New Trends in Dental Biomechanics with Photonics Technologies
Abstract
:1. Introduction
2. Conventional Technologies
3. Photonic Technologies
3.1. Photoelasticity
3.1.1. Working Principles
- For stress (or strain) induced birefringence, the normally-incident polarized light is split into two components along the principal stress directions in a plane perpendicular to the direction of light propagation and are transmitted only along these planes through the model.
- The velocities of light transmission along these directions are directly proportional to the intensities of the respective principal stresses.
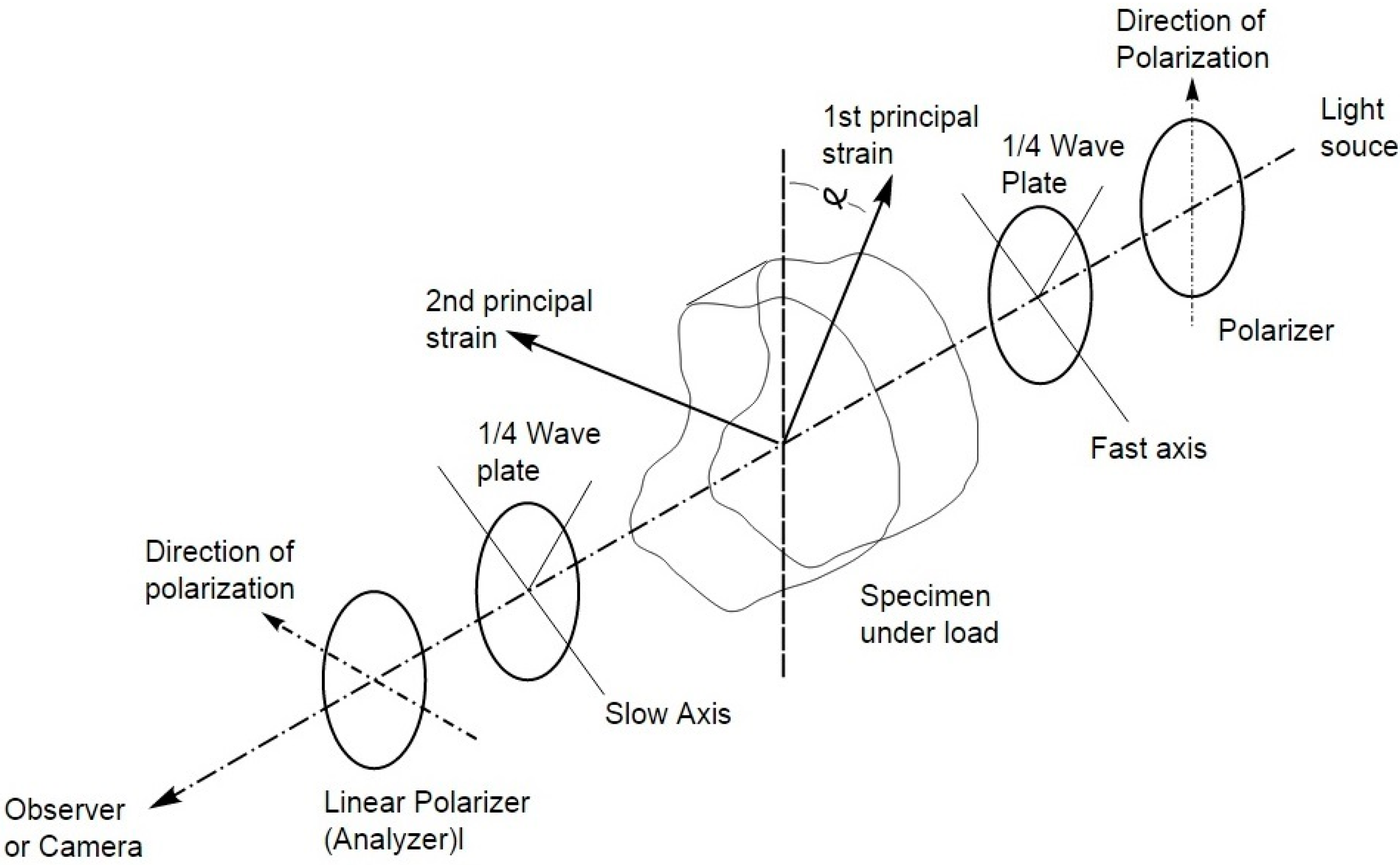
3.1.2. Dental Applications
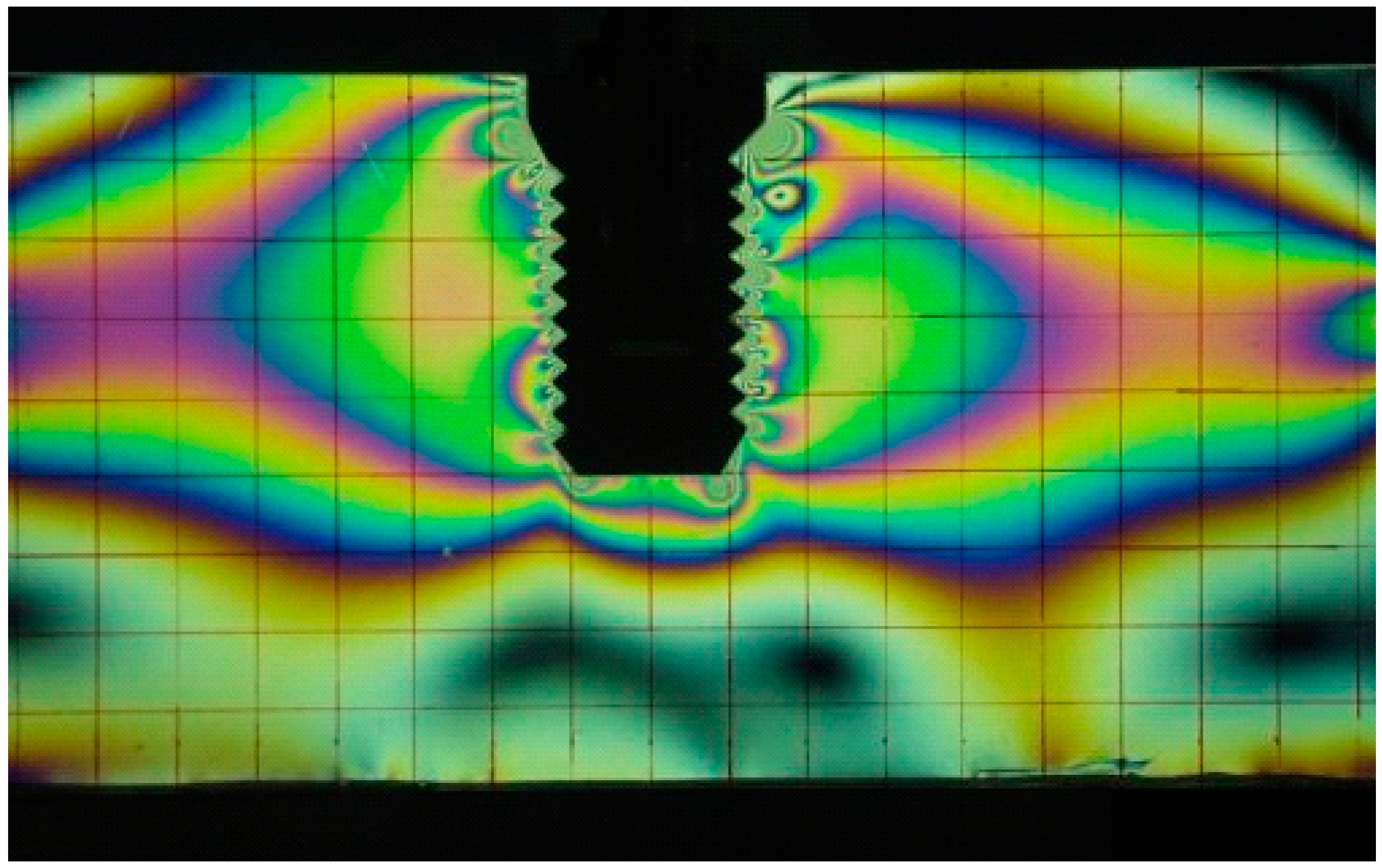
3.2. Interferometric Techniques
3.2.1. Moiré Interferometry
3.2.2. Electronic Speckle Pattern Interferometry (ESPI)
3.3. LASERS
3.3.1. Working Principles
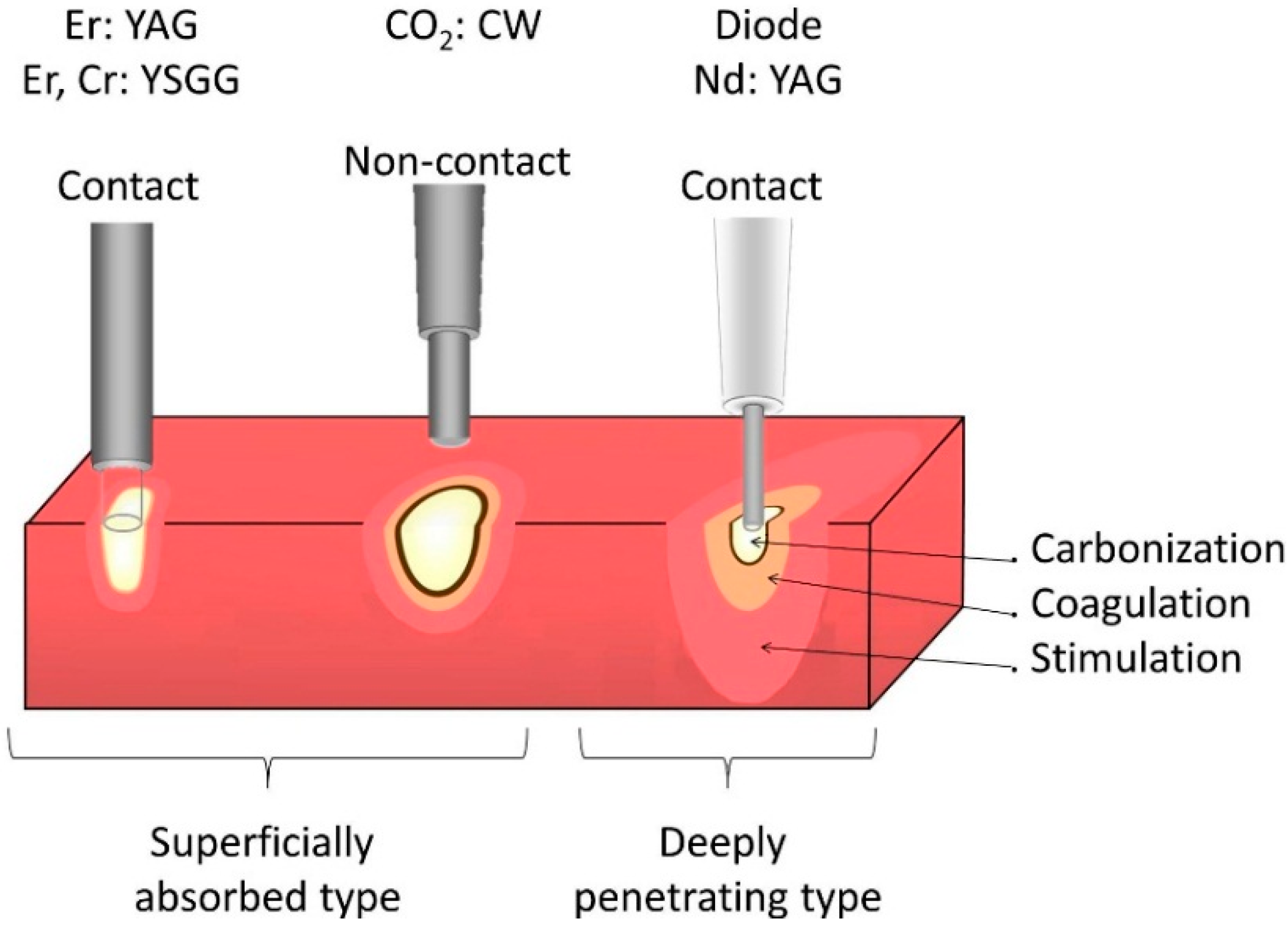
3.3.2. Dental Applications
3.4. Optical Coherence Tomography (OCT)
3.4.1. Working Principles
3.4.2. Dental Applications
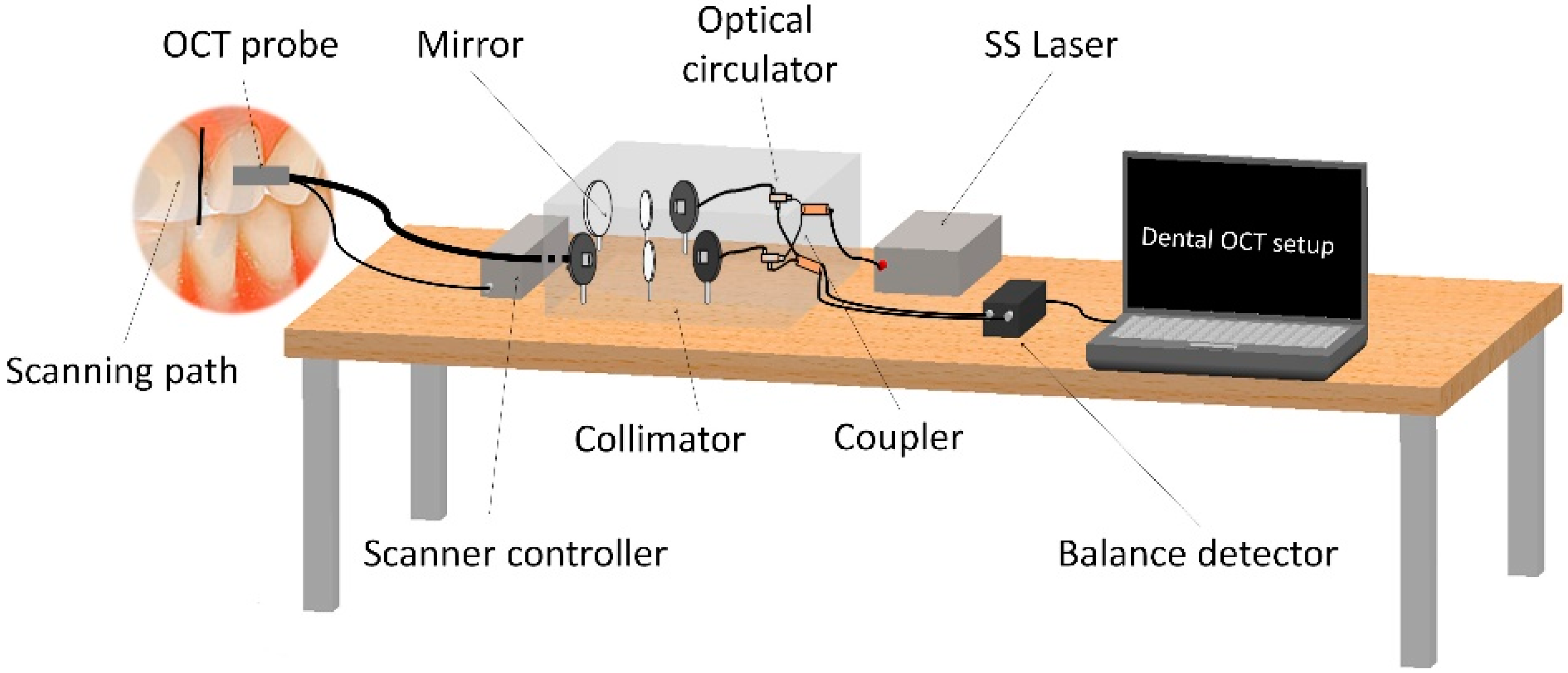
3.5. Fiber Optic Sensors (FOS)
3.5.1. Working Principles
3.5.2. Dental Applications
Prosthetics

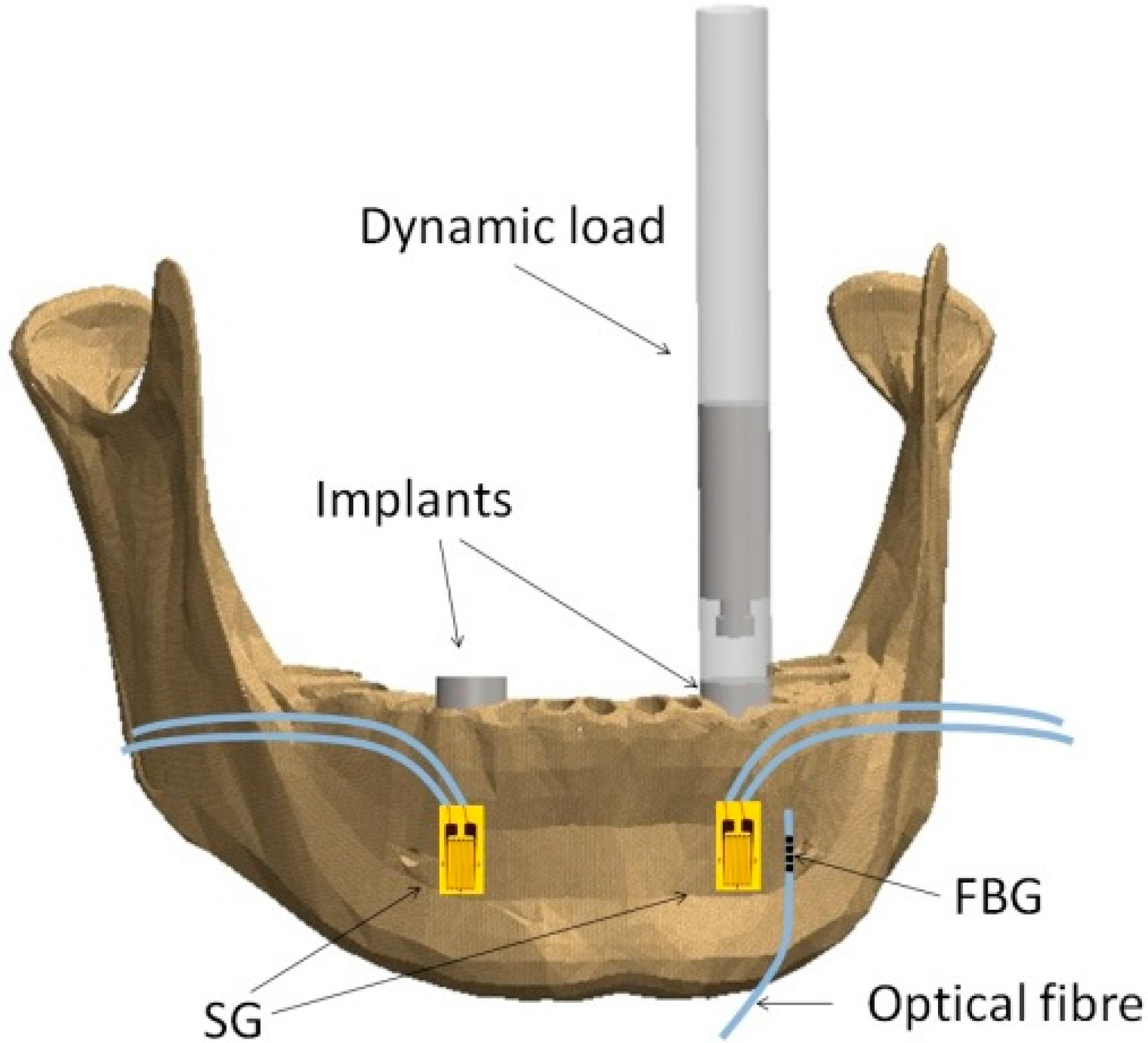
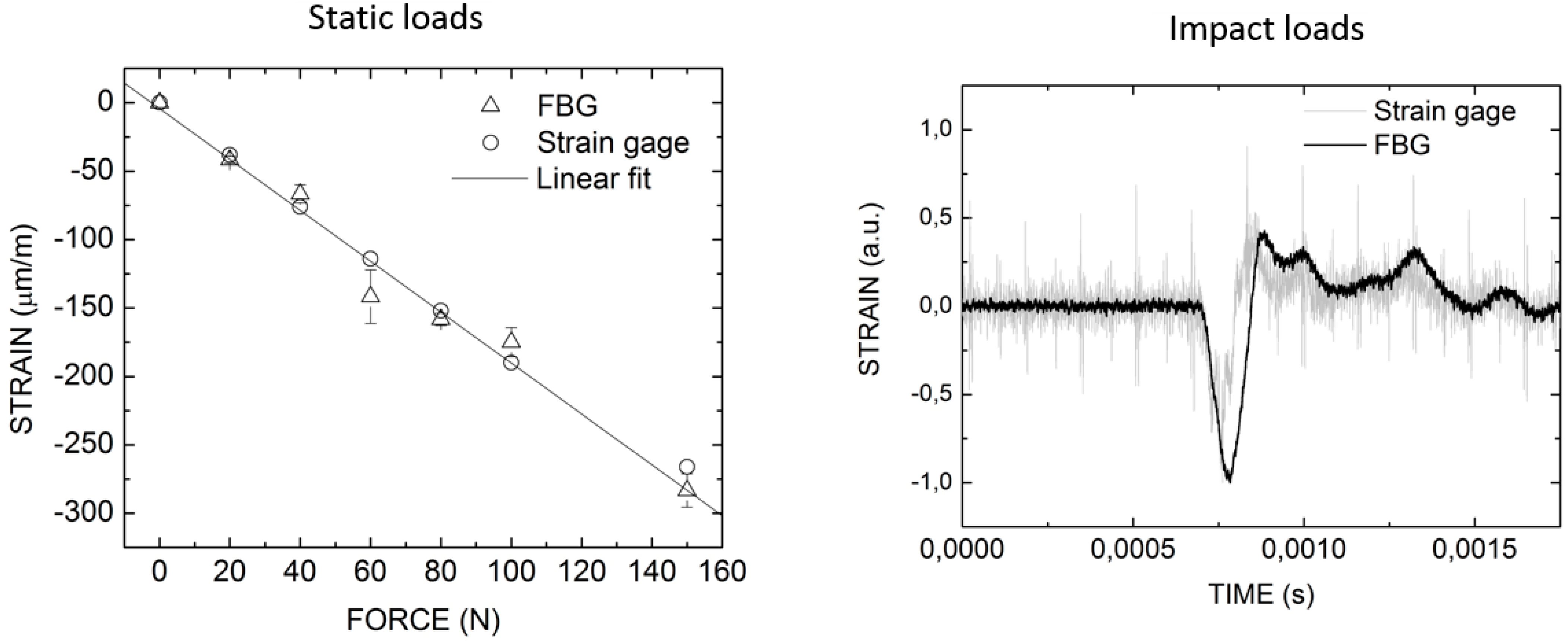
Implantology
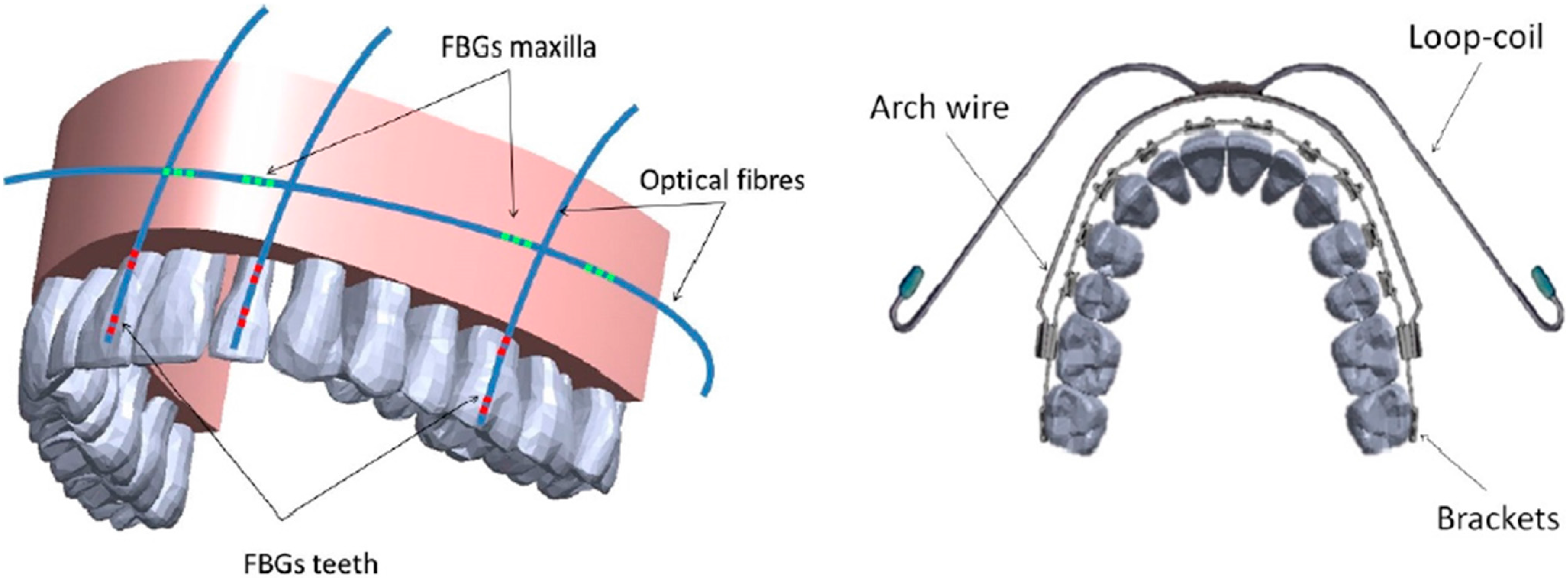
Orthodontics
Dental Materials
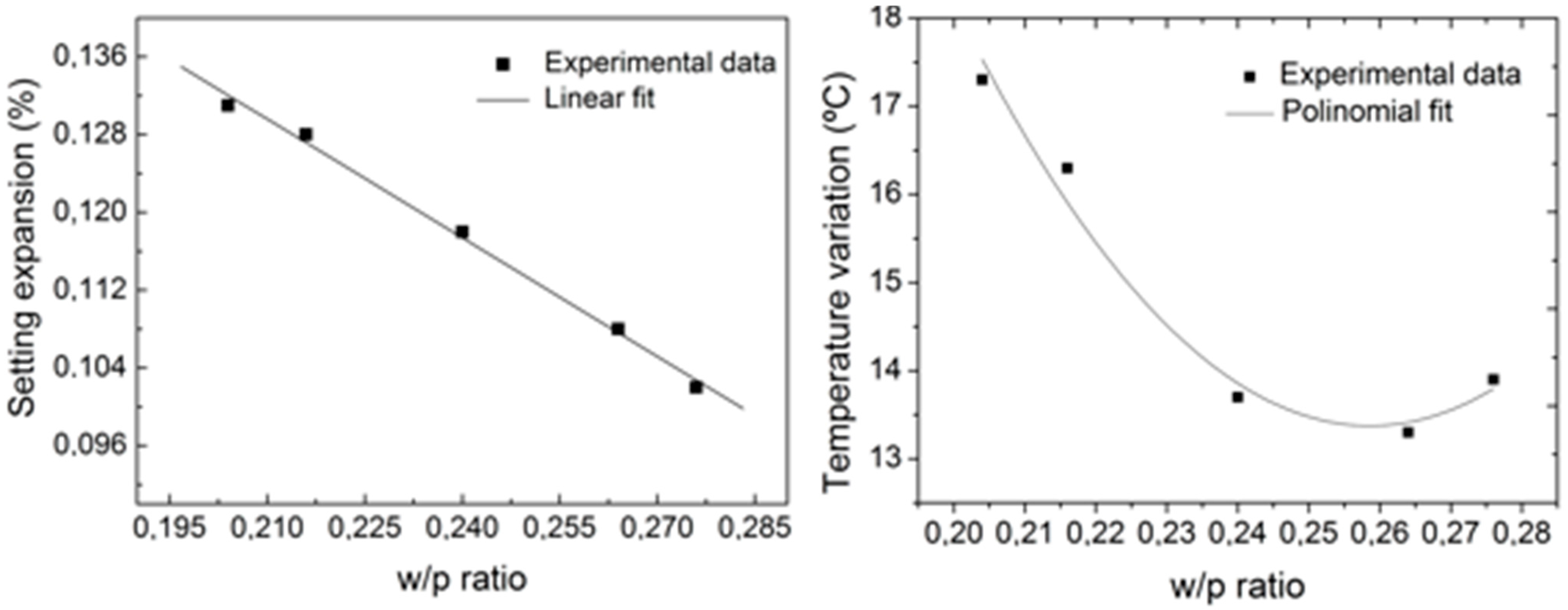
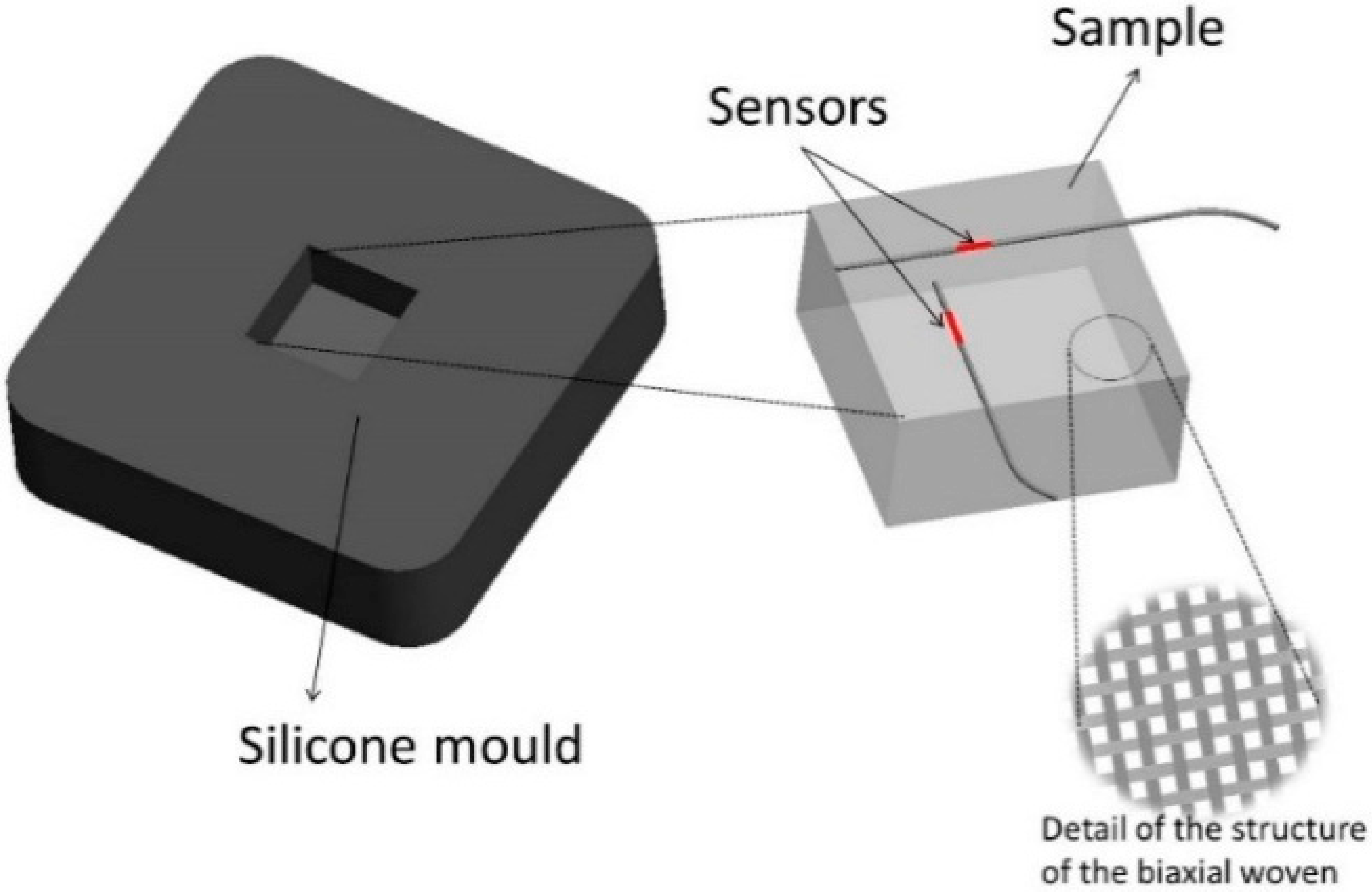
Other Applications
4. Final Remarks
| Photonic Technology | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Photoelasticity [29,30,31,32,33,34,35,36,38,39,40,41,42,43,44,45] |
|
|
|
| Moiré Fringe [52,53,54] |
|
|
|
| ESPI [56,58,59,61,62,63,64,65,66,67,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
|
|
|
| Lasers [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] |
|
|
|
| OCT [104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] |
|
|
|
| FOS [103,104,105,106,107,108,109,110,111,112,113,114,116,117,118,119,120,121,122,124,125,126,127,128,129,130,131,132,133,134,135,137,138,139] |
|
|
|
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lynch, C.D.; O’Sullivan, V.R.; McGillycuddy, C.T. Pierre Fauchard: The father of modern dentistry. Br. Dent. J. 2006, 201, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, B.R. Applications of Interferometry and Optical Metrology in Dentistry. In Applications of Optics in Medicine and Biology; Herron, R.E., Ed.; SPIE: San Diego, CA, USA, 1977. [Google Scholar]
- Zhou, Z.R.; Zheng, J. Tribology of dental materials: A review. J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Morin, D.L.; Douglas, W.H.; Cross, M.; DeLong, R. Biophysical stress analysis of restored teeth: Experimental strain measurement. Dent. Mater. 1988, 4, 41–48. [Google Scholar] [CrossRef]
- Perez-Gonzalez, A.; Gonzalez-Lluch, C.; Sancho-Bru, J.L.; Cervantes, P.; Iserte-Vilar, J. Biomechanical Models of Endodontic Restorations. In Theoretical Biomechanics; Klika, V., Ed.; InTech: Vukovar, Croatia, 2011; pp. 133–160. [Google Scholar]
