A Facile Approach to Preparing Molecularly Imprinted Chitosan for Detecting 2,4,6-Tribromophenol with a Widely Linear Range
Abstract
:1. Introduction
2. Experimental
2.1. Instruments and Reagents
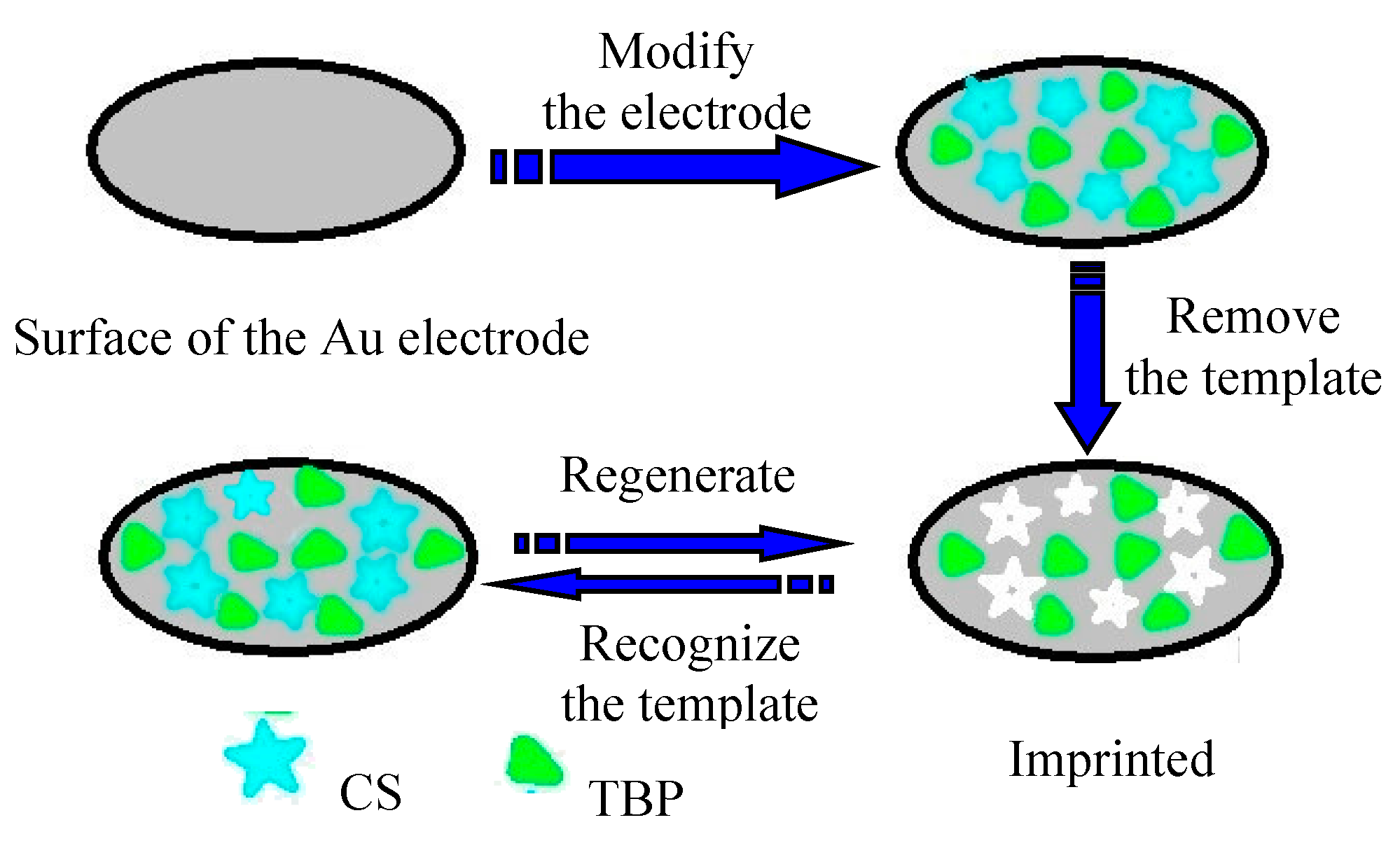
2.2. Preparation of TBP Imprinting Polymer Sensor (MIP/Au)
2.3. Measurements
3. Results and Discussion
3.1. Preparation of MIP/Au Sensor
3.2. Scanning Electron Microscopy
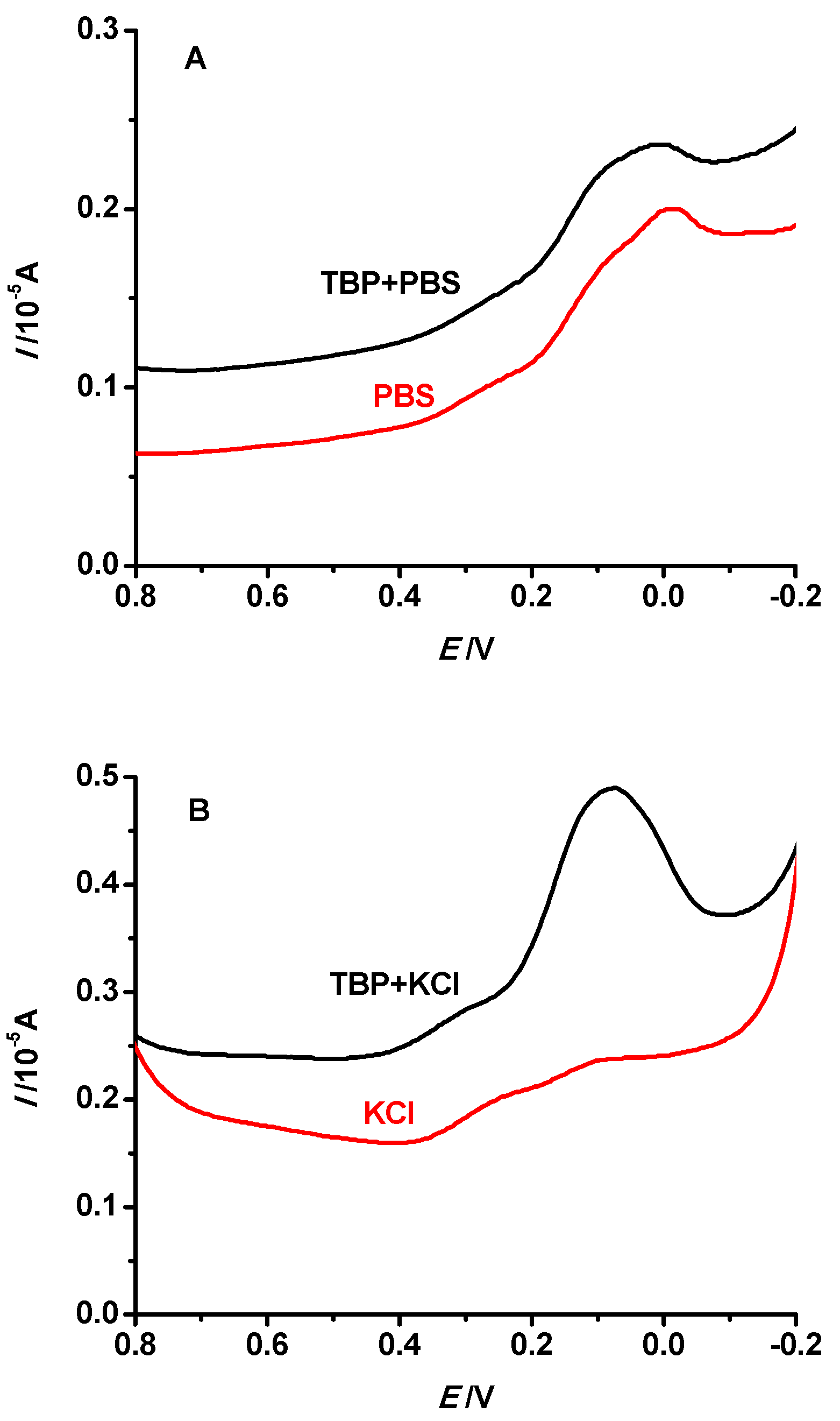
3.3. Supporting Electrolyte
3.4. Electrochemical Impedance Spectroscopy
3.5. Performance of the Sensor
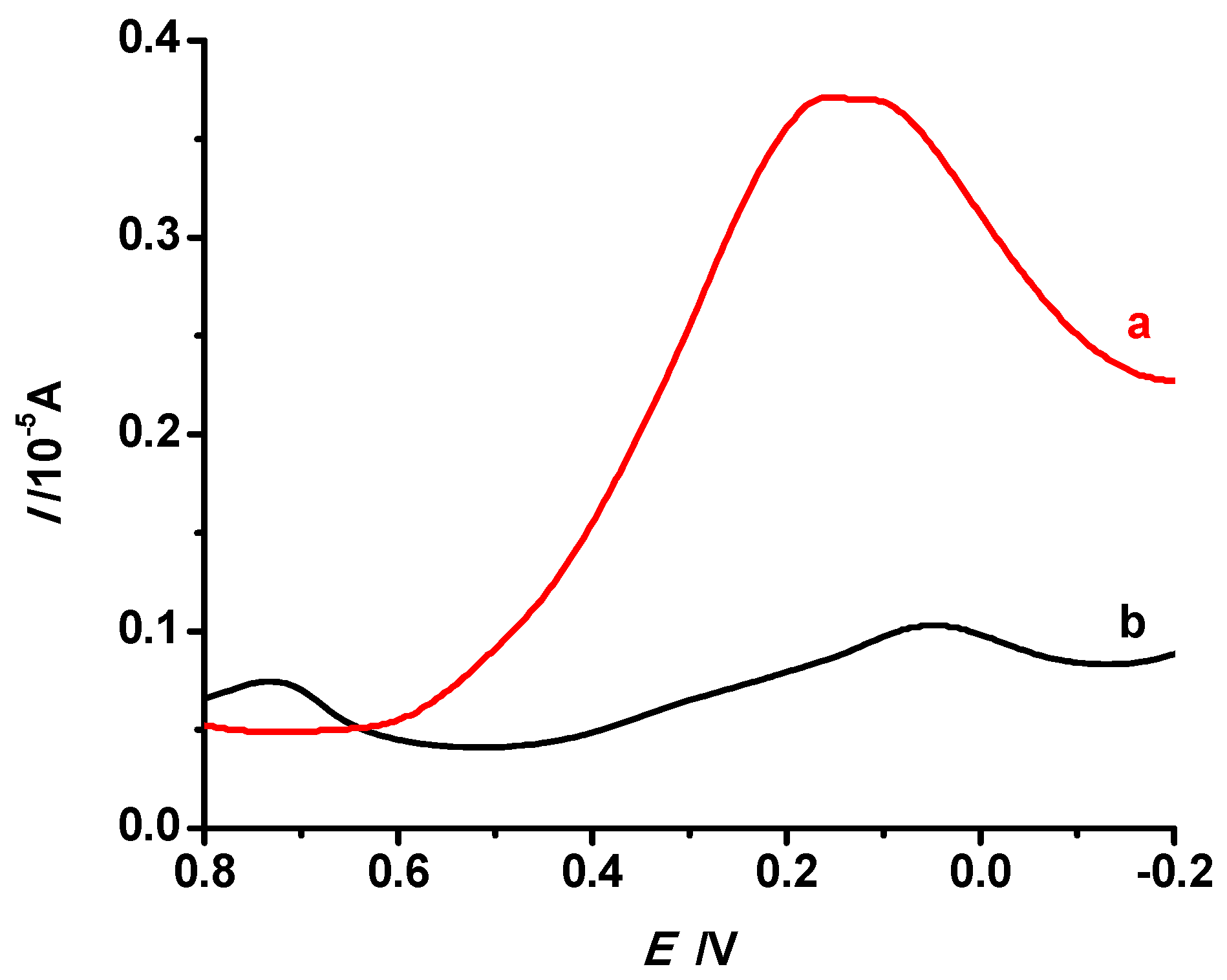
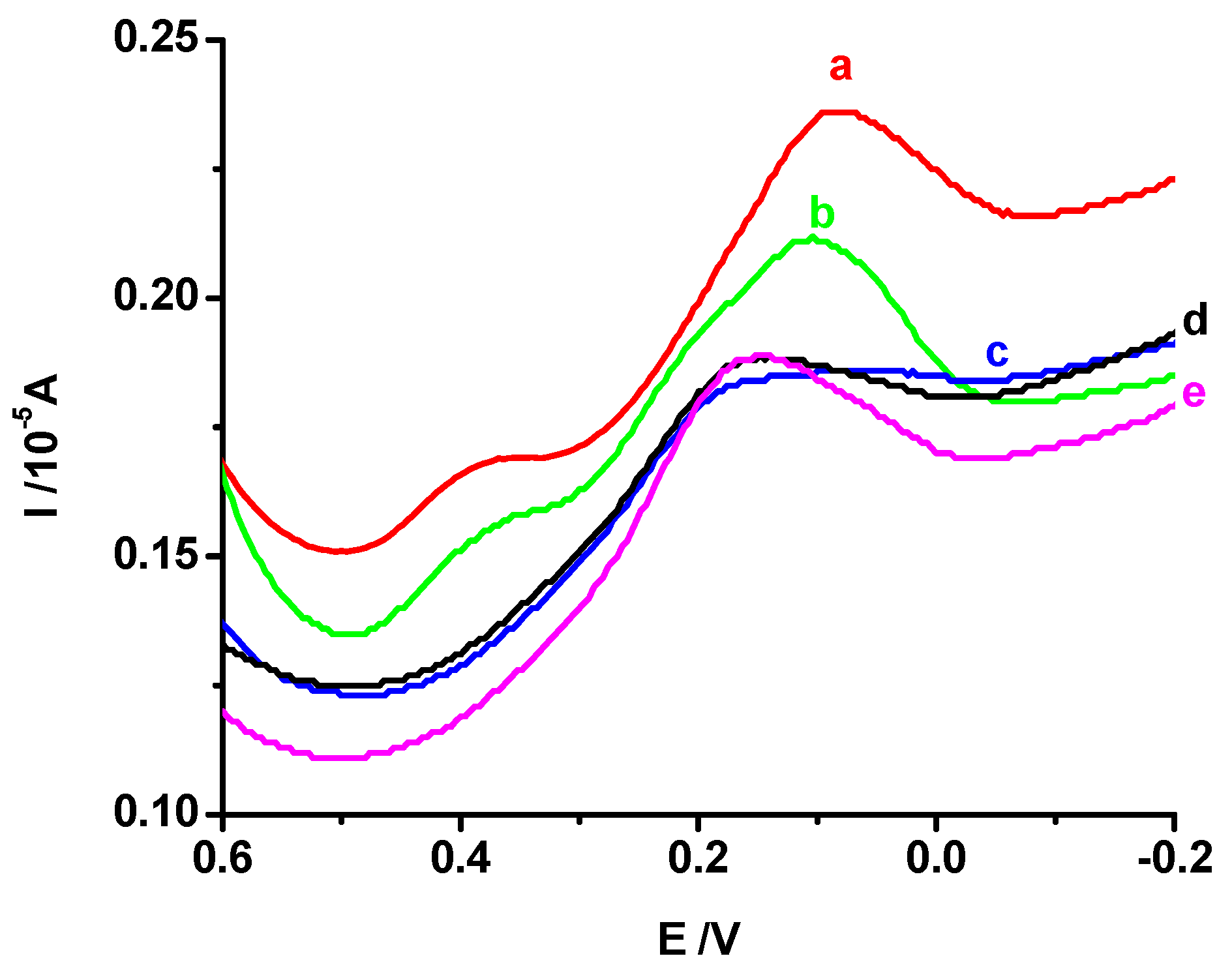
3.5.1. Effect of Imprinting
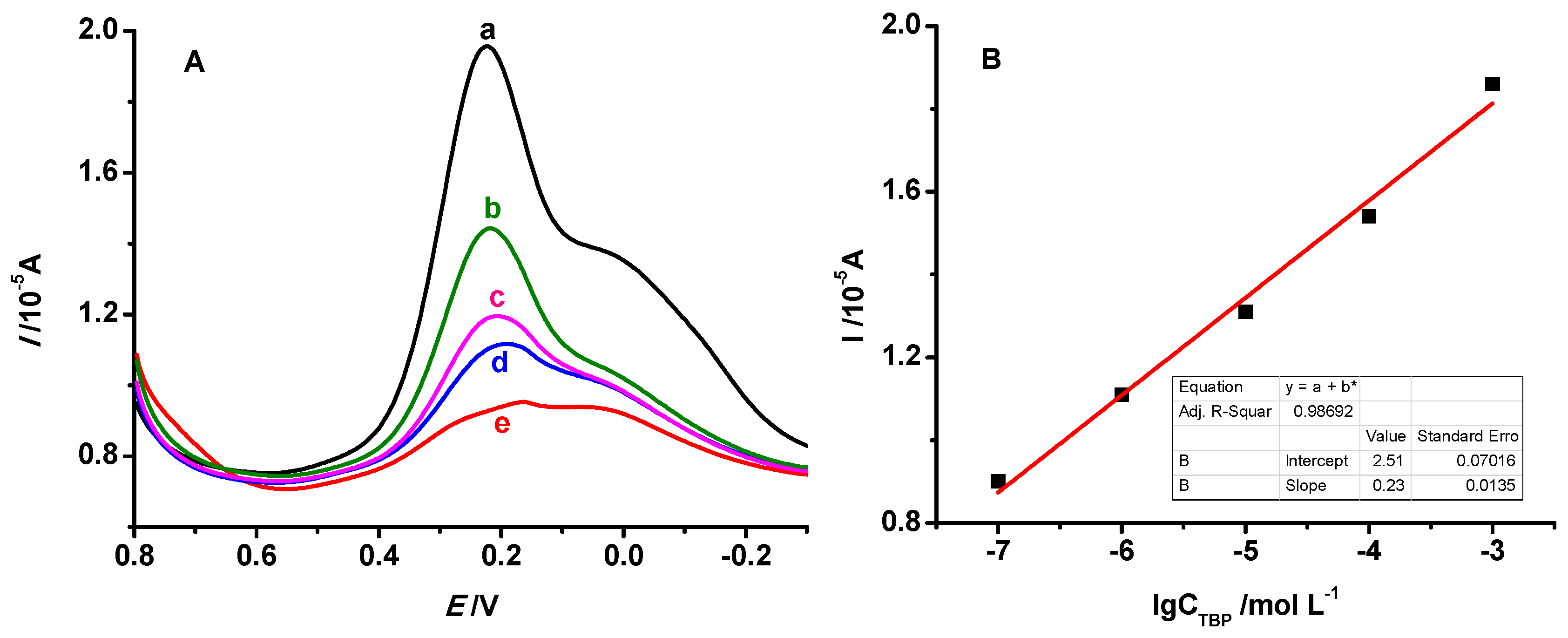
3.5.2. The Linear Range and Detection Limit of MIP/Au
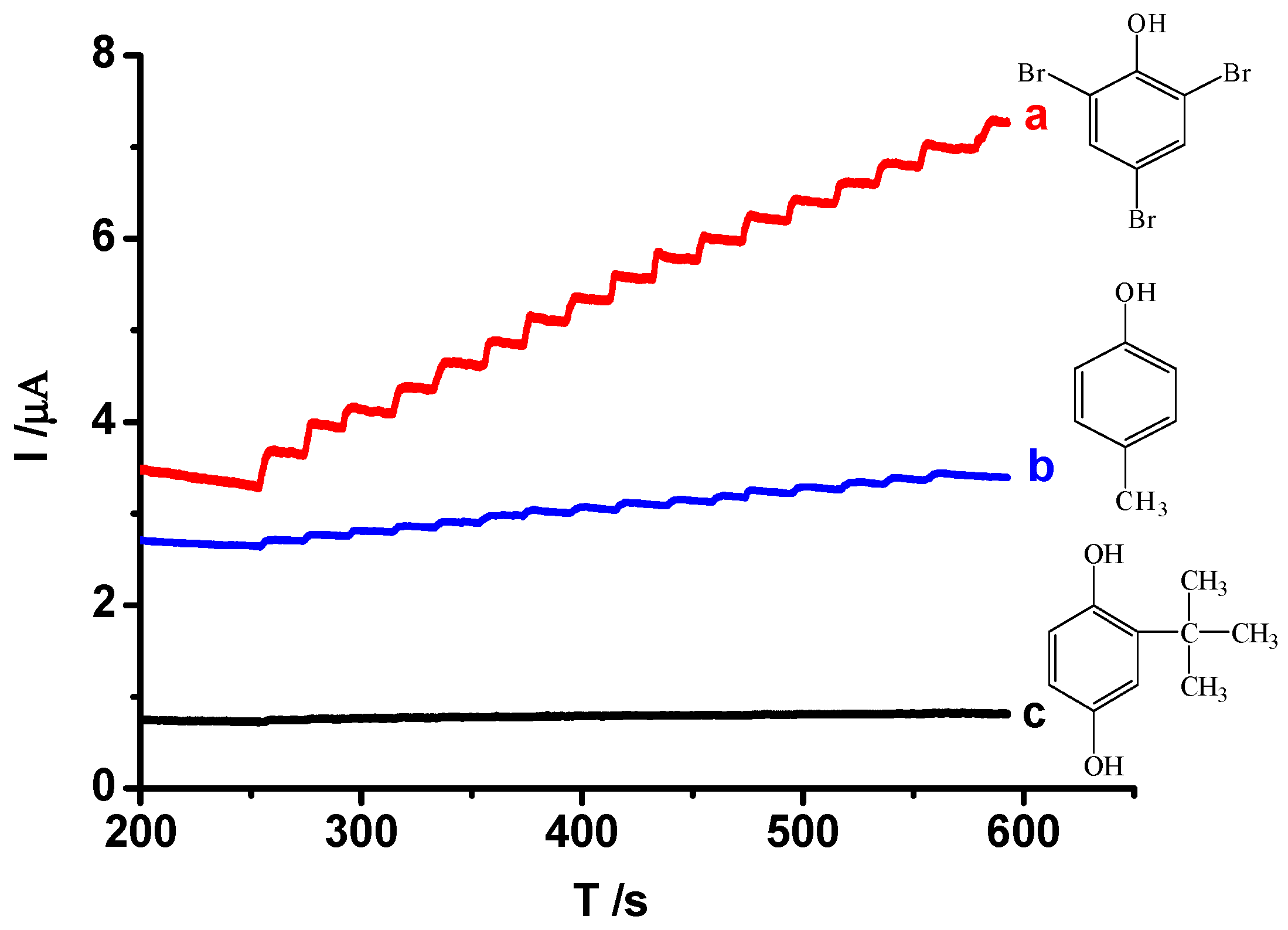
3.5.3. The Selectivity Performance of MIP/Au
3.5.4. Regeneration and Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cantón, R.F.; Sanderson, J.T.; Letcher, R.J.; Bergman, Å.; van den Berg, M. Inhibition and induction of aromatase (CYP19) activity by brominated flame. Toxicol. Sci. 2005, 88, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, C.S.; Yu, L.Q.; Zhou, B.S. Chronic exposure to environmental levels of tribromophenol impairs zebrafish reproduction. Toxicol Appl. Pharm. 