Enhanced Iron and Selenium Uptake in Plants by Volatile Emissions of Bacillus amyloliquefaciens (BF06)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Assays of Microbial VOCs
2.3. Measurement of Total Chlorophyll Content and Chlorophyll Fluorescence
2.4. Determination of Fe and Se Content
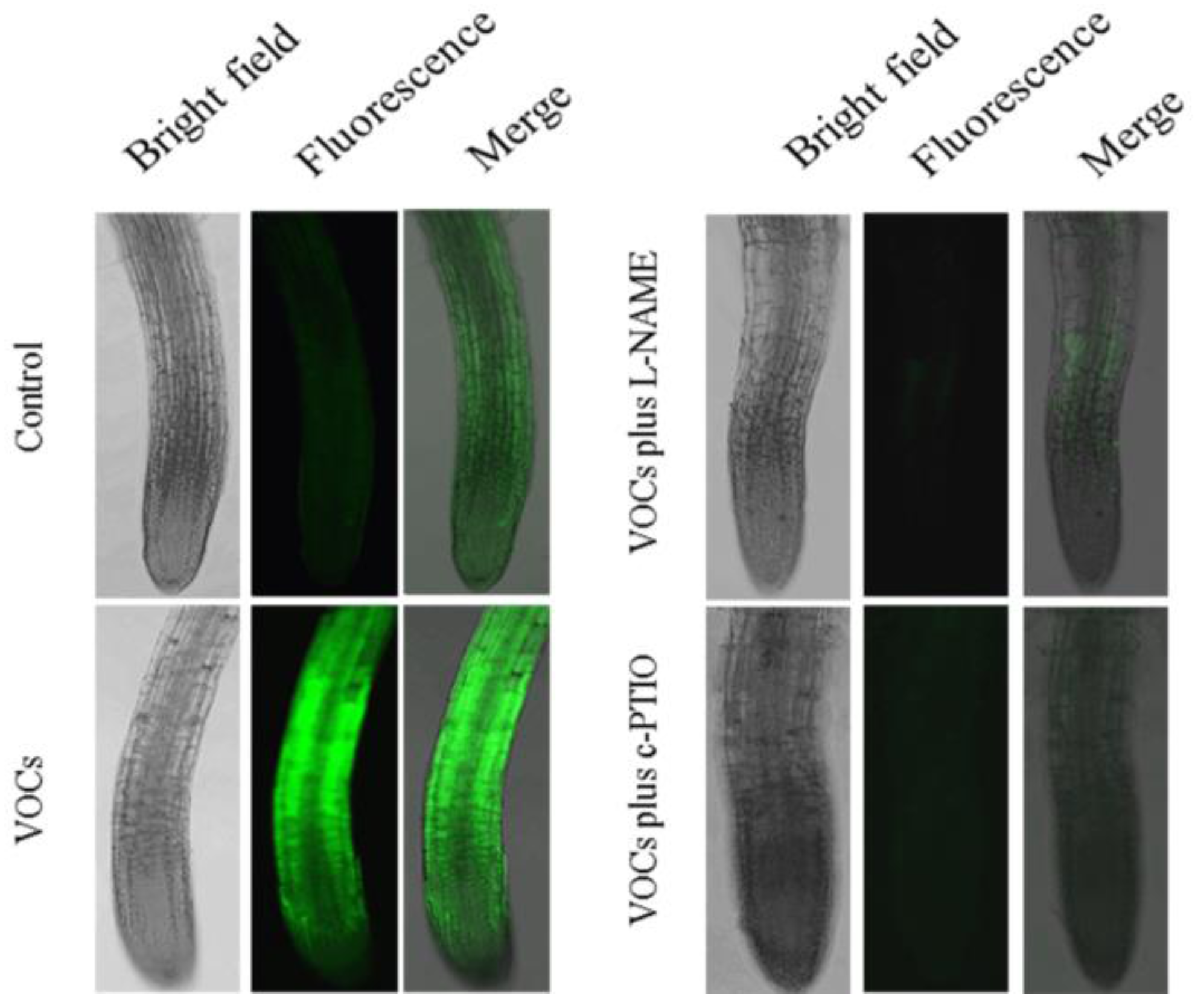
2.5. In Vivo Localization of NO in Plant Roots
2.6. RNA-Sequencing (RNA-Seq) Analysis
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT–PCR) Analyses
2.8. Ferric-Chelate Reductase (FCR) Activity
2.9. Statistical Analyses
3. Results
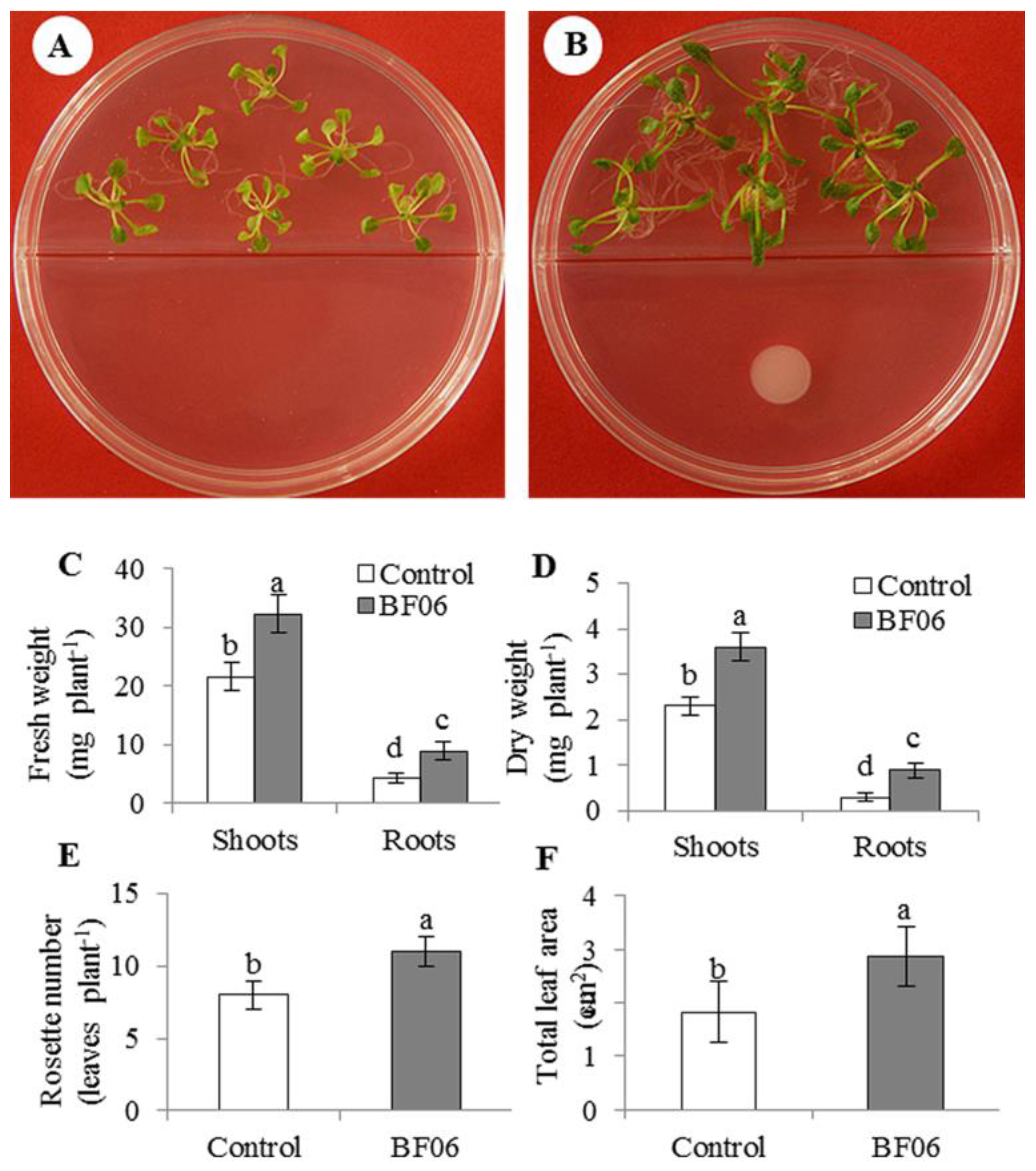