- Cerqueira, N.M.; Özcan, M.; Dent, M.; Gonçalves, M.; da Rocha, D.M.; Vasconcellos, D.K.; Bottino, M.A.; Yener-Salihoğlu, E. A strain gauge analysis of microstrain induced by various splinting methods and acrylic resin types for implant impressions. Int. J. Maxillofac. Implant. 2012, 27, 341–345. [Google Scholar]
- Carvalho, L.; Vaz, M.A.; Simões, J.A. Mandibular strains induced by conventional and novel dental implants. J. Strain Anal. Eng. 2004, 39, 291–297. [Google Scholar] [CrossRef]
- Şahin, S.; Çehreli, M.C.; Yalçin, E. The influence of functional forces on the biomechanics of implant-supported prostheses—A review. J. Dent. 2002, 30, 271–282. [Google Scholar] [CrossRef]
- Craig, R.G.; Peyton, F. Measurement of strains in fixed bridges with electronic strain gauges. J. Dent. Res. 1967, 46, 615–619. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Enamel-A “metallic-like” deformable biocomposite. J. Dent. 2007, 35, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Dauvillier, B.S.; Feilzer, A.J.; de Gee, A.J.; Davidson, C.L. Visco-elastic parameters of dental restorative materials during setting. J. Dent. Res. 2000, 79, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Attar, N.; Tam, L.E.; McComb, D. Mechanical and physical properties of contemporary dental luting agents. J. Prosthet. Dent. 2003, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yap, A.; Koh, W.; Tsai, K.; Lim, C. Measurement of Poisson's ratio of dental composite restorative materials. Biomaterials 2004, 25, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Khera, S.C.; Ralston, J.L.; Chang, K.H. Stresses at the dentinoenamel junction of human teeth—A finite element investigation. J. Prosthet. Dent. 1991, 66, 541–549. [Google Scholar] [CrossRef]
- Versluis, A.; Tantbirojn, D.; Pintado, M.R.; DeLong, R.; Douglas, W.H. Residual shrinkage stress distributions in molars after composite restoration. Dent. Mater. 2004, 20, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Magne, P. Efficient 3D finite element analysis of dental restorative procedures using micro-CT data. Dent. Mater. 2007, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.N.; Pavan, P.G.; Ruggero, A.L. Evaluation of stress induced in peri-implant bone tissue by misfit in multi-implant prosthesis. Dent. Mater. 2006, 22, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Tan, K.; Liu, G. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [PubMed]
- El-Anwar, M.; El-Zawahry, M. A three dimensional finite element study on dental implant design. J. Gen. Eng. Biotechnol. 2011, 9, 77–82. [Google Scholar] [CrossRef]
- Maceri, F.; Martignoni, M.; Vairo, G. Mechanical behaviour of endodontic restorations with multiple prefabricated posts: A finite-element approach. J. Biomech. 2007, 40, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Pegoretti, A.; Fambri, L.; Zappini, G.; Bianchetti, M. Finite element analysis of a glass fibre reinforced composite endodontic post. Biomaterials 2002, 23, 2667–2682. [Google Scholar] [CrossRef]
- Ho, M.; Lee, S.; Chen, H.; Lee, M. Three-dimensional finite element analysis of the effects of posts on stress distribution in dentin. J. Prosthet. Dent. 1994, 72, 367–372. [Google Scholar] [CrossRef]
