Biotrickling Filtration of Air Contaminated with 1-Butanol
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
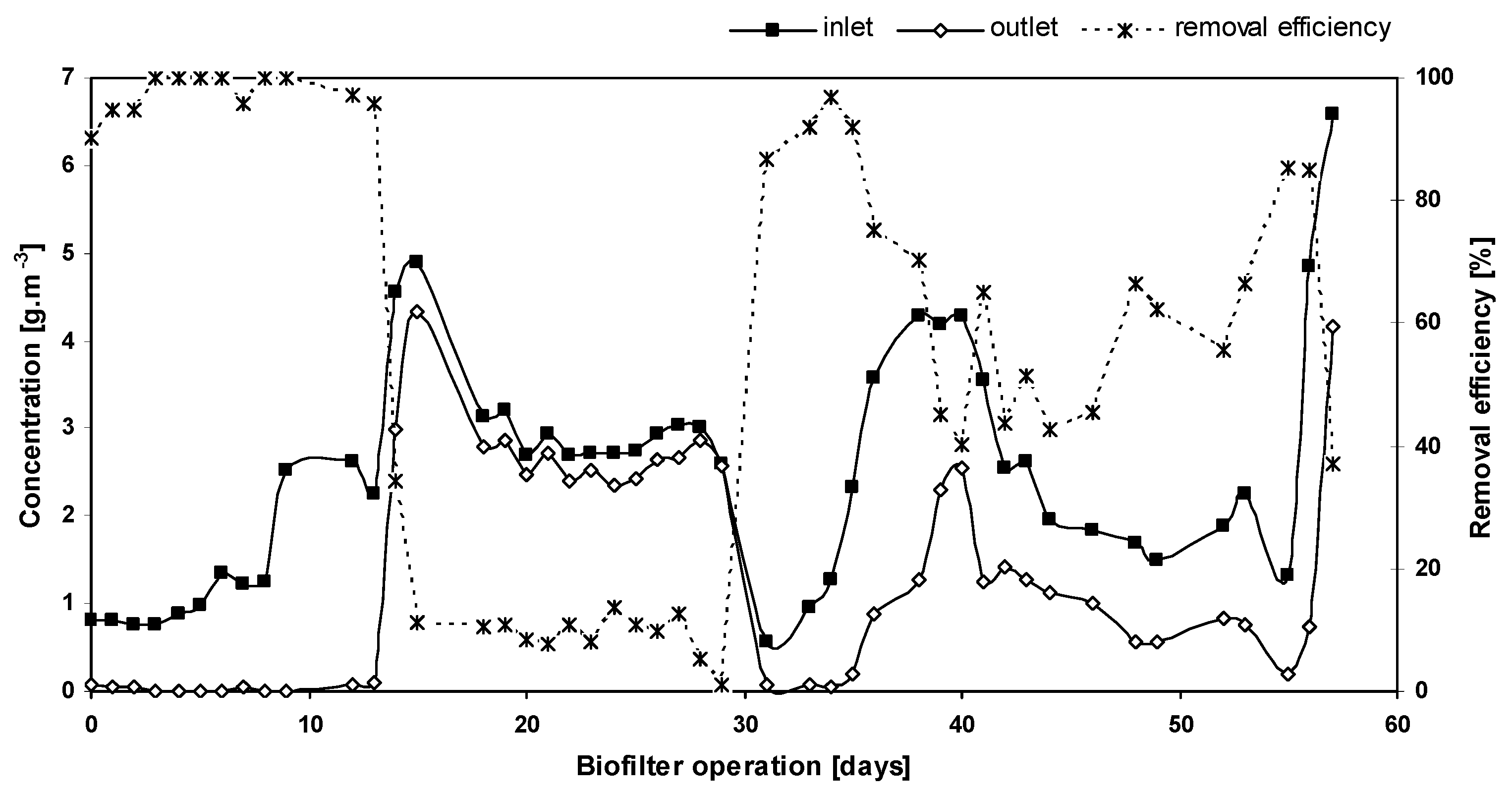
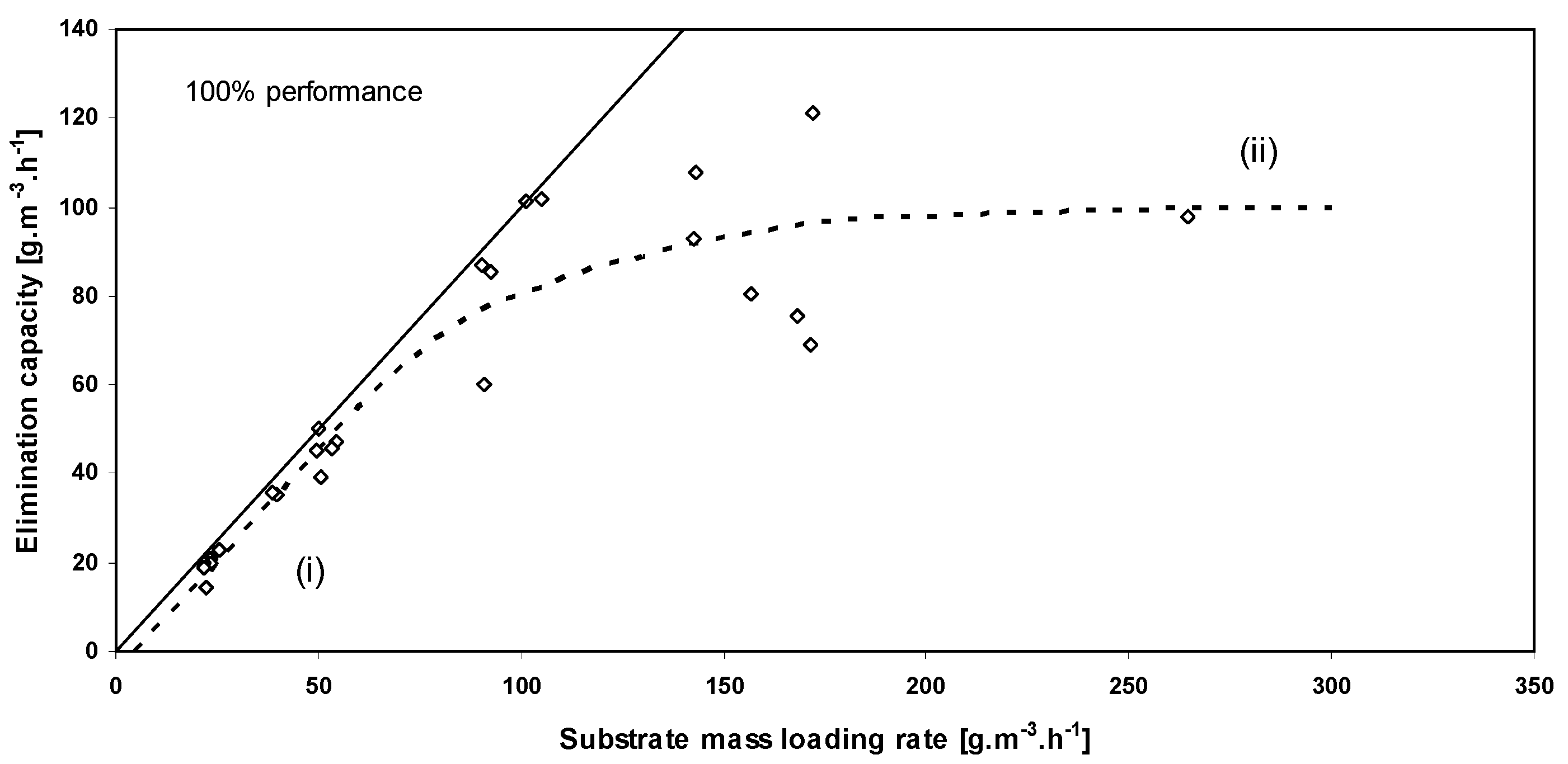
3.1. Effect of Gas Flow Rate and Inlet Concentration
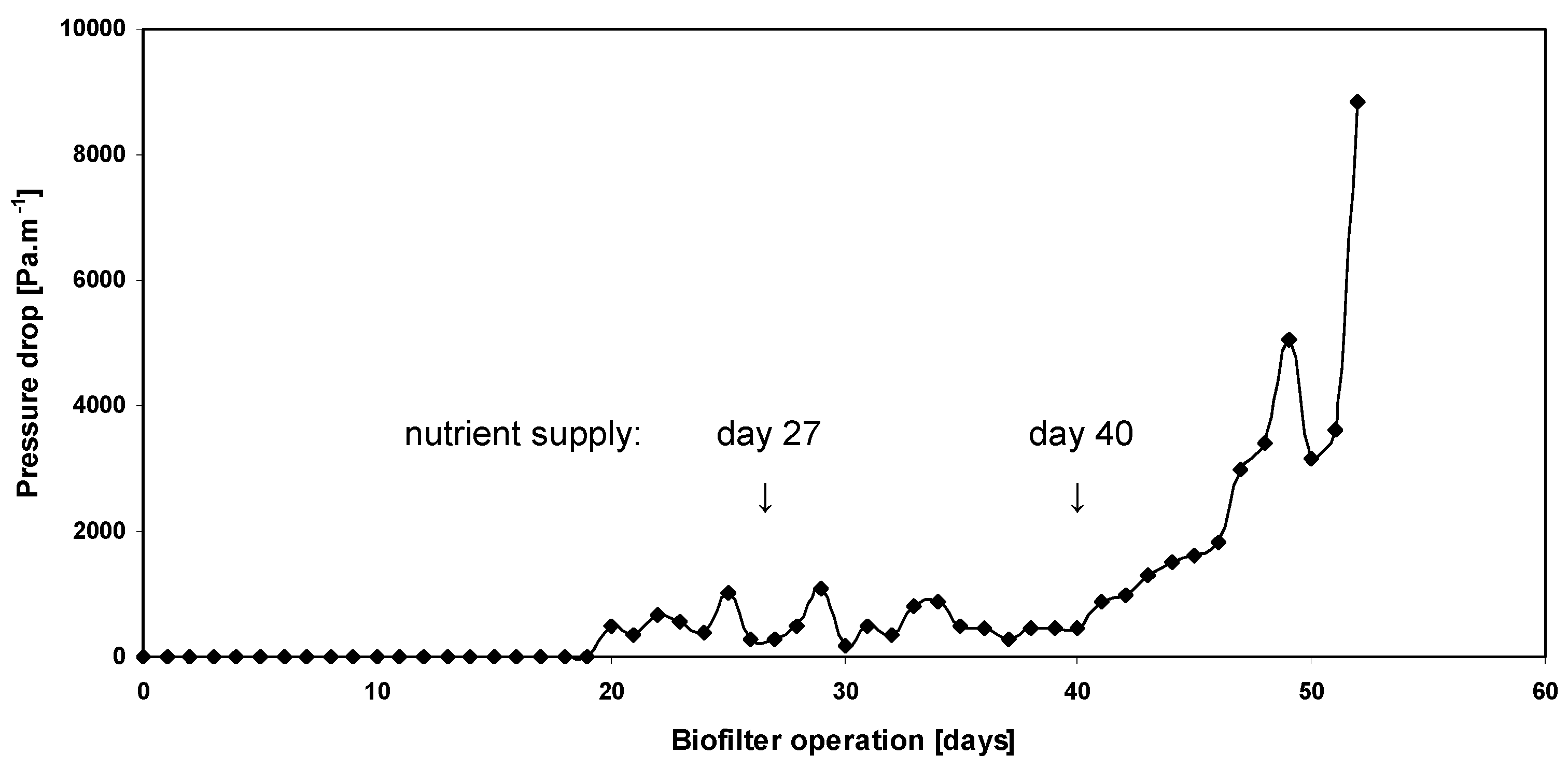
3.2. Evolution of the Pressure Drop
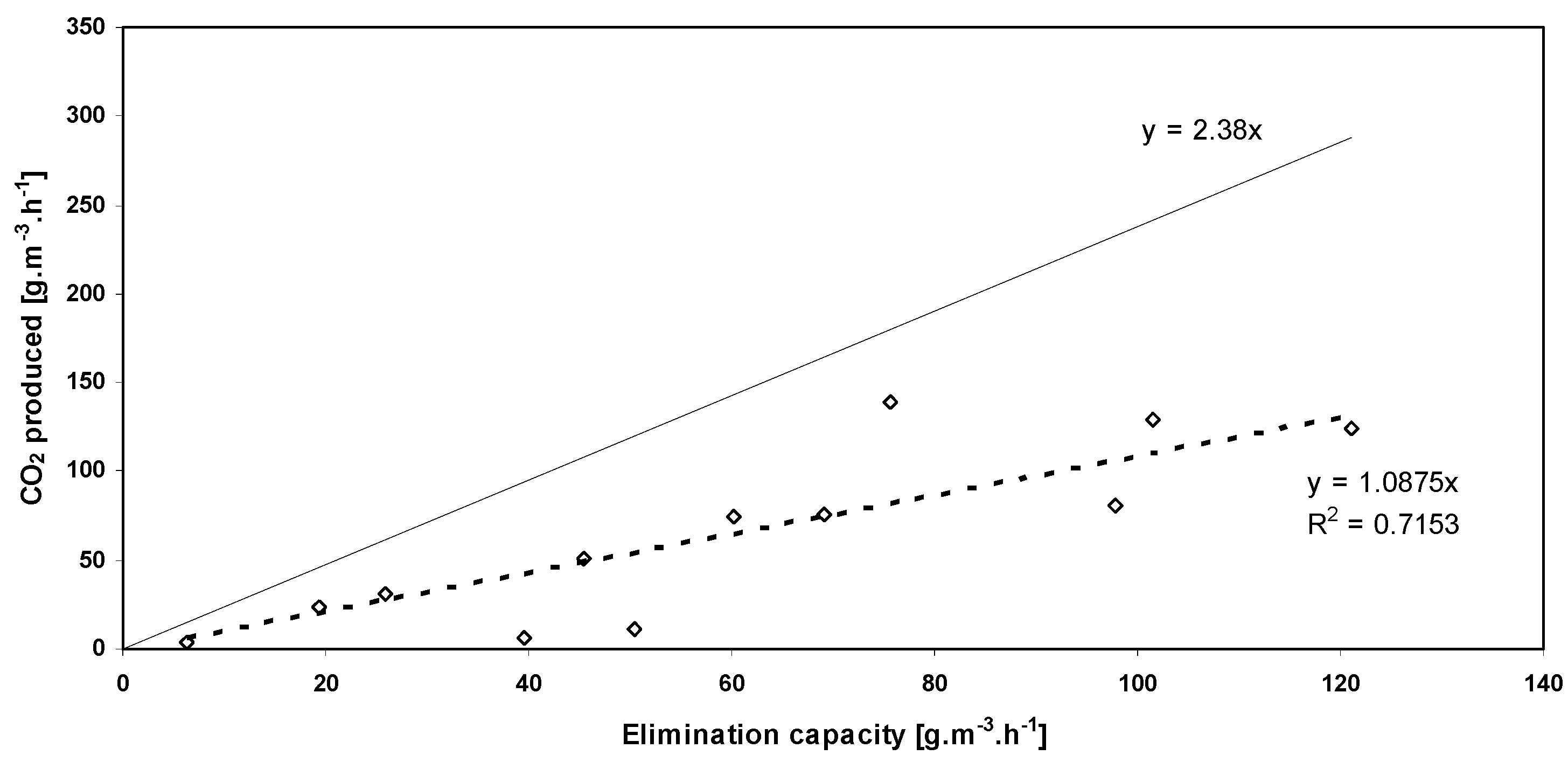
3.3. CO2 Production
3.4. Comparisons of Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartacek, J.; Kennes, C.; Lens, P.N.L. Biotechniques for Air Pollution Control; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Cooper, C.D.; Alley, F.C. Air Pollution Control: A Design Approach, 3rd ed.; Waveland Press Inc.: Long Grove, IL, USA, 2002. [Google Scholar]
- Borwankar, D.S.; Anderson, W.A.; Fowler, M.W. A technology assessment tool for evaluation of VOC abatement technologies from solvent based industrial coating operations. In Air Quality—New Perspective; Badilla, G.L., Valdez, B., Schorr, M., Eds.; InTech Open: Rijeka, Croatia, 2016. [Google Scholar]
- Iranpour, R.; Cox, H.H.; Deshusses, M.A.; Schroeder, E.D. Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ. Prog. Sustain. Energy 2005, 24, 254–267. [Google Scholar] [CrossRef]