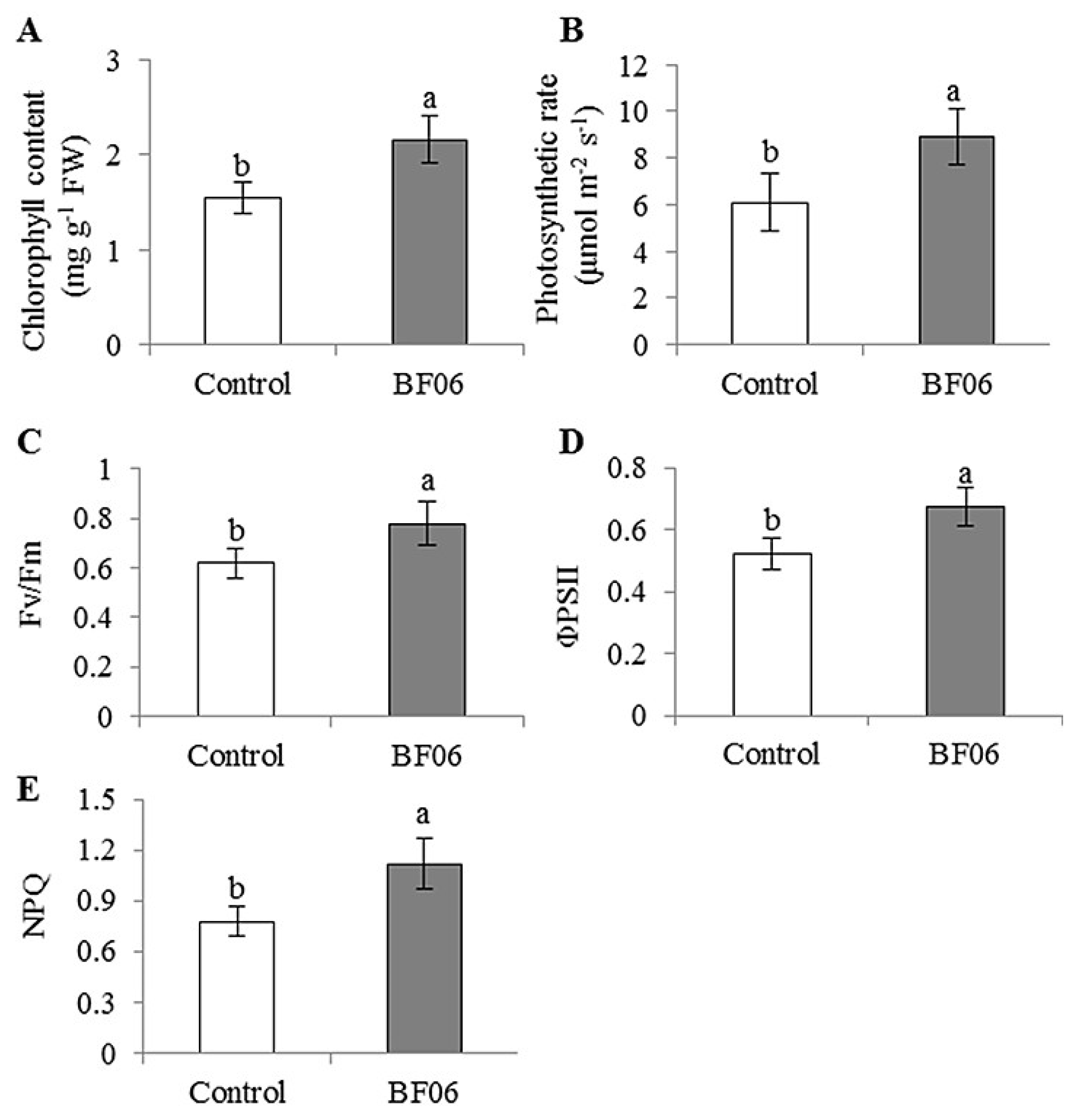
3.1. Effects of VOCs Released by BF06 on Plant Growth and Photosynthesis
3.2. Transcriptomic Analysis of BF06 VOCs-Regulated Gene Profiles
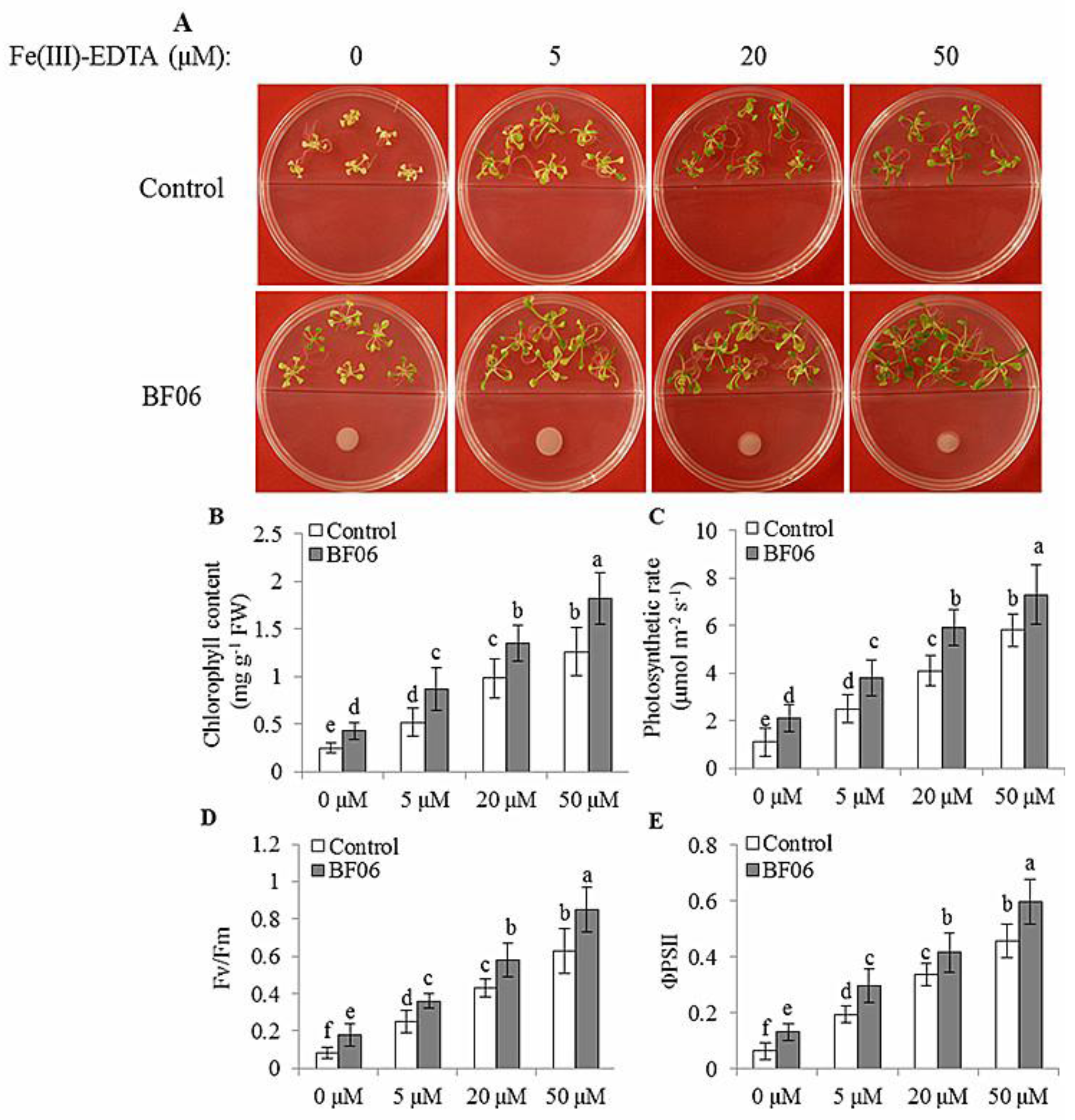
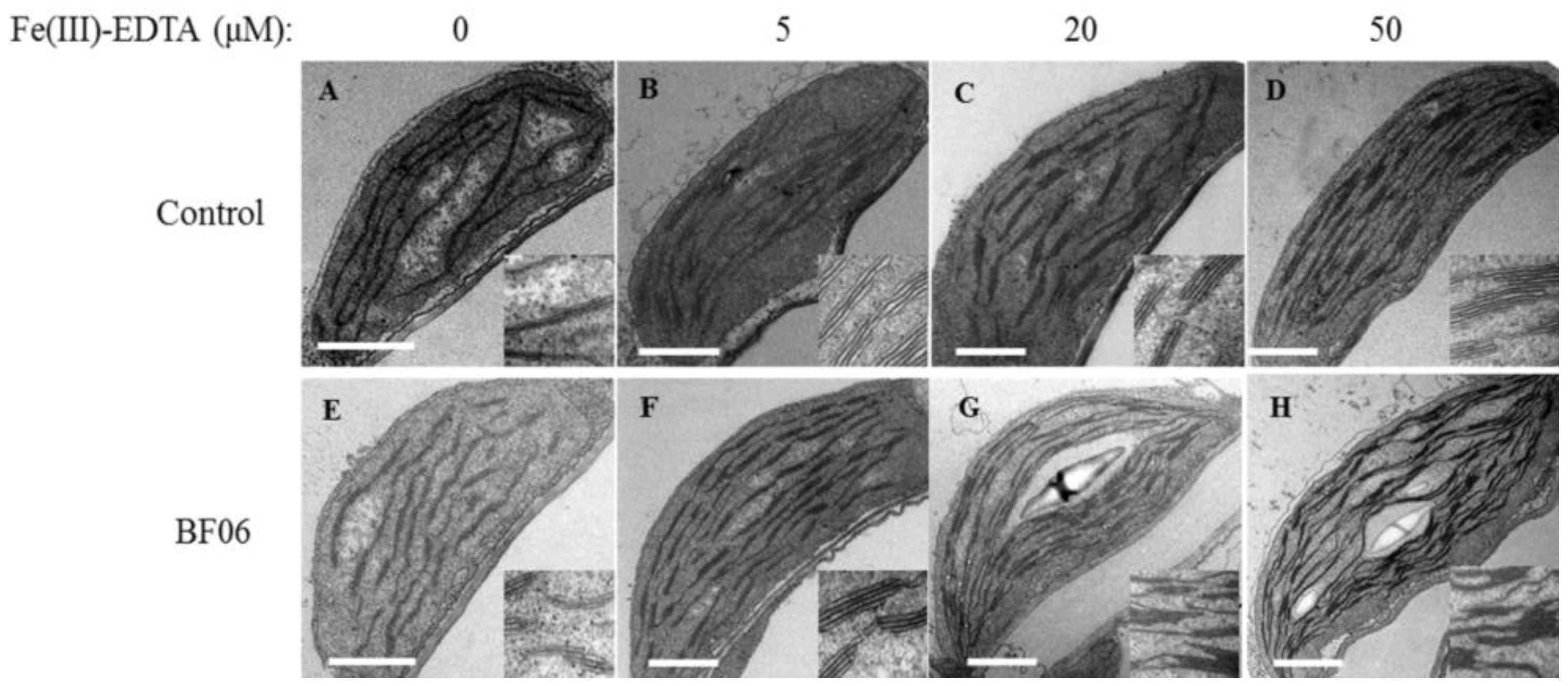
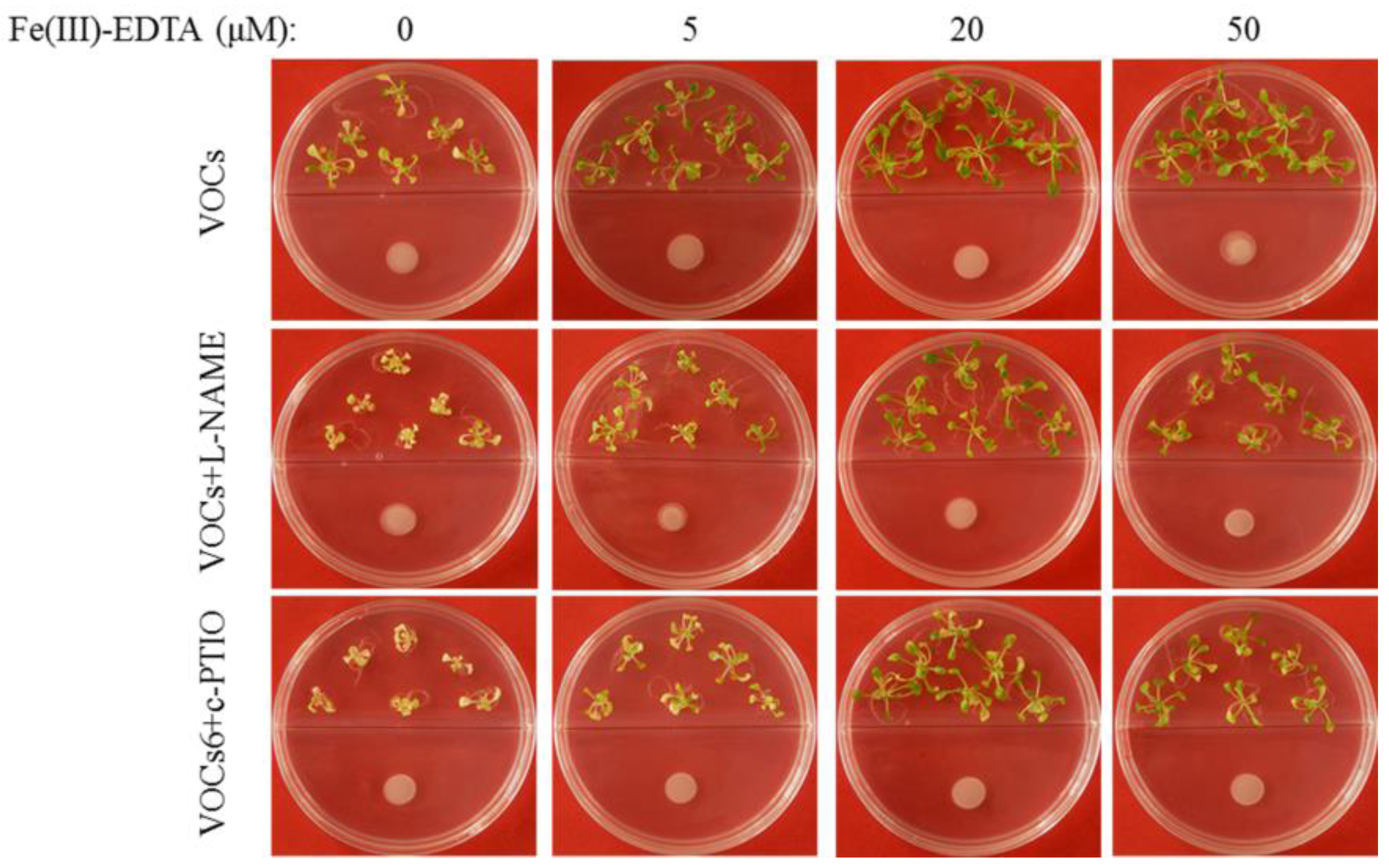
3.3. BF06 VOCs Enhance the Tolerance of Arabidopsis to Fe Deficiency
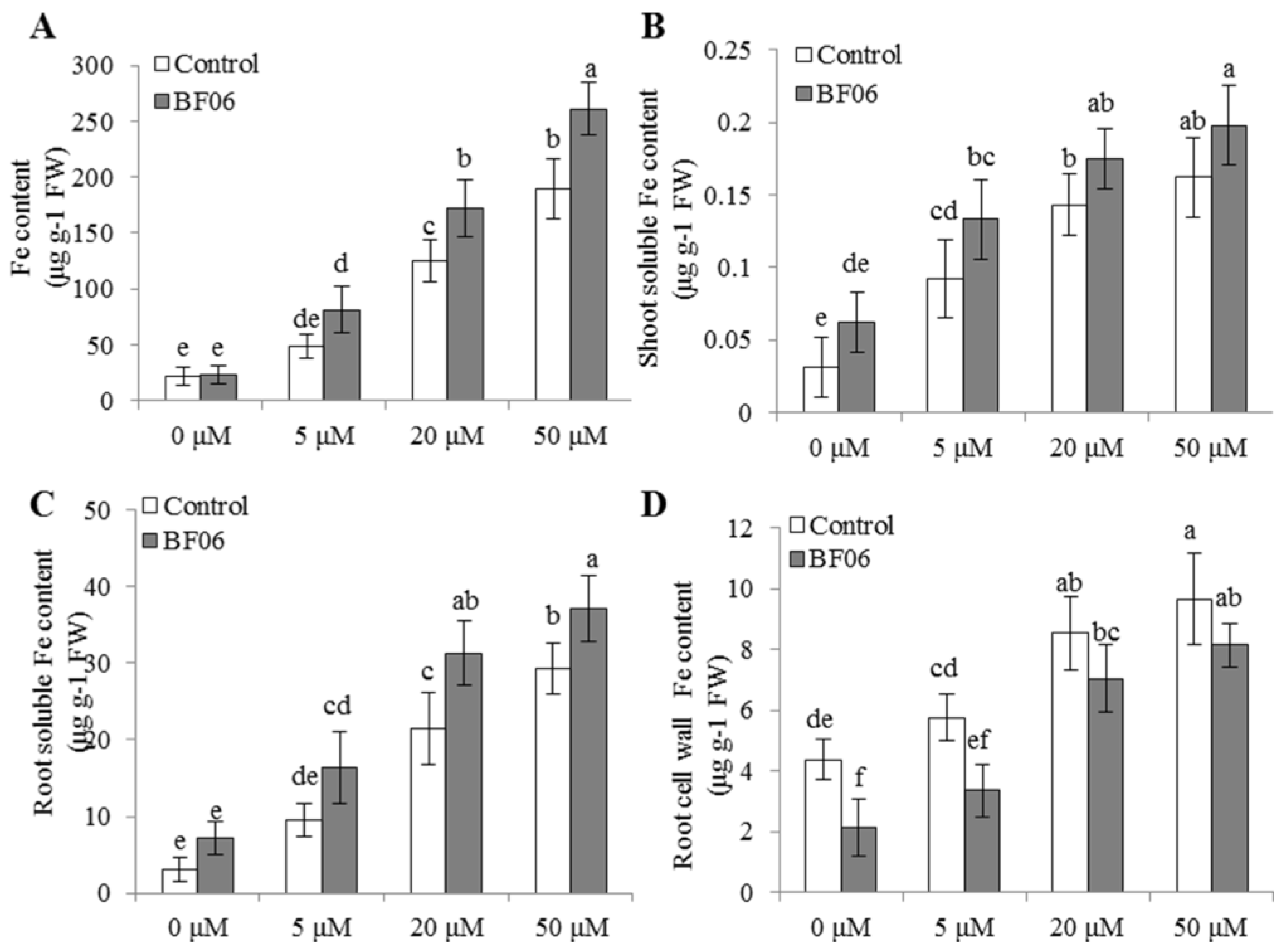
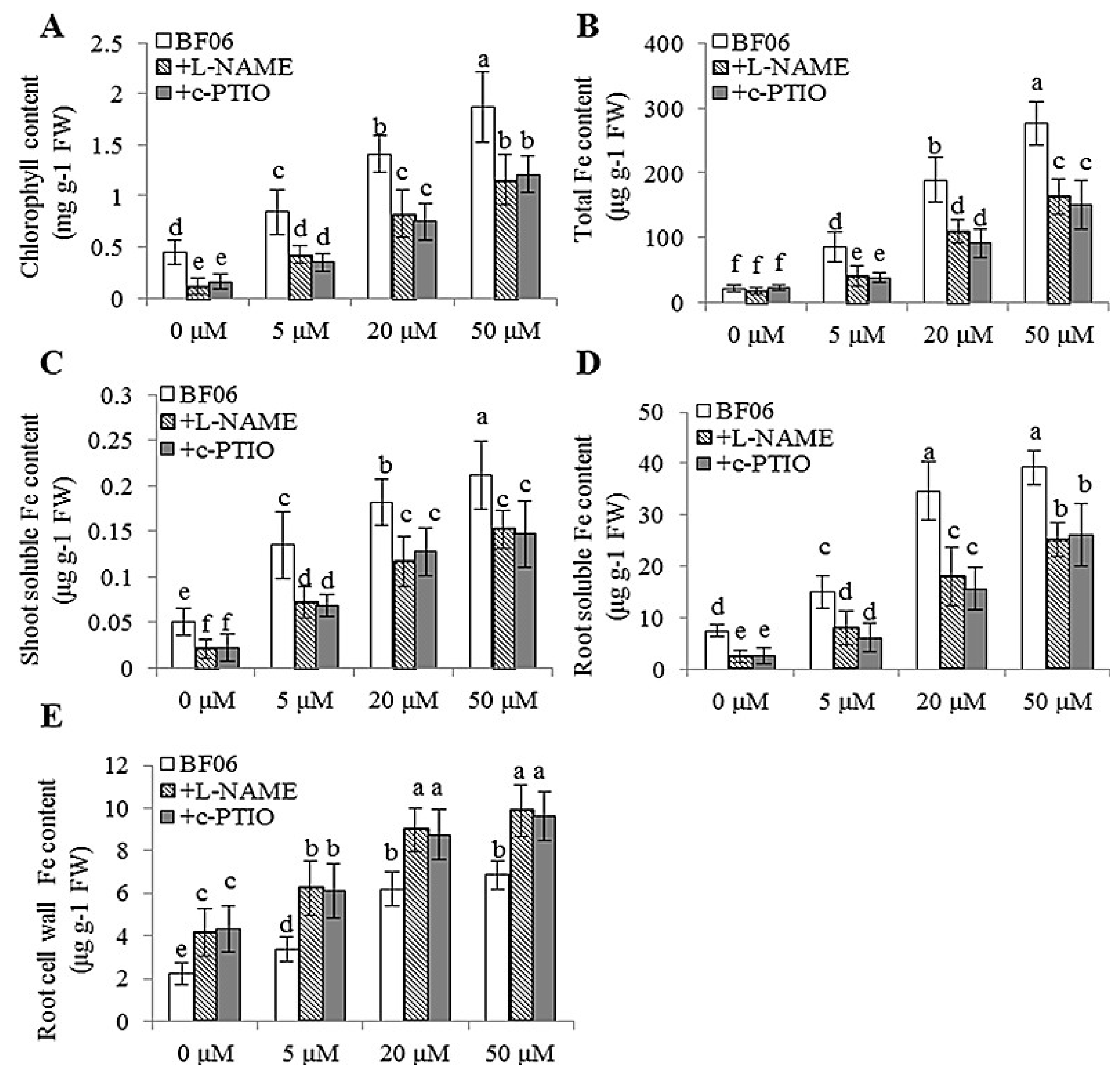
3.4. VOCs-Induced NO Accumulation Promotes Fe Absorption and Availability
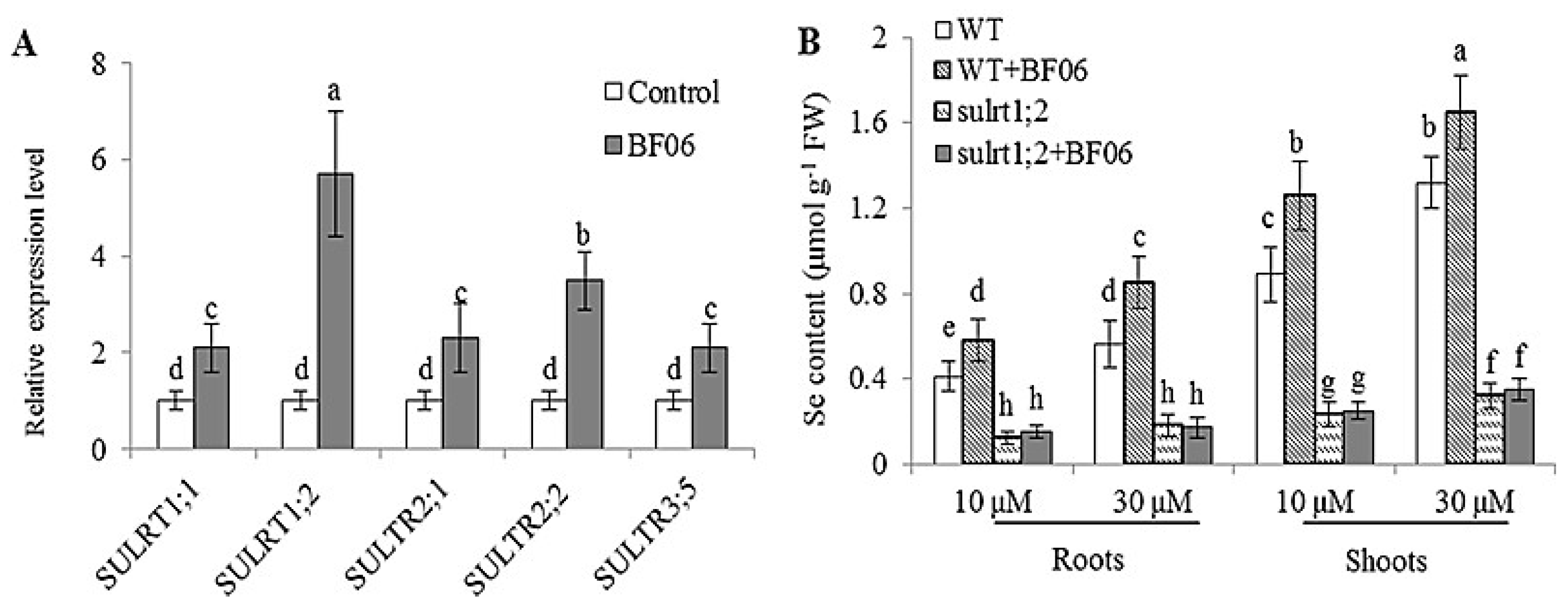
3.5. Effects of BF06 VOCs on Se Accumulation in Plants
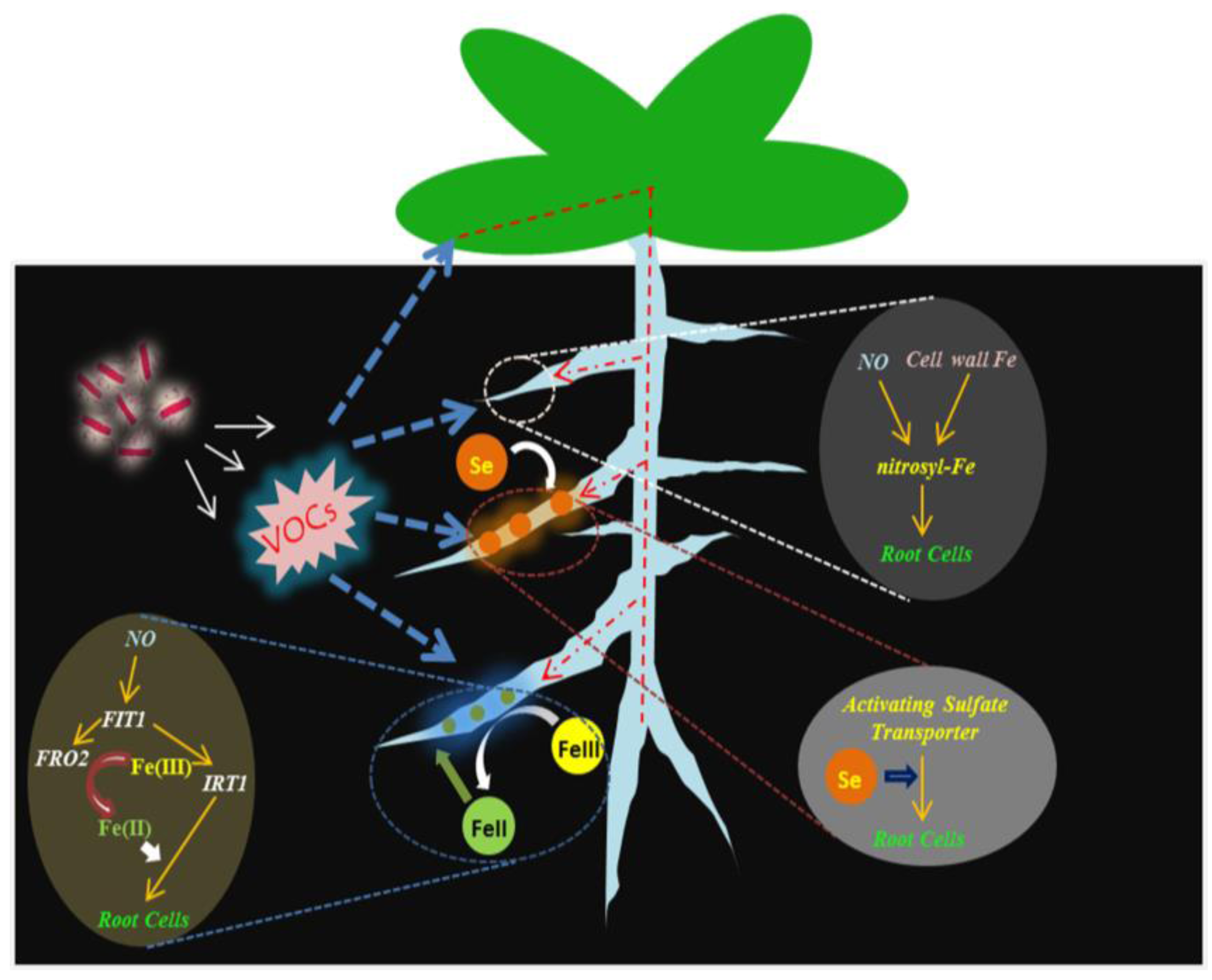