- Zak, B. Photoelastic analysis in der orthodontischen mechanik. Oesterr. Z Stomatol. 1935, 35, 22–37. [Google Scholar]
- Noonan, M.A. The use of photoelasticity in a study of cavity preparations. J. Dent. Child 1949, 16, 24–28. [Google Scholar] [PubMed]
- Mahler, D.B.; Peyton, F.A. Photoelasticity as a research technique for analyzing stresses in dental structures. J. Dent. Res. 1955, 34, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Farah, J.W.; Craig, R.G.; Sikarskie, D.L. Photoelastic and finite element stress analysis of a restored axisymmetric first molar. J. Biomech. 1973, 6, 511–520. [Google Scholar] [CrossRef]
- Lehman, M.L.; Meyer, M.L. Relationship of dental caries and stress: Concentrations in teeth as revealed by photoelastic tests. J. Dent. Res. 1966, 45, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Assunção, W.G.; Barão, V.; Tabata, L.; Gomes, E.; Delben, J.; dos Santos, P. Biomechanics studies in dentistry: Bioengineering applied in oral implantology. J. Craniofac. Surg. 2009, 20, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G.; El-Ebrashi, M.K.; LePeak, P.J.; Peyton, F.A. Experimental stress analysis of dental restorations: Part I. Two-dimensional photoelastic stress analysis of inlays. J. Prosthet. Dent. 1967, 17, 277–291. [Google Scholar] [CrossRef]
- Farah, J.W.; Craig, R.G. Reflection photoelastic stress analysis of a dental bridge. J. Dent. Res. 1971, 50, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Mattison, G.D. Photoelastic stress analysis of cast-gold endodontic posts. J. Prosthet. Dent. 1982, 48, 407–411. [Google Scholar] [CrossRef]
- Johnson, E.; Castaldi, C.; Gau, D.; Wysocki, G. Stress pattern variations in operatively prepared human teeth, studied by three-dimensional photoelasticity. J. Dent. Res. 1968, 47, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Standlee, J.P.; Caputo, A.A.; Hanson, E.C. Retention of endodontic dowels: Effects of cement, dowel length, diameter, and design. J. Prosthet. Dent. 1978, 39, 401–405. [Google Scholar] [CrossRef]
- Asundi, A.; Kishen, A. A strain gauge and photoelastic analysis of in vivo strain and in vitro stress distribution in human dental supporting structures. Arch. Oral Biol. 2000, 45, 543–550. [Google Scholar] [CrossRef]
- Asundi, A.; Kishen, A. Advanced digital photoelastic investigations on the tooth-bone interface. J. Biomed. Opt. 2001, 6, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, J.F.; Caputo, A.; Furstman, L. Root tipping: A photoelastic-histopathologic correlation. Am. J. Orthod. 1975, 67, 1–10. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Zhang, J. Photoelastic study of the effects of occlusal surface morphology on tooth apical vertical bite forces. J. Contemp. Dent. Pract. 2004, 5, 74–93. [Google Scholar] [PubMed]
- Carvalho, L.; Merino, J.; Vaz, M.A.; Simões, J.A. A photoelastic analysis to assess the role of dental implant design and its relation to occlusion loads transferred to the surrounding bone. In Proceeding of the 46 eme Congrès Groupement International pour la Recherche Scientifique en Stomatologie et Odontologie, Paredes, Portugal, 25–27 April 2002; pp. 66–67.