- Delhoménie, M.C.; Heitz, M. Biofiltration of air: A review. Crit. Rev. Biotechnol. 2005, 25, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Rene, E.R.; Veiga, M.C. Bioprocesses for air pollution control. J. Chem. Technol. Biotechnol. 2009, 84, 1419–1436. [Google Scholar] [CrossRef]
- Schiavon, M.; Ragazzi, M.; Rada, E.C.; Torretta, V. Air pollution control through biotrickling filters: A review considering operational aspects and expected performance. Crit. Rev. Biotechnol. 2016, 36, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Arulneyam, D.; Swaminathan, T. Biodegradation of ethanol vapour in a biofilter. Bioprocess Eng. 2000, 22, 63–67. [Google Scholar] [CrossRef]
- Chan, W.C.; Lin, Y.S. Compounds interaction on the biodegradation of butanol mixture in a biofilter. Bioresour. Technol. 2010, 101, 4234–4237. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Lai, Y.Z. Biodegradation kinetic behaviours of n-butyl alcohol and sec-butyl alcohol in a composite bead biofilter. Process Biochem. 2009, 44, 593–596. [Google Scholar] [CrossRef]
- Kwon, H.M.; Yeom, S.H. Design of a Biofilter Packed with Crab Shell and Operation of the Biofilter Fed with Leaf Mold Solution as a Nutrient. Biotechnol. Bioprocess Eng. 2009, 14, 248–255. [Google Scholar] [CrossRef]