4. Discussion
4.1. A Promoting Effect of BF06 VOCs on Plant Growth
4.2. NO Is Essential for mVOCs-Induced Plant Fe Acquisition
4.3. BF06 VOCs Regulates Plant Uptake of Se by Upregulating of Sulfate Transporter Genes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Rodriguez-Kabana, R.; Zehnder, G.W.; Murphy, J.; Sikora, E.; Fernandez, C. Plant root-bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Aust. J. Plant Pathol. 1999, 28, 27–33. [Google Scholar] [CrossRef]
- Lin, W.; Okon, Y.; Hardy, R.W.F. Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl. Environ. Microbiol. 1983, 45, 1775–1779. [Google Scholar] [PubMed]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Phys. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Follet-Gueye, M.L.; Vicré-Gibouin, M.; Hawes, M. Root border cells and secretions as critical elements in plant host defense. Curr. Opin. Plant Biol. 2013, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Schroth, M.N. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 1986, 76, 386–389. [Google Scholar] [CrossRef]
- MacDonald, E.M.S.; Powell, G.K.; Regier, D.A.; Glass, N.L.; Roberto, F.; Kosuge, T.; Morris, R.O. Secretion of zeatin, ribosylzeatin and ribosyl-1″-methylzeatin by Pseudomonas savastanoi plasmid coded cytokinin biosynthesis. Plant Physiol. 