Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
| Properties | Waste Oil Feedstock |
|---|---|
| Density (g/mL @ 15 °C) | 0.892 |
| Molecular weight (g/g mole) | 912.4 |
| Saponification value (mg KOH/g oil) | 188.76 |
| Acid value (mg KOH/g oil) | 1.91 |
| Water content (wt%) | 0.10 |
2.2. Activated Carbon Preparation
2.3. Catalyst Preparation
2.4. Characterization of Activated Carbon and Catalyst
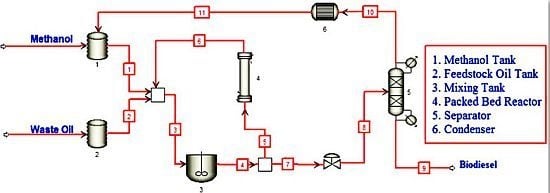
2.5. Instrumentation and Catalytic Reaction Procedure
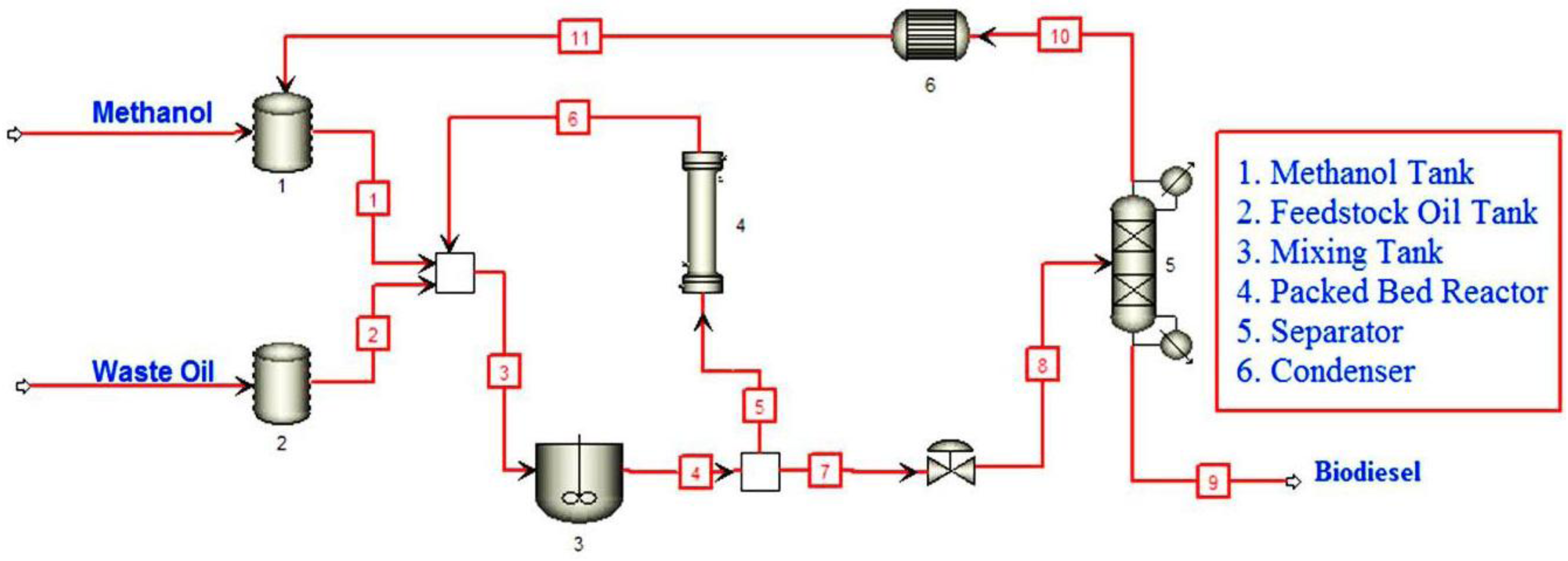
2.6. Analysis of Final Product
3. Results and Discussion
3.1. Characterization of KOH/JS Catalyst
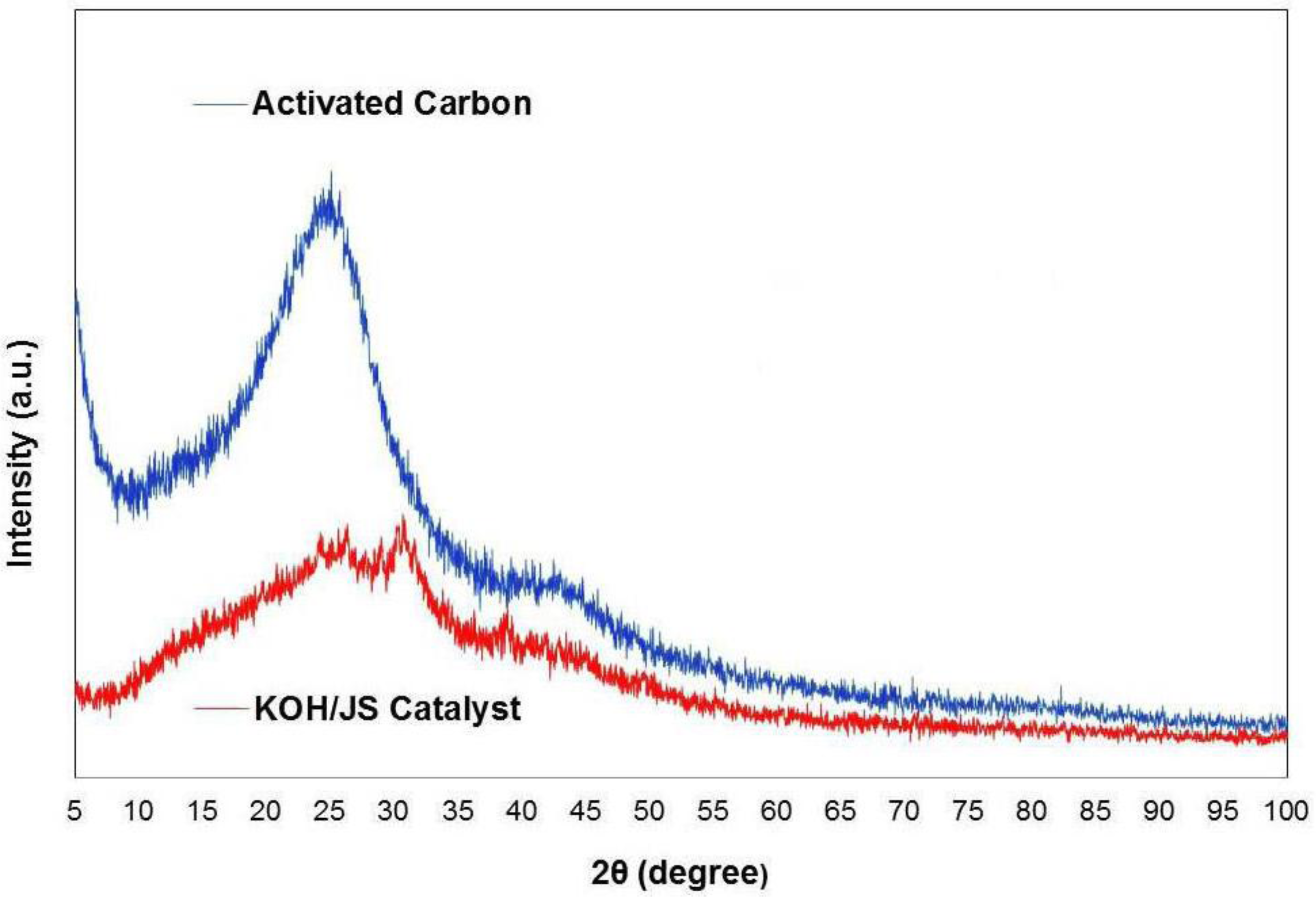
| Physical Property | Material | |
|---|---|---|
| Activated Carbon | KOH/JS Catalyst | |
| BET surface area (m2/g) | 927.85 | 275.83 |
| Pore volume (m3/g) | 0.923 | 0.209 |
| Mean pore diameter (A°) | 44.86 | 93.71 |
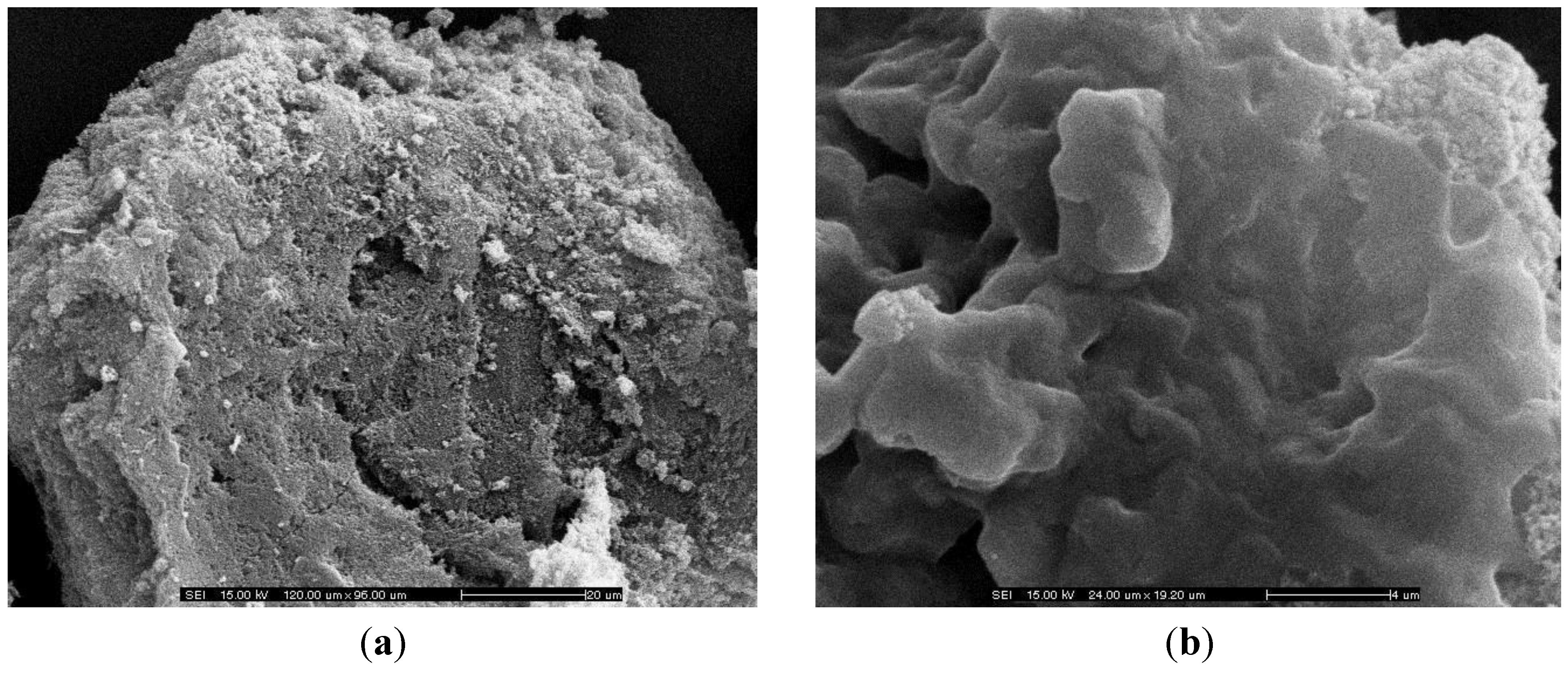
3.2. Influence of Various Parameters on the Yield of Biodiesel

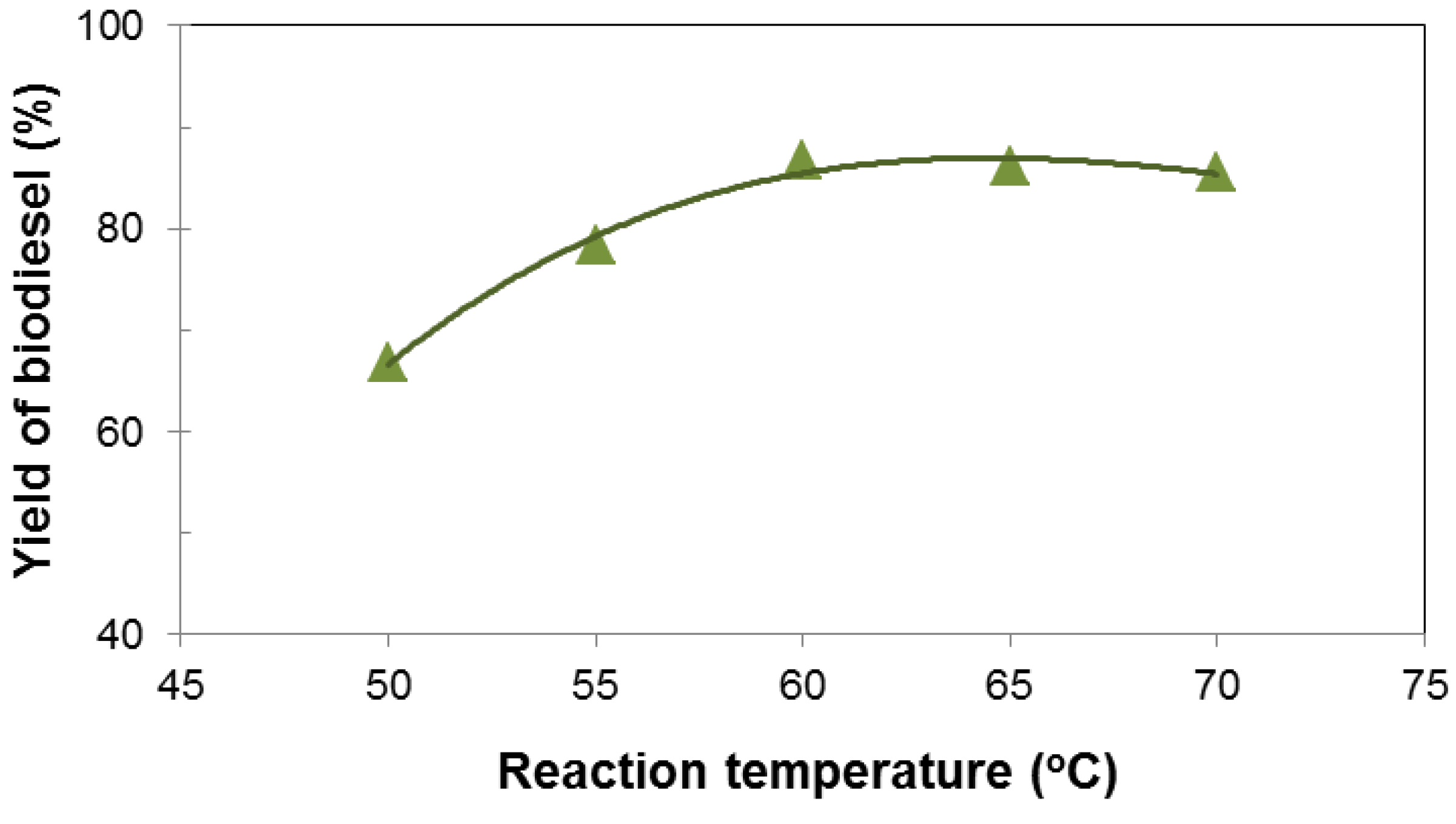
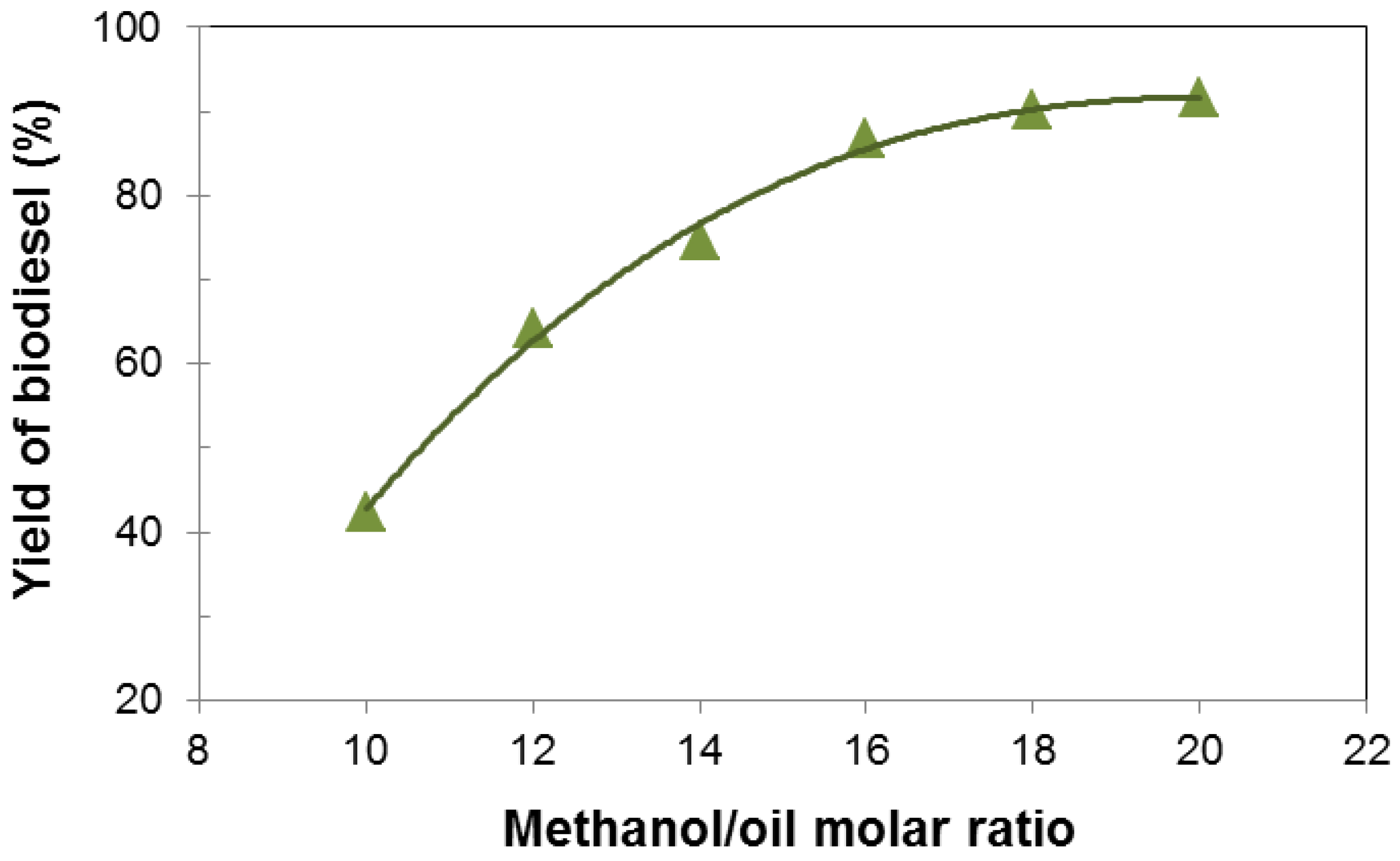
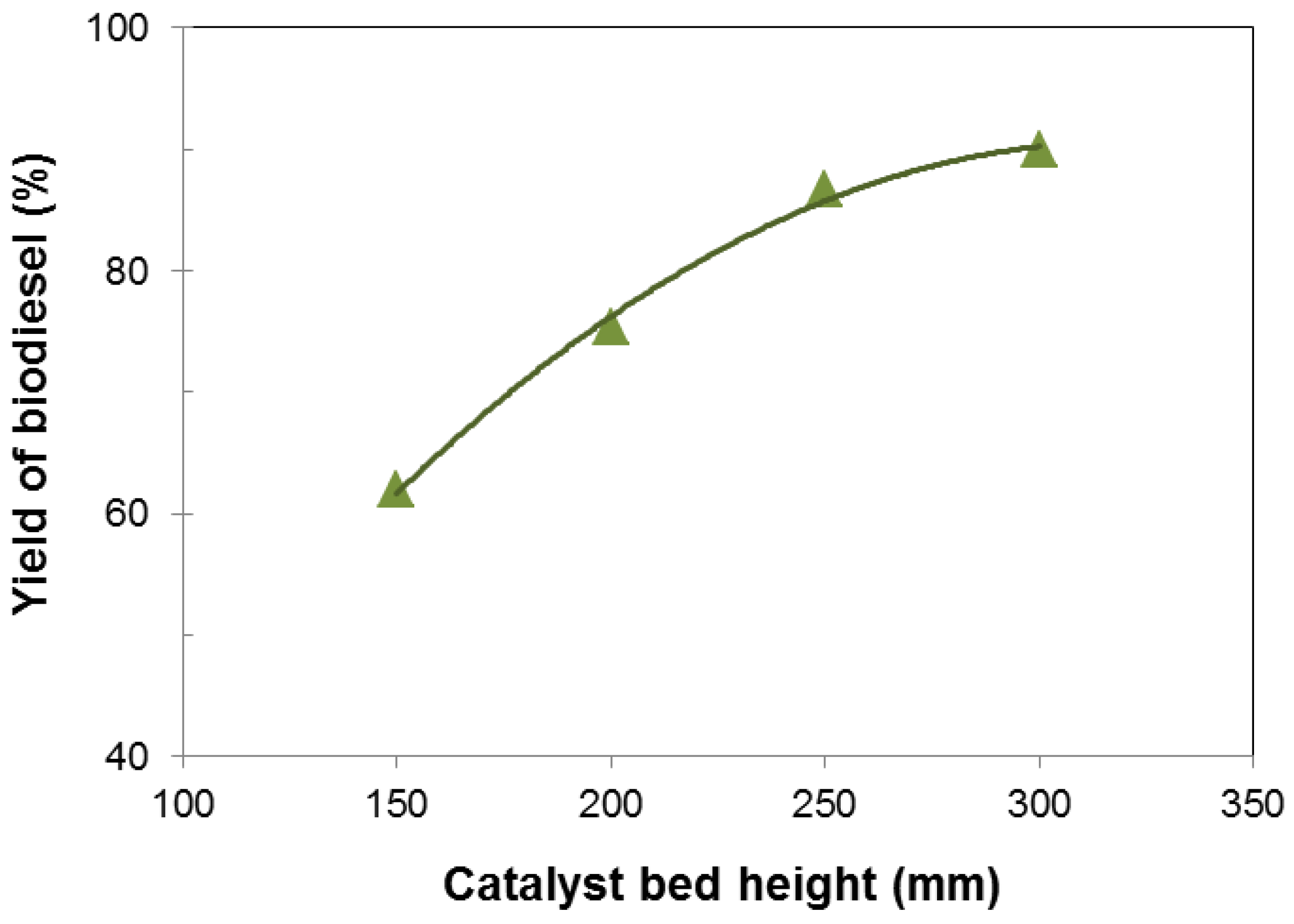