- Kam, S.K.; Kim, J.K.; Lee, M.G. Removal characteristics of mixed gas of ethyl acetate and 2-butanol by a biofilter packed with Jeju scoria. Korean J. Chem. Eng. 2011, 28, 1019–1022. [Google Scholar] [CrossRef]
- Kibazohi, O.; Yun, S.I.; Anderson, W.A. Removal of hexane in biofilters packed with perlite and a peat-perlite mixture. World J. Microbiol. Biotechnol. 2004, 20, 337–343. [Google Scholar] [CrossRef]
- Jorio, H.; Bibeau, L.; Heitz, M. Biofiltration of Air Contaminated by Styrene: Effect of Nitrogen Supply, Gas Flow Rate, and Inlet Concentration. Environ. Sci. Technol. 2000, 34, 1764–1771. [Google Scholar] [CrossRef]
- Grove, J.A.; Zhang, H.; Anderson, W.A.; Moo-Young, M. Estimation of Carbon Recovery and Biomass Yield in the Biofiltration of Octane. Environ. Eng. Sci. 2009, 26, 1497–1502. [Google Scholar] [CrossRef]
- Heinze, U.; Friedrich, C.G. Respiratory activity of biofilms: Measurement and its significance for the elimination of n-butanol from waste gas. Appl. Microbiol. Biotechnol. 1997, 48, 411–416. [Google Scholar] [CrossRef]
- Fitch, M.W.; England, E.; Zhang, B. 1-Butanol removal from a contaminated airstream under continuous and diurnal loading conditions. J. Air Waste Manag. Assoc. 