2010, 243, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, J.; Wang, L.; Yang, X.; Shi, L.; Liu, D. Endocrine disrupting effects of TBBPA and TBP on pelteobagrus fulvidraco. J. Ecol. Rural Environ. 2016, 32, 1012–1017. [Google Scholar]
- Chuang, H.Y.; Joyce Ma, M.C.; Kim, J.S. Seasonal distribution of bromophenols in selected Hong Kong seafood. J. Agric. Food Chem. 2003, 51, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Belyea, J.; Gilvey, L.B.; Davis, M.F.; Godek, M.; Sit, T.L.; Lommel, S.A.; Franzen, S. Enzyme function of the globin dehaloperoxidase from Amphitrite ornata is activated by substrate binding. Biochemistry 2005, 48, 15637–15644. [Google Scholar] [CrossRef] [PubMed]
- Blythe, J.W.; Heitz, A.; Joll, C.A.; Kagi, R.I. Determination of trace concentrations of bromophenols in water using purge-and-trap after in situ acetylation. J. Chromatogr. A 2006, 1102, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Polo, M.; Llompart, M.; Garcia-Jares, C.; Gomez-Noya, G.; Bollain, M.H.; Cela, R. Development of a solid-phase microextraction method for the analysis of phenolic flame retardants in water samples. J. Chromatogr. A 2006, 1124, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Alquie, L.; Kaisa, J.P.; Reed, G.; Gilmor, T.; Vas, G. Method