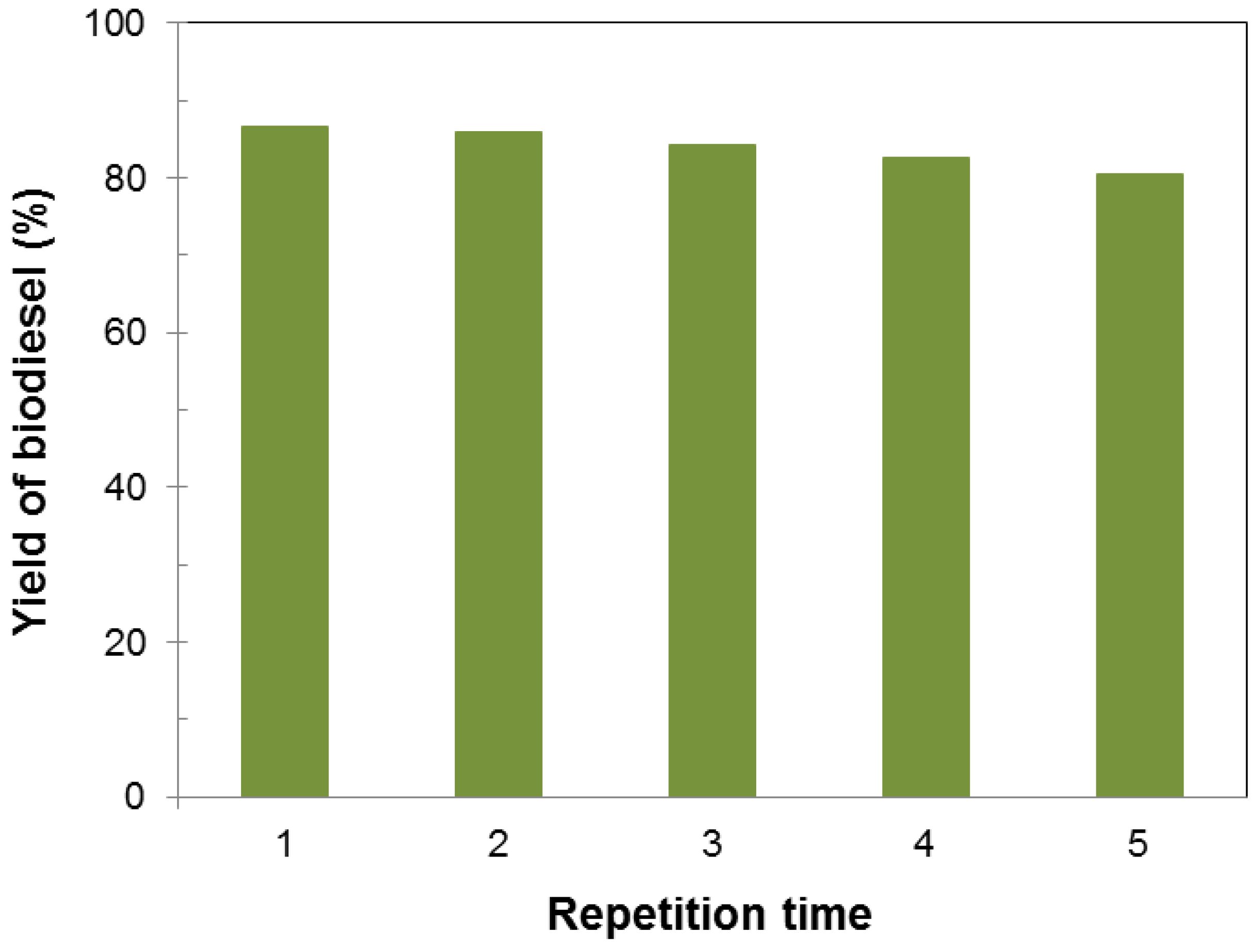
3.3. Characterization and Fuel Properties of Biodiesel
| Fuel Properties | Biodiesel in This Work |
|---|---|
| Kinematic viscosity (mm2/s @ 40 °C) | 4.9 |
| Density (g/mL @ 15 °C) | 0.874 |
| Flash Point (°C) | 166 |
| Cloud Point (°C) | 11 |
| Pour Point (°C) | 7 |
| Acid value (mg KOH/g oil) | 0.80 |
| Water content (%) | 0.03 |
4. Conclusions
Acknowledgments
References
- Jiang, J.J.; Tan, C.S. Biodiesel production from coconut oil in supercritical methanol in the presence of cosolvent. J. Taiwan Inst. Chem. Eng. 2012, 43, 102–107. [Google Scholar] [CrossRef]
- Manh, D.V.; Chen, Y.H.; Chang, C.C.; Chang, M.C.; Chang, C.Y. Biodiesel production from Tung oil and blended oil via ultrasonic transesterification process. J. Taiwan Inst. Chem. Eng. 2011, 42, 640–644. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, C.; Wang, W.; Wu, Y.; Yu, F.; Chi, R.; Zhang, J. Continuous production of biodiesel from soybean oil using supercritical methanol in a vertical tubular reactor: I. Phase holdup and distribution of intermediate product along the axial direction. Chin. J. Chem. Eng. 2010, 18, 626–629. [Google Scholar]