2002, 52, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Li, C.; Heber, A.J.; Ni, J.; Huang, H. Biofiltration of a mixture of ethylene, ammonia, n-butanol, and acetone gases. Bioresour. Technol. 2013, 127, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Ondarts, M.; Hort, C.; Sochard, S.; Platel, V.; Moynault, L.; Seby, F. Evaluation of compost and a mixture of compost and activated carbon as biofilter media for the treatment of indoor air pollution. Environ. Technol. 2012, 33, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Feizi, F.; Nasernejad, B.; Zamir, S.M. Effect of operating temperature on transient behaviour of a biofilter treating waste-air containing n-butanol vapor during intermittent loading. Environ. Technol. 2016, 37, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Zhang, L.; Tian, S.; Sun, P.T.-C.; Xie, W. A pilot-study on treatment of a waste gas containing butyl acetate, n-butyl alcohol and phenylacetic acid from pharmaceutical factory by bio-trickling filter. Biochem. Eng. J. 2007, 37, 42–48. [Google Scholar] [CrossRef]
- Liu, S.; Qureshi, N. How microbes tolerate ethanol and butanol. New Biotechnol. 2009, 26, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Veiga, M.C. Bioreactors for Waste Gas Treatment; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Estrada, J.M.; Quijano, G.; Lebrero, R.; Munoz, R. Step-feed