development and validation for the determination of 2,4,6-tribromoanisole, 2,4,6-tribromophenol, 2,4,6-trichloroanisole, and 2,4,6-trichlorophenol in various drug products using stir bar sorptive extraction and gas chromatography–tandem mass spectrometry detection. J. Chromatogr. A 2012, 1262, 196–204. [Google Scholar] [PubMed]
- Osmani, Q.; Hughes, H.; McLoughlin, P. Probing the recognition of molecularly imprinted polymer beads. J. Mater. Sci. 2012, 47, 2218–2227. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Lia, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.P.; Prasad, A. Molecularly imprinted polymer based enantioselective sensing devices: A review. Anal. Chim. Acta 2015, 853, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.; Hantao, L.W.; Mogollon, N.; Braga, S. New materials and trends in sorbents for solid-phase extraction. Trends Anal. Chem. TrAC 2013, 43, 14–23. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Wang, S.; Liu, Z.; Aisa, H.A. A new microemulsion approach for producing molecularly imprinted polymers with selective recognition cavities for gallic acid. Anal. Methods 2013, 7, 10256–10265. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, G.Q.; Zhang, Y.; Guo, X.Z.; Zhang, H.Q. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed. 2013, 52, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Chandra, B.K.C.; Sosnowska, M.; Sobczak, J.W.; Nesterov, V.N.; D’Souza, F.; Kutner, W. Nicotine molecularly imprinted polymer: Synergy of coordination and hydrogen bonding. Biosens. Bioelectron. 2015, 64, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Antonacci, A.; Lambreva, M.D.; Litescu, S.C.; Rea, G. Synthetic biology and biomimetic chemistry as converging technologies fostering a new generation of smart biosensors. Biosens. Bioelectron. 2015, 74, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Han, W.; Yan, Y.; Li, X.; Li, C.; Hu, B. A novel molecular imprinted nanosensor based quartz crystal microbalance for determination of kaempferol. Anal. Methods 2016, 8, 2434–2440. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical detection of sulfanilamide using pencil graphite electrode based on molecular imprinting technology. Electroanalysis 2014, 26, 2328–2336. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Alexander, S. Design and fabrication of molecularly imprinted polymer-based potentiometric sensor from the surface modified multiwalled carbon nanotube for the determination of lindane (γ-hexachlorocyclohexane), an organochlorine pesticide. Biosens. Bioelectron. 