- Gan, M.; Pan, D.; Ma, L.; Yue, E.; Hong, J. The kinetics of the esterification of free fatty acids in waste cooking oil using Fe2(SO4)3/C catalyst. Chin. J. Chem. Eng. 2009, 17, 83–87. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zhang, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Simasatitkul, L.; Siricharnsakunchai, P.; Patcharavorachot, Y.; Assabumrungrat, S.; Arpornwichanop, A. Reactive distillation for biodiesel production from soybean oil. Korean J. Chem. Eng. 2011, 28, 649–655. [Google Scholar]
- Vicente, G.; Martinez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid amorphous-zirconia catalysis in fixed bed reactor. Biomass Bioenerg. 2006, 30, 870–873. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, Y.H.; Lin, R.H.; Shang, N.C.; Chang, C.Y.; Chia-Chi Chang, C.C.; Chiang, P.C.; Hud, C.Y. Biodiesel production in a rotating packed bed using K/γ-Al2O3 solid catalyst. J. Taiwan Inst. Chem. Eng. 2011, 42, 937–944. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar]
- Hameed, B.H.; Goh, C.S.; Chin, L.H. Process optimization for methyl ester production from waste cooking oil using activated carbon supported potassium fluoride. Fuel Process. Technol. 2009, 90, 1532–1537. [Google Scholar]
- Wang, J.; Chen, K.; Chen, C. Biodiesel production from soybean oil catalyzed by K2SiO3/C. Chin. J. Catal. 2011, 32, 1592–1596. [Google Scholar] [CrossRef]
- Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew Energy 2009, 34, 1145–1150. [Google Scholar]
- Fang, L.; Zhang, K.; Li, X.; Wu, H.; Wu, P. Preparation of a carbon-silica mesoporous composite functionalized with sulfonic acid groups and its application to the production of biodiesel. Chin. J. Catal. 2012, 33, 114–122. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Process. Technol. 2010, 91, 1378–1385. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Utilization of biodiesel waste as a renewable resource for activated carbon: Application to environmental problems. Renew. Sustain. Energy Rev. 2009, 13, 2495–2504. [Google Scholar] [CrossRef]
- Nunes, A.A.; Franca, A.S.; Oliveira, L.S. Activated carbons from waste biomass: An alternative use for biodiesel production solid residues. Bioresour. Technol. 2009, 100, 1786–1792. [Google Scholar] [CrossRef]