- Caputo, A.A.; Standlee, J.P. Biomechanics in Clinical Dentistry; Quintessence Pub. Co.: Chicago, IL, USA, 1987. [Google Scholar]
- Cehreli, M.; Duyck, J.; de Cooman, M.; Puers, R.; Naert, I. Implant design and interface force transfer: A photoelastic and strain-gauge analysis. Clin. Oral Implant. Res. 2004, 15, 249–257. [Google Scholar] [CrossRef]
- Aguiar, F.J.; Tiossi, R.; Macedo, A.; Mattos, M.; Ribeiro, R.; Rodrigues, R. Photoelastic analysis of stresses transmitted by universal cast to long abutment on implant-supported single restorations under static occlusal loads. J. Craniofac. Surg. 2012, 23, 2019–2023. [Google Scholar] [PubMed]
- Ochiai, K.; Ozawa, S.; Caputo, A.; Nishimura, R. Photoelastic stress analysis of implant-tooth connected prostheses with segmented and nonsegmented abutments. J. Prosthet. Dent. 2003, 89, 495–502. [Google Scholar] [CrossRef]
- Celik, G.; Uludag, B. Photoelastic stress analysis of various retention mechanisms on 3-implant-retained mandibular overdentures. J. Prosthet. Dent. 2007, 97, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Markarian, R.A.; Ueda, C.; Sendyk, C.; Laganá, D.; Souza, R. Stress distribution after installation of fixed frameworks with marginal gaps over angled and parallel implants: A photoelastic analysis. J. Prosthodont. 2007, 16, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kinomoto, Y.; Torii, M.; Takeshige, F.; Ebisu, S. Polymerization contraction stress of resin composite restorations in a model class I cavity configuration using photoelastic analysis. J. Esthet. Restor. Dent. 2000, 12, 309–319. [Google Scholar] [CrossRef]
- Asvanund, P.; Morgano, S.M. Photoelastic stress analysis of different prefabricated post-and-core materials. Dent. Mater. J. 2011, 30, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Soltani, F.; Vafaei, F.; Khoshhal, M.; Ayatollahi, M.R.; Soltani, N.; Nejati, M. Comparison of stresses induced by Fiber Post, Parapost and Casting Post in root canals by Photoelasticity method. Iran. Endod. J. 2010, 5, 11–16. [Google Scholar] [PubMed]
- Kishen, A.; Asundi, A. Photomechanical investigations on post endodontically rehabilitated teeth. J. Biomed. Opt. 2002, 7, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.I.; Condos, S.; Musikant, B.L.; Deutsch, A.S. Pilot study comparing the photoelastic stress distribution for four endodontic post systems. J. Oral Rehabill. 1996, 23, 679–685. [Google Scholar] [CrossRef]
- Wood, J.D.; Wang, R.; Weiner, S.; Pashley, D.H. Mapping of tooth deformation caused by moisture change using moiré interferometry. Dent. Mater. 2003, 19, 159–166. [Google Scholar] [CrossRef]
- Wang, R.Z.; Weiner, S. Strain-structure relations in human teeth using Moiré fringes. J. Biomech. 1998, 31, 135–141. [Google Scholar] [CrossRef]