biofiltration: A low cost alternative configuration for off-gas treatment. Water Res. 2013, 47, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Sorial, G.A.; Smith, F.L.; Suidan, M.T.; Pandit, A.; Biswas, P.; Brenner, R.C. Evaluation of trickle-bed air biofilter performance for styrene removal. Water Res. 1998, 32, 1593–1603. [Google Scholar] [CrossRef]
- Kim, D.; Sorial, G.A. Nitrogen utilization and biomass yield in trickle bed air biofilters. J. Hazard. Mater. 2010, 182, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Rene, E.R.; López, M.E.; Veiga, M.C.; Kennes, C. Performance of a fungal monolith bioreactor for the removal of styrene from polluted air. Bioresour. Technol. 2010, 101, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Thalasso, F. Waste Gas Biotreatment Technology. J. Chem. Technol. Biotechnol. 1998, 72, 303–319. [Google Scholar] [CrossRef]
- Mohseni, M.; Allen, D.G. Biofiltration of mixtures of hydrophilic and hydrophobic volatile organic compounds. Chem. Eng. Sci. 2000, 55, 1545–1558. [Google Scholar] [CrossRef]
- Eshraghi, M.; Parnian, P.; Zamir, S.M.; Halladj, R. Biofiltration of n-butanol vapor at different operating temperatures: Experimental study and mathematical modeling. Int. Biodeterior. Biodegrad. 2017, 119, 361–367. [Google Scholar] [CrossRef]