- Kurniawan, A.; Ismadji, S. Potential utilization of Jatropha curcas L. press-cake residue as new precursor for activated carbon preparation: Application in methylene blue removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2011, 42, 826–836. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Gratuito, M.K.B.; Panyathanmaporn, T.; Chumnanklang, R.A.; Sirinuntawittaya, N.; Dutta, A. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar]
- Tongpoothorn, W.; Sriuttha, M.; Homchan, P.; Chanthai, S.; Ruangviriyachai, C. Preparation of activated carbon derived from Jatropha curcas fruit shell by simple thermo-chemical activation and characterization of their physico-chemical properties. Chem. Eng. Res. Des. 2011, 89, 335–340. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. A packed bed membrane reactor for production of biodiesel using activated carbon supported catalyst. Bioresour. Technol. 2011, 102, 1095–1102. [Google Scholar]
- Alba-Rubio, A.C.; Vila, F.; Alonso, D.M.; Ojeda, M.; Mariscal, R.; Granados, M.L. Deactivation of organosulfonic acid functionalized silica catalysts during biodiesel synthesis. Appl. Catal. Environ. 2010, 95, 279–287. [Google Scholar] [CrossRef]
- Jiang, S.T.; Zhang, F.J.; Pan, L.J. Sodium phosphate as a solid catalyst for biodiesel preparation. Braz. J. Chem. Eng. 2010, 27, 137–144. [Google Scholar]
- Pechyen, C.; Atong, D.; Ahtong, D.; Sricharoenchaikul, V. Investigation of pyrolyzed chars from physic nut waste for the preparation of activated carbon. J. Solid Mech. Mater. Eng. 2007, 1, 498–507. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Nakweang, C. Preparing activated carbon from palm shell for biodiesel fuel production. Chiang Mai J. Sci. 2011, 38, 572–578. [Google Scholar]
- Feng, Y.; Zhang, A.; Li, J.; He, B. A continuous process for biodiesel production in a fixed bed reactor packed with cation-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2011, 102, 3607–3609. [Google Scholar]
- Lu, P.; Yuan, Z.; Li, L.; Wang, Z.; Luo, W. Biodiesel from different oil using fixed-bed and plug-flow reactors. Renew Energy 2010, 35, 283–287. [Google Scholar]