- Wouters, F.R.; Jon-And, C.; Abramson, N.; Olsson, L.; Frithiof, L.; Söder, P.O.; Dirtoft, I. Measurement of gingival swelling from dental casts by generation of a moiré pattern with laser light. J. Dent. Res. 1988, 67, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Madjarova, V.; Kadono, H.; Toyooka, S. Dynamic electronic speckle pattern interferometry (DESPI) phase analyses with temporal Hilbert transform. Opt. Express 2003, 11, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Zhu, L.; Wu, S.; Wang, Y. Review of electronic speckle pattern interferometry (ESPI) for three dimensional displacement measurement. Chin. J. Mech. Eng. 2014, 27, 1–13. [Google Scholar] [CrossRef]
- Jacquot, P. Speckle interferometry: A review of the principal methods in use for experimental mechanics applications. Strain 2008, 44, 57–69. [Google Scholar] [CrossRef]
- Lang, H.; Rampado, M.; Müllejans, R.; Raab, W.H. Determination of the dynamics of restored teeth by 3D electronic speckle pattern interferometry. Lasers Surg. Med. 2004, 34, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Tan, A.C.; Quan, C. Non-destructive characterization of resin-based filling materials using Electronic Speckle Pattern Interferometry. Dent. Mater. 2004, 20, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanis, N. The Submicron Dimensional Behavior of Post-Cure Dental Composites: An Electronic Speckle Pattern-Correlation Interferometry Study. Ph.D. Thesis, Medizinische Fakultät Charité, Universitätsmedizin Berlin, Berlin, Germany, 25 October 2013. [Google Scholar]
- Chattah, N.L.; Shahar, R.; Weiner, S. Design strategy of minipig molars using Electronic Speckle Pattern Interferometry: Comparison of deformation under load between the tooth-mandible complex and the isolated tooth. Adv. Mater. 2009, 21, 413–418. [Google Scholar] [CrossRef]
- Zaslansky, P.; Currey, J.D.; Friesem, A.A.; Weiner, S. Phase shifting speckle interferometry for determination of strain and Young᾿s modulus of mineralized biological materials: A study of tooth dentin compression in water. J. Biomed. Opt. 2005, 10. [Google Scholar] [CrossRef] [PubMed]
- Fages, M.; Slangen, P.; Raynal, J.; Corn, S.; Turzo, K.; Margerit, J.; Cuisinier, F.J. Comparative mechanical behavior of dentin enamel and dentin ceramic junctions assessed by speckle interferometry (SI). Dent. Mater. 2012, 28, e229–e238. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Murukeshan, V.M.; Krishnakumar, V.; Asundi, A. Analysis on the nature of thermally induced deformation in human dentine by electronic speckle pattern interferometry (ESPI). J. Dent. 2001, 29, 531–537. [Google Scholar] [CrossRef]
- Shahar, R.; Zaslansky, P.; Barak, M.; Friesem, A.A.; Currey, J.D.; Weiner, S. Anisotropic Poisson᾿s ratio and compression modulus of cortical bone determined by speckle interferometry. J. Biomech. 2007, 40, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Botto, A.P.; Carvalho, L.; Monteiro, J.; Ramos, N.V.; Vaz, M.; Hecke, M.B.; Ustrell, J. Evaluation of the displacements transmitted to a pig jaw, by orthodontic and orthopaedic devices. In Proceeding of the International Conference on BIODENTAL Engineering, Porto, Portugal, 26–27 June 2009.