2015, 64, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cong, J.; Liu, J.; Gao, Y.; Liu, X. A facile approach for synthesizing molecularly imprinted graphene for ultrasensitive and selective electrochemical detecting 4-nitrophenol. Anal. Chim. Acta. 2015, 864, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. Trends Anal. Chem. TrAC 2004, 23, 36–48. [Google Scholar] [CrossRef]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP sensors—The electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Paim, L.L.; Zhang, X.; Wang, S.; Stradiotto, N.R. An electrochemical sensor for reducing sugars based on a glassy carbon electrode modified with electropolymerized molecularly imprinted poly-ophenylenediamine film. Electroanalysis 2014, 26, 1612–1622. [Google Scholar] [CrossRef]
- Özcan, L.; Şahin, Y. Determination of paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. Sens. Actuators B Chem. 2007, 127, 362–369. [Google Scholar] [CrossRef]
- Cetó, X.; Saint, C.; Chow, C.W.K.; Voelcker, N.H.; Prieto-Simón, B. Electrochemical detection of N-nitrosodimethylamine using a molecular imprinted polymer. Sens. Actuators B Chem. 2016, 237, 613–620. [Google Scholar] [CrossRef]
- Granado, V.L.; Gutiérrez-Capitán, M.; Fernández-Sánchez, C.; Gomes, M.T.; Rudnitskaya, A.; Jimenez-Jorquera, C. Thin-film electrochemical sensor for diphenylamine detection using molecularly imprinted polymers. Anal. Chim. Acta 2014, 809, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Twu, Y.K.; Chang, I.T.; Ping, C.C. Preparation of novel chitosan scaffolds by electrochemical process. Carbohydr. Polym. 2005, 62, 113–119. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, D.; Fu, G. Enhanced surface imprinting of lysozyme over a new kind of magnetic chitosan submicrospheres. J. Colloid Interface Sci. 2015, 440, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.; Jin, F.; Jin, M.; et al. Competitive fluorescence assay for specific recognition of atrazine bymagnetic molecularly imprinted polymer based on Fe3O4-chitosan. Carbohydr. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Liu, B.; Lian, H.T.; Sun, X.Y. Preparation and application of urea electrochemical sensor based on chitosan molecularly imprinted films. Electroanalysis 2011, 23, 1454–1461. [Google Scholar] [CrossRef]