- Samart, C.; Sreetongkittikul, P.; Sookman, C. Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica. Fuel Process. Technol. 2009, 90, 922–925. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2007, 86, 906–910. [Google Scholar] [CrossRef]
- Bo, X.; Guomin, X.; Lingfeng, C.; Ruiping, W.; Lijing, G. Transesterification of palm oil with methanol to biodiesel over a KF/Al2O3 heterogeneous base catalyst. Energy Fuels 2007, 21, 3109–3112. [Google Scholar]
- Wan, T.; Yu, P.; Gong, S.; Li, Q.; Luo, Y. Application of KF/MgO as a heterogeneous catalyst in the production of biodiesel from rapeseed oil. Korean J. Chem. Eng. 2008, 25, 998–1003. [Google Scholar] [CrossRef]
- Long, T.; Deng, Y.; Gan, S.; Chen, J. Application of choline chloride·xZnCl2 ionic liquids for preparation of biodiesel. Chin. J. Chem. Eng. 2010, 18, 322–327. [Google Scholar]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Pin-Ngern, K.; Tonprasert, N.; Dangnuan, S. Production of fatty acid methyl ester by esterification of waste frying oil with methanol using acidified silica as heterogeneous. J. Biobased Mater. Bioenerg. 2012, in press. [Google Scholar]