- Chuang, S.F.; Chen, T.Y.; Chang, C.H. Application of digital image correlation method to study dental composite shrinkage. Strain 2008, 44, 231–238. [Google Scholar] [CrossRef]
- Li, J.; Fok, A.S.L.; Satterthwaite, J.; Watts, D.C. Measurement of the full-field polymerization shrinkage and depth of cure of dental composites using digital image correlation. Dent. Mater. 2009, 25, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.F.; Chang, C.H.; Chen, T.Y. Spatially resolved assessments of composite shrinkage in MOD restorations using a digital-image-correlation technique. Dent. Mater. 2011, 27, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lau, A.; Fok, A.S.L. Application of digital image correlation to full-field measurement of shrinkage strain of dental composites. Appl. Phys. Eng. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Lau, A.; Li, J.; Heo, Y.C.; Fok, A. A study of polymerization shrinkage kinetics using digital image correlation. Dent. Mater. 2015, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Tiossi, R.; de Torres, E.; Rodrigues, R.; Conrad, H.; de Mattos, M.G.; Fok, A.S.; Ribeiro, R.F. Comparison of the correlation of photoelasticity and digital imaging to characterize the load transfer of implant-supported restorations. J. Prosthet. Dent. 2014, 112, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Dickinson, A.; Holst, S.; Holst, A. Biomechanical methods applied in dentistry: A comparative overview of photoelastic examinations, strain gauge measurements, finite element analysis and three-dimensional deformation analysis. Eur. J. Prosthodont. Restor. Dent. 2009, 17, 50–57. [Google Scholar] [PubMed]
- Downs, J.C.; Convissar, R.; Anagnostaki, E.; Sun, G.; Hoopingarner, C.R. Lasers in Fixed Prosthetic and Cosmetic Reconstruction. In Principles and Practice of Laser Dentistry, 2nd ed.; Convissar, R.A., Ed.; Elsevier Inc.: New York, NY, USA, 2016; pp. 89–106. [Google Scholar]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. J. Periodontol. 2015, 68, 217–269. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. The current status of laser applications in dentistry. Aust. Dent. J. 2003, 48, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Dederich, D.N.; Bushick, R.D. Lasers in dentistry. J. Am. Dent. Assoc. 2004, 135, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Cozean, C.; Arcoria, C.; Pelagalli, J.; Powell, G.L. Dentistry for the 21st century? Erbium:YAG laser for teeth. J. Am. Dent. Assoc. 1997, 128, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Seka, W.D.; Featherstone, J.D.B.; Fried, D.; Visuri, S.R.; Walsh, J.T. Laser ablation of dental hard tissue: From explosive ablation to plasma-mediated ablation. In Proceeding of the SPIE 2672, Lasers in Dentistry II, 144, San Jose, CA, USA, 27 January 1996.
- Fried, D.; Zuerlein, M.; Featherstone, J.D.B.; Seka, W.; Duhn, C.; McCormack, S.M. IR laser ablation of dental enamel: Mechanistic dependence on the primary absorber. Appl. Surf. Sci. 1998, 127–129, 852–856. [Google Scholar] [CrossRef]
- Perhavec, T.; Gorkič, A.; Bračun, D.; Diaci, J. A method for rapid measurement of laser ablation rate of hard dental tissue. Opt. Laser Technol. 2009, 41, 397–402. [Google Scholar] [CrossRef]
- Featherstone, J.D.; Nelson, D.G. Laser effects on dental hard tissues. Adv. Dent. Res. 1987, 1, 21–26. [Google Scholar] [PubMed]
- Rao, G.; Tripthi, P.S.; Srinivasan, K. Hemostatic effect of the CO2 laser over excision of an intraoral hemangioma. Int. J. Laser Dent. 2012, 2, 74–77. [Google Scholar] [CrossRef]
- Angeletti, P.; Pereira, M.D.; Gomes, H.C.; Hino, C.T.; Ferreira, L.M. Effect of low-level laser therapy (GaAlAs) on bone regeneration in midpalatal anterior suture after surgically assisted rapid maxillary expansion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shimizu, N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 525–532. [Google Scholar] [CrossRef]
- Krause, L.S.; Cobb, C.M.; Rapley, J.W.; Killoy, W.J.; Spencer, P. Laser irradiation of bone. I. An in vitro study concerning the effects of the CO2 laser on oral mucosa and subjacent bone. J. Periodontol. 1997, 68, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A. Potential applications of Erbium:YAG laser in periodontics. J. Periodontal Res. 2004, 39, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A. Clinical application of Erbium:YAG laser in periodontology. J. Int. Acad. Periodontol. 2008, 10, 22–30. [Google Scholar] [PubMed]
- Sculean, A.; Schwarz, F.; Becker, J. Anti-infective therapy with an Er:YAG laser: Influence on peri-implant healing. Expert Rev. Med. Devices 2005, 2, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Aoki, A.; Oda, S.; Yoneyama, T.; Ishikawa, I. Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J. Clin. Laser Med. Surg. 2003, 21, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Oyster, D.K.; Parker, W.B.; Gher, M.E. CO2 lasers and temperature changes of titanium implants. J. Periodontol. 1995, 66, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.C.; Bernier, J.L.; Sprague, W.G. Laser and the dental pulp. J. Am. Dent. Assoc. 1971, 83, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, K.D.; Klitzman, B.; Burkes, E.J.; Hoke, J.; Moshonov, J. Pulpal response to cavity preparation with the Er:YAG and Mark III free electron lasers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 695–702. [Google Scholar] [CrossRef]
- Wigdor, H.; Abt, E.; Ashrafi, S.; Walsh, J.T. The effect of lasers on dental hard tissues. J. Am. Dent. Assoc. 1993, 124, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. The current status of low level laser therapy in dentistry. Part 2. Hard tissue applications. Aust. Den. J. 1997, 42, 302–306. [Google Scholar] [CrossRef]
- Deppe, H.; Horch, H.-H. Laser applications in oral surgery and implant dentistry. Lasers Med. Sci. 2007, 22, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bitter, K.; Noetzel, J.; Volk, C.; Neumann, K.; Kielbassa, A. Bond strength of fiber posts after the application of Erbium:Yttrium-Aluminum-Garnet Laser treatment and gaseous ozone to the root canal. J. Endodont. 2008, 34, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Dichtl, S.; Hitzenberger, C.K.; Sattmann, H.; Robl, B.; Moritz, A.; Fercher, A.F.; Sperr, W. Polarization-sensitive optical coherence tomography of dental structures. Caries Res. 2000, 34, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical coherence tomography—Principles and applications. Rep. Prog. Phys. 2003, 66. [Google Scholar] [CrossRef]
- Canjau, S.; Todea, C.; Negrutiu, M.L.; Sinescu, C.; Topala, F.I.; Marcauteanu, C.; Manescu, A.; Duma, V.-F.; Bradu, A.; Podoleanu, A.G. Optical coherence tomography for non-invasive ex vivo investigations in dental medicine—A joint group experience (Review). СТМ 2015, 7, 97–115. [Google Scholar] [CrossRef]
- Todea, C.; Negrutiu, M.L.; Balabuc, C.; Sinescu, C.; Topala, F.I.; Marcauteanu, C.; Canjau, S.; Semez, G.; Podoleanu, A.G. Optical coherence tomography applications in dentistry. TMJ 2010, 60, 5–17. [Google Scholar]
- Hsieh, Y.; Ho, Y.; Lee, S.; Chuang, C.; Tsai, J.; Lin, K.; Sun, C.-W. Dental optical coherence tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ho, Y.; Lee, S. Sensing of tooth microleakage based on dental optical coherence tomography. J. Sens. 2015, 2015. [Google Scholar] [CrossRef]
- Todea, C.; Balabuc, C.; Sinescu, C.; Filip, L.; Kerezsi, C.; Calniceanu, M.; Negrutiu, M.; Bradu, A.; Hughes, M.; Podoleanu, A.G. En face optical coherence tomography investigation of apical microleakage after laser-assisted endodontic treatment. Lasers Med. Sci. 2010, 25, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sinescu, C.; Hughes, M.; Bradu, A.; Negrutiu, M.; Todea, C.; Antonie, S.; Laissue, P.; Rominu, M.; Podoleanu, A. Implant bone interface investigated with a non-invasive method: Optical coherence tomography. In Proceedings of Biophotonics: Photonic Solutions for Better Health Care Jürgen Popp, Strasbourg, France, 7 April 2008.