- Duan, H.; Li, L.; Wang, X.; Wang, Y.; Li, J.; Luo, C. CdTe quantum dots@luminol as signal amplification system for chrysoidine with chemiluminescence-chitosan/graphene oxide-magnetite-molecularly imprinting sensor. Spectrochim. Acta A 2016, 153, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, D.; Huang, L.; Wu, Z.; Xiang, S.; Chen, S. Sensing 2,4,6-tribromophenol based on molecularly imprinted technology. Monatsh. Chem. 2015, 146, 485–491. [Google Scholar] [CrossRef]
- Wu, Z. Preparation of Tribromophenol Molecularly Imprinted Sensor [D]; Fujian Normal University: Fuzhou, China, 2014. [Google Scholar]
- Ma, X.; Chen, R.; Zheng, X.; Youn, H.; Chen, Z. Preparation of molecularly imprinted CS membrane for recognizing naringin in aqueous media. Polym. Bull. 2011, 66, 853–863. [Google Scholar] [CrossRef]
- Cui, Z.; Xiang, Y.; Zhang, T. Investigation on proton conductivity behavior of sulfuric acid cross-linked chitosan membrane. Acta. Chim. Sinica 2007, 65, 1902–1906. [Google Scholar]
- Piletsky, S.A.; Panasyuk, T.L.; Piletskaya, E.V. Receptor and transport properties of imprinted polymer membranes—A review. J. Membr. Sci. 1999, 157, 263–278. [Google Scholar] [CrossRef]
- Li, S.; Du, D.; Huang, J.; Tu, H.; Yang, Y.; Zhang, A. One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/AuNPs hybrid film and its application in the selective determination of p-nitrophenol. Analyst 2013, 138, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Deng, J.; Xiao, X.L.; Ding, L.; Yuan, Y.L.; Li, H.; Li, X.T.; Yan, X.N.; Wang, L.L. Electrochemical sensor based on a poly(para-aminobenzoic acid) film modified glassy carbon electrode for the determination of melamine in milk. Electrochim. Acta 2011, 56, 4595–4602. [Google Scholar] [CrossRef]
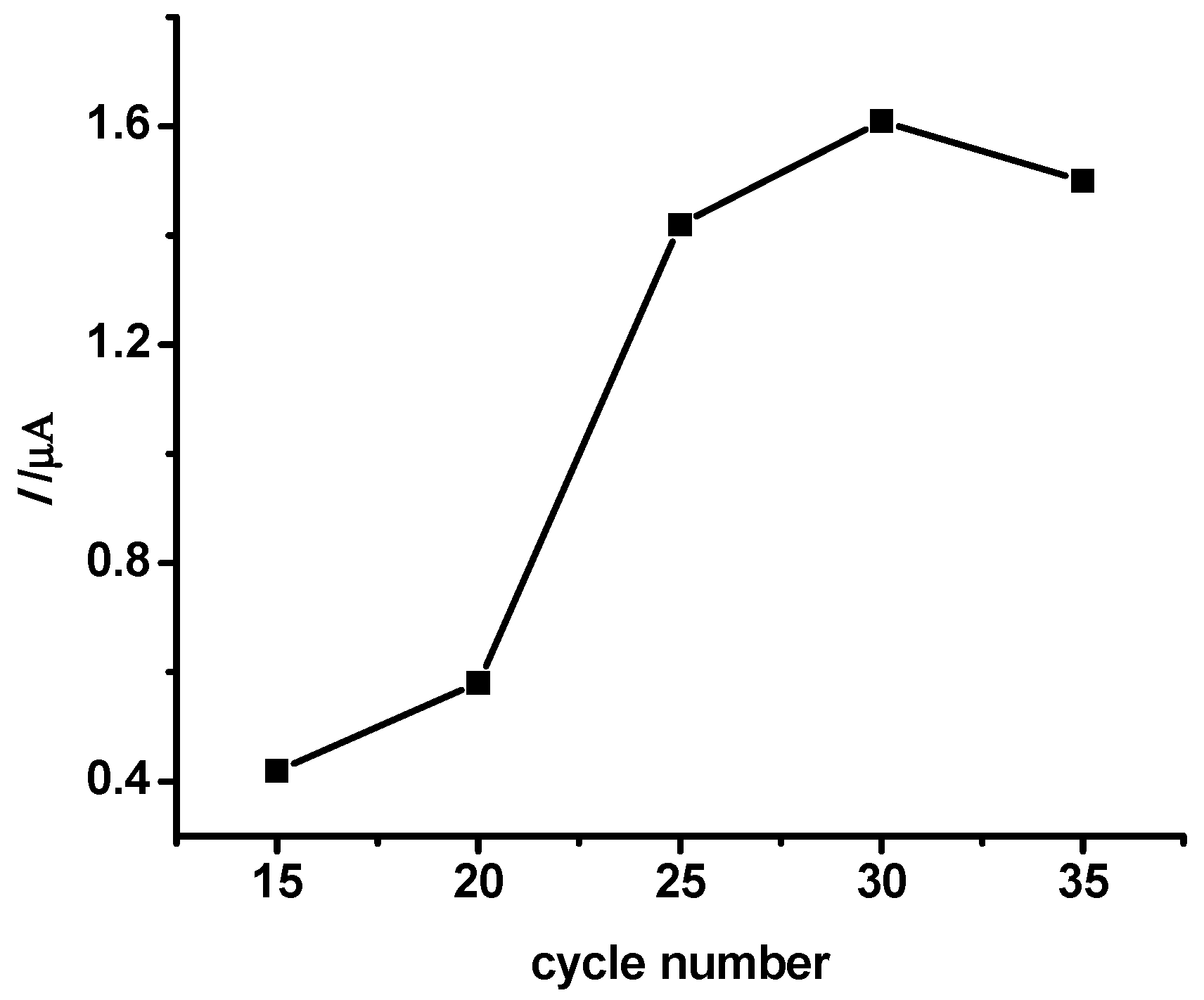



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Lu, Y.; Wu, Z.; Li, M.; Xiang, S.; Ma, X.; Zhang, Z. A Facile Approach to Preparing Molecularly Imprinted Chitosan for Detecting 2,4,6-Tribromophenol with a Widely Linear Range. Environments 2017, 4, 30. https://doi.org/10.3390/environments4020030
Huang L, Lu Y, Wu Z, Li M, Xiang S, Ma X, Zhang Z. A Facile Approach to Preparing Molecularly Imprinted Chitosan for Detecting 2,4,6-Tribromophenol with a Widely Linear Range. Environments. 2017; 4(2):30. https://doi.org/10.3390/environments4020030
Chicago/Turabian StyleHuang, Limei, Yaqi Lu, Zhenyue Wu, Meishan Li, Shengchang Xiang, Xiuling Ma, and Zhangjing Zhang. 2017. "A Facile Approach to Preparing Molecularly Imprinted Chitosan for Detecting 2,4,6-Tribromophenol with a Widely Linear Range" Environments 4, no. 2: 30. https://doi.org/10.3390/environments4020030