- Buasri, A.; Ksapabutr, B.; Panapoy, M.; Chaiyut, N. Synthesis of biofuel from palm stearin using an activated carbon supported catalyst in packed column reactor. Adv. Sci. Lett. 2012, in press. [Google Scholar]
- Buasri, A.; Ksapabutr, B.; Panapoy, M.; Chaiyut, N. Biodiesel production from waste cooking palm oil using calcium oxide supported on activated carbon as catalyst in a fixed bed reactor. Korean J. Chem. Eng. 2012, 29, pp. 1–5. Available online: http://www.springer.com/chemistry/journal/11814 (accessed on 24 May 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Rodklum, C.; Chaikwan, T.; Kumphan, N. Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst. Appl. Sci. 2012, 2, 641-653. https://doi.org/10.3390/app2030641
Buasri A, Chaiyut N, Loryuenyong V, Rodklum C, Chaikwan T, Kumphan N. Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst. Applied Sciences. 2012; 2(3):641-653. https://doi.org/10.3390/app2030641
Chicago/Turabian StyleBuasri, Achanai, Nattawut Chaiyut, Vorrada Loryuenyong, Chao Rodklum, Techit Chaikwan, and Nanthakrit Kumphan. 2012. "Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst" Applied Sciences 2, no. 3: 641-653. https://doi.org/10.3390/app2030641