- Sinescu, C.; Negrutiu, M.L.; Todea, C.; Balabuc, C.; Filip, L.; Rominu, R.; Bradu, A.; Hughes, M.; Podoleanu, A.G. Quality assessment of dental treatments using en-face optical coherence tomography. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef] [PubMed]
- Fried, D.; Staninec, M.; Darling, C.; Kang, H.; Chan, K. Monitoring tooth demineralization using a cross polarization optical coherence tomographic system with an integrated MEMS scanner. Proc. SPIE Int. Soc. Opt. Eng. 2012. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Everett, M.; Colston, B.W.; da Silva, L.B.; Otis, L.L. Fiber Optic Based Optical Coherence Tomography (OCT) for Dental Applications; Fourth Pacific Not thwest Fiber Optic Sensor Workshop: Portland, OR, USA, 1998. [Google Scholar]
- Colston, B.; Everett, M.; da Silva, L.; Otis, L.L.; Stroeve, P.; Nathel, H. Imaging of hard- and soft-tissue structure in the oral cavity by optical coherence tomography. Appl. Opt. 1998, 37, 3582–3585. [Google Scholar] [CrossRef] [PubMed]
- Feldchtein, F.; Gelikonov, V.; Iksanov, R.; Gelikonov, G.; Kuranov, R.; Sergeev, A.; Gladkova, N.; Ourutina, M.; Reitze, D.; Warren, J. In vivo OCT imaging of hard and soft tissue of the oral cavity. Opt. Express 1998, 3, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Darling, C.; Fried, D. Nondestructive monitoring of the repair of enamel artificial lesions by an acidic remineralization model using polarization-sensitive optical coherence tomography. Dent. Mater. 2012, 28, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, D.H.B.; Margallo-Balbás, E.; French, P.J.; Pandraud, G.; Breedveld, P.; Dankelman, J. Micro-optics assembly in dental drill as a platform for imaging and sensing during surgical drilling. In Proceedings of the IEEE Sensors, Kona, HI, USA, 1–4 November 2010; pp. 265–268.
- Lin, C.L.; Kuo, W.C.; Chang, Y.H.; Yu, J.J.; Lin, Y.C. Examination of ceramic/enamel interfacial debonding using acoustic emission and optical coherence tomography. Dent. Mater. 2014, 30, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kuo, W.C.; Yu, J.J.; Huang, S.F. Examination of ceramic restorative material interfacial debonding using acoustic emission and optical coherence tomography. Dent. Mater. 2013, 29, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.; Montes, M.; Gomes, A.; Mota, C.; Campello, S.; Freitas, A. Marginal analysis of resin composite restorative systems using optical coherence tomography. Dent. Mater. 2011, 27, e213–e223. [Google Scholar] [CrossRef] [PubMed]
- De Melo, L.S.A.; de Araujo, R.E.; Freitas, A.Z.; Zezell, D.; Vieira, J.N.D.; Girkin, J.; Hall, A.; Carvalho, M.T.; Gomes, A.S. Evaluation of enamel dental restoration interface by optical coherence tomography. J. Biomed. Opt. 2005, 10. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, P.; Palin, W.; Shortall, A.; Wang, R. Time-resolved simultaneous measurement of group index and physical thickness during photopolymerization of resin-based dental composite. J. Biomed. Opt. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Boadi, J.; Fernandes, J.; Mittar, S.; Hearnden, V.; Lu, Z.; MacNeil, S.; Thornhill, M.H.; Murdoch, C.; Hunter, K.D.; Matcher, S.J. Imaging of 3D tissue-engineered models of oral cancer using 890 and 1300 nm optical coherence tomography. CTM 2015, 7, 60–68. [Google Scholar] [CrossRef]
- Tjin, S.C.; Tan, Y.K.; Yow, M.; Lam, Y.Z.; Hao, J. Recording compliance of dental splint use in obstructive sleep apnoea patients by force and temperature modelling. Med. Biol. Eng. Comput. 2001, 39, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Mishra, V.; Bhalla, A.; Singh, N.; Jain, S.C.; Garg, H.; Raviprakash, S.; Grewal, N.; Kapur, P. Fiber Bragg grating sensor for measurement of impact absorption capability of mouthguards. Dent. Traumatol. 2011, 27, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.C.; Ramos, A.; Carvalho, L.; Nogueira, R.N.; Ballu, A.; Mesnard, M. Fibre Bragg grating sensing and finite element analysis of the biomechanics of the mandible. In Proceeding of the 17th International Conference in Optical Fibre Sensors, Bruges, Belgium, 30 August 2005; pp. 102–105.
- Carvalho, L.; Silva, J.C.C.; Nogueira, R.N.; Pinto, J.L.; Kalinowski, H.J.; Simões, J.A. Application of Bragg grating sensors in dental biomechanics. J. Strain Anal. Eng. 2006, 41, 411–416. [Google Scholar] [CrossRef]
- Carvalho, P.; Abe, L.; Schiller, M.W.; Carvalho, L.; Simões, J.A. FEA and Experimental FBG Sensing System for the Analysis of Different Dental Implant Concepts. In Proceeding of the 5th World Congress of Biomechanics, Munich, Germany, 29 July–4 August 2006; p. S568.
- Schiller, M.W.; Abe, I.; Carvalho, P.; Lopes, P.; Carvalho, L.; Nogueira, R.N. On the Use of FBG Sensors to Assess the Performance of a Dental Implant System. In Proceeding of the Optical Fiber Sensors, Cancun, Mexico, 23–26 October 2006.
- Silva, J.; Carvalho, L.; Nogueira, R.; Simões, J.; Pinto, J.; Kalinowski, H. FBG applied in dynamic analysis of an implanted cadaveric mandible. In Proceeding of the Second European Workshop on Optical Fibre Sensors, Santander, Spain, 9–11 June 2004; pp. 226–229.
- Fangaia, S.; Almeida, M.; Carvalho, L.; Nicolau, P. In Vitro Pilot Study on Bone Heating During Surgical Implant Bed Preparation. In Biodental Engineering II; Taylor & Francis Group, Ed.; CRC Press: London, UK, 2013; pp. 93–96. [Google Scholar]
- Milczewski, M.S.; da Silva, J.C.C.; Martelli, C.; Grabarski, L.; Abe, I.; Kalinowski, H.J. Force monitoring in a maxilla model and dentition using optical fiber bragg gratings. Sensors 2012, 12, 11957–11965. [Google Scholar] [CrossRef] [PubMed]
- Milczewski, M.S.; Martelli, C.; Canning, J.; Kalinowski, H.J.; Simões, J.A.; Stevenson, M.; Talaia, P. Orthodontic forces sensing with polymer PCF. In Proceeding of the Third European Workshop on Optical Fibre Sensors, 66191M, Napoly, Italy, 4 July 2007.
- Trannin, P.; Milczewski, M.; de Oliveira, W.; Filho, O.G.; Lopes, S.; Kalinowski, H. Orthodontic mechanics using mini-implant measured by FBG. In Proceeding of the Fifth Asia-Pacific Optical Sensors Conference, Jeju, Korea, 20 July 2015; pp. 96551N–96551N-4.
- Milczeswki, M.S.; Silva, J.C.C.; Abe, I.; Simões, J.A.; Paterno, A.S.; Kalinowski, H.J. Measuring Orthodontic Forces with HiBi FBG Sensors. In Proceding of the Optical Fiber Sensors, Cancun, Mexico, 23 October 2006.