| Type of Process | Type of VOC | Elimination Capacity (g m−3 h−1) | Reference |
|---|---|---|---|
| Biofilter | Ethanol | 53–219 | [29] |
| Biofilter | Methanol | 113 | [29] |
| Biofilter | Ethanol | 195 | [9] |
| Biotrickling | Methanol | 1916 | [29] |
| Biofilter | n-Butanol and sec-butanol | 56 and 21 | [11] |
| Biofilter | Methanol | 250 | [30] |
| Biotrickling | Butanol | 102 | [17] |
| Biofilter | Butanol (transient) | 131 | [21] |
| Biofilter + biotrickling | Butanol (in mixture) | 2 | [19] |
| Biofilter | Butanol | 162 | [31] |
| Biotrickling | Butanol (in mixture) | 317 | [22] |
| Biotrickling | Butanol | 100 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, T.; Anderson, W.A. Biotrickling Filtration of Air Contaminated with 1-Butanol. Environments 2017, 4, 57. https://doi.org/10.3390/environments4030057
Schmidt T, Anderson WA. Biotrickling Filtration of Air Contaminated with 1-Butanol. Environments. 2017; 4(3):57. https://doi.org/10.3390/environments4030057
Chicago/Turabian StyleSchmidt, Thomas, and William A. Anderson. 2017. "Biotrickling Filtration of Air Contaminated with 1-Butanol" Environments 4, no. 3: 57. https://doi.org/10.3390/environments4030057





