | >1.7 | 100% | 59% |
| Roche TnT | 0.75 (0.62–0.86) | >9 | 69% | 80% |
| NT-proBNP | 0.59 (0.45–0.72) | >6.3 | 75% | 49% |
| Composite SHDEcho | ||||
| Singulex TnI | 0.79 (0.66–0.88) | >1.2 | 74% | 77% |
| Abbott TnI | 0.82 (0.71–0.9) | >1.6 | 84% | 68% |
| Roche TnT | 0.62 (0.49–0.73) | >8 | 58% | 70% |
| NT-proBNP | 0.74 (0.62–0.84) | >18 | 58% | 92% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.K.M.; Dugo, C.; Whalley, G.; Wynne, Y.; Semple, H.; Smith, K.; Cleave, P.; Christiansen, J.; To, A.; Amir, N.; et al. Diagnostic Utility of High Sensitivity Troponins for Echocardiographic Markers of Structural Heart Disease. Med. Sci. 2018, 6, 17. https://doi.org/10.3390/medsci6010017
Wang TKM, Dugo C, Whalley G, Wynne Y, Semple H, Smith K, Cleave P, Christiansen J, To A, Amir N, et al. Diagnostic Utility of High Sensitivity Troponins for Echocardiographic Markers of Structural Heart Disease. Medical Sciences. 2018; 6(1):17. https://doi.org/10.3390/medsci6010017
Chicago/Turabian StyleWang, Tom Kai Ming, Clementina Dugo, Gillian Whalley, Yvonne Wynne, Heather Semple, Kevin Smith, Peter Cleave, Jonathan Christiansen, Andrew To, Nezar Amir, and et al. 2018. "Diagnostic Utility of High Sensitivity Troponins for Echocardiographic Markers of Structural Heart Disease" Medical Sciences 6, no. 1: 17. https://doi.org/10.3390/medsci6010017