- Carvalho, L.; Roriz, P.; Frazão, O.; Marques, M.B. Evaluation of the performance of orthodontic devices using FBG sensors. J. Phys.: Conf. Ser. 2015, 605. [Google Scholar] [CrossRef]
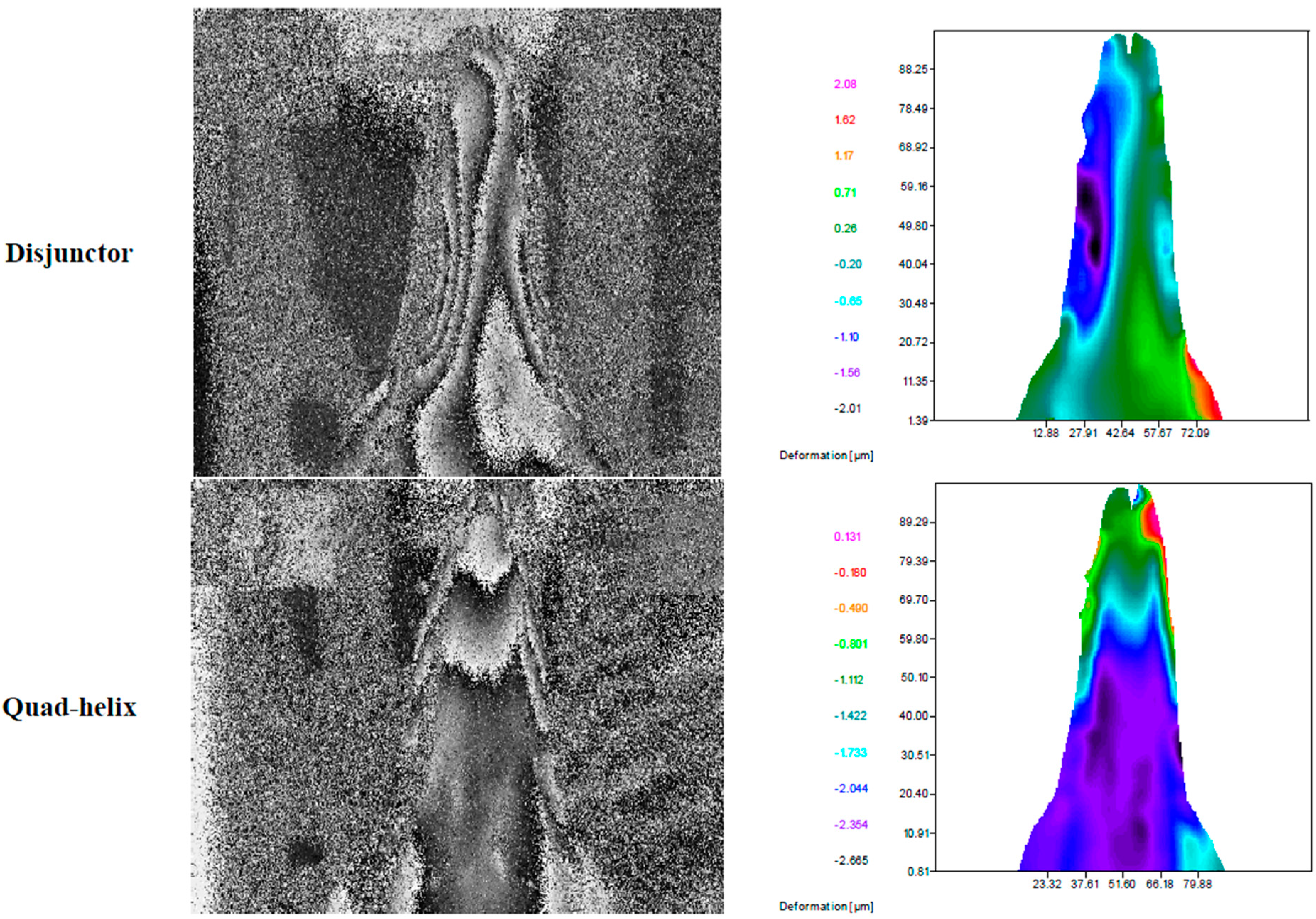
- Botto, A.P.; Carvalho, L.; Ustrell, J.; Pinto, J.L. Evaluation and comparison of strains transmitted to a pig jaw with fiber Bragg grating sensors, by the orthodontic devices Quad-Helix and Disjunctor. In Proceeding of the 54ème Congrès Annuel du GIRSO, La Grande Motte, France, 22–24 April 2010.
- Alberto, N.; Carvalho, L.; Lima, H.; Antunes, P.; Nogueira, R.; Pinto, J.L. Characterization of different water/powder ratios of dental gypsum using fiber Bragg grating sensors. Dent. Mater. J. 2011, 30, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Milczewski, M.S.; da Silva, J.C.C.; Abe, I.; Carvalho, L.; Nogueira, R.N.; Paterno, A.S.; Kalinowski, H.J.; Pinto, J.L. Determination of setting expansion of dental materials using fibre optical sensing. Meas. Sci. Technol. 2006, 17, 1152–1156. [Google Scholar] [CrossRef]
- Ottevaere, H.; Tabak, M.; Bartholomees, F.; de Wilde, W.P. A novel optical characterization technique for monitoring the stress build-up in dental cements. In Proceeding of Symposium IEEE/LEOS Benelux Chapter, 2000, Delft, The Netherlands, 15 July 2014; pp. 83–86.
- Ottevaere, H.; Tabak, M.; Chah, K.; Mégret, P.; Thienpont, H. Dental composite resins: Measuring the polymerization shrinkage using optical fiber Bragg grating sensors. In Proceeding of the SPIE 8439, Optical Sensing and Detection II, Brussels, Belgium, 16 April 2012.
- Anttila, E.J.; Krintilä, O.H.; Laurila, T.K.; Lassila, L.V.J.; Vallittu, P.K.; Hernberg, R.G.R. Evaluation of polymerization shrinkage and hydroscopic expansion of fiber-reinforced biocomposites using optical fiber Bragg grating sensors. Dent. Mater. 2008, 24, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Colston, B.W.; Gutierrez, D.M.; Everett, M.J.; Brown, S.B.; Langry, K.C.; Cox, R.; Johnson, P. W.; Roe, J.N. Intraoral Fiber-Optic-Based Diagnostic for Periodontal Disease. In Proceeding of International Symposium on Biomedical Optics, San Jose, CA, USA, 21–25 February 2000.
- Carvalho, L.; Alberto, N.; Gomes, P.; Nogueira, R.; Pinto, J.L.; Fernandes, M.H. In the trail of a new bio-sensor for measuring strain in bone: Osteoblastic biocompatibility. Biosens. Bioelectron. 2011, 26, 4046–4052. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakih, E.; Osman, N.; Adikan, F. The use of fiber bragg grating sensors in biomechanics and rehabilitation applications: The state-of-the-art and ongoing research topics. Sensors 2012, 12, 12890–12926. [Google Scholar] [CrossRef] [PubMed]
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simões, J.A. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: A review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, N.; Tiwari, U.; Kapur, P. Fiber grating sensors in medicine: Current and emerging applications. Sens. Actuator A: Phys. 2011, 167, 279–290. [Google Scholar] [CrossRef]
- Peterson, J.I.; Vurek, G.G. Fiber-optic sensors for biomedical applications. Science 1984, 224, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Roriz, P.; Frazao, O.; Lobo-Ribeiro, A.B.; Santos, J.L.; Simoes, J.A. Review of fiber-optic pressure sensors for biomedical and biomechanical applications. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Roriz, P.; Ramos, A.; Santos, J.L.; Simões, J.A. Fiber optic intensity-modulated sensors: A review in biomechanics. Photonic Sens. 2012, 2, 315–330. [Google Scholar] [CrossRef]
- Zawawi, M.A.; O᾿Keffe, S.; Lewis, E. Intensity-modulated fiber optic sensor for health monitoring applications: A comparative review. Sens. Rev. 2013, 33, 57–67. [Google Scholar] [CrossRef]
- Elsarnagawy, T.; El-Wakad, M. Investigating the prospective use of fibre-optics-based sensors in dentistry. Technisch. Mess. Tech. Mess. 2008, 75, 207–211. [Google Scholar]
- Keiser, G.; Xiong, F.; Cui, Y.; Shum, P.P. Review of diverse optical fibers used in biomedical research and clinical practice. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, L.; Roriz, P.; Simões, J.; Frazão, O. New Trends in Dental Biomechanics with Photonics Technologies. Appl. Sci. 2015, 5, 1350-1378. https://doi.org/10.3390/app5041350
Carvalho L, Roriz P, Simões J, Frazão O. New Trends in Dental Biomechanics with Photonics Technologies. Applied Sciences. 2015; 5(4):1350-1378. https://doi.org/10.3390/app5041350
Chicago/Turabian StyleCarvalho, Lídia, Paulo Roriz, José Simões, and Orlando Frazão. 2015. "New Trends in Dental Biomechanics with Photonics Technologies" Applied Sciences 5, no. 4: 1350-1378. https://doi.org/10.3390/app5041350