1986, 82, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Nicander, B.; Granhall, U.; Tillberg, E. Cytokinin production by Paenibacillus polymyxa. Soil Biol. Biochem. 1999, 31, 1847–1852. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, Z.Y.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J.F. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, L.; Orlando, J.; Rodríguez, M.I.; Chulze, S.; Etcheverry, M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005, 156, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Guo, J.S.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.Y.; Wang, J.F. Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol. Biochem. 2016, 105, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Grayston, S.; Chanway, C. N2-fixation and seedling growth promotion of lodgepole pine by endophytic Paenibacillus polymyxa. Microb. Ecol. 2013, 66, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Farag, M.A.; Park, H.B.; Kloepper, J.W.; Lee, S.H.; Ryu, C.M. Induced resistance by a long-chain bacterial volatile: Elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, e48744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A soil bacteria regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Zhang, H.; Ryu, C.M. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M.; Kim, Y.C. 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wunsche, H.; Baldwin, I.T. Dimethyl disulfide produced by the naturally associated bacterium Bacillus spB55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda Mdel, C.; Macías-Rodríguez, L.; Santoyo, G.; Farías-Rodríguez, R.; Valencia-Cantero, E. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 2013, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Abadia, J. Function of iron in chloroplasts. J. Plant Nutr. 1986, 9, 609–646. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, noxious, and not readily available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Fett, J.P.; Guerinot, M.L. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, Y.; Sugahara, K. Active extrusion of protons and exudation of carboxylic acids in response to iron deficiency by roots of chickpea (Cicer arietinum L.). Plant Soil 1997, 189, 49–55. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. The essential basic helix–loop–helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Lotfi, M.; Dalmiya, N.; Sethuraman, K.; Deitchler, M.; Geibel, S.; Gillenwater, K.; Gilman, A.; Mason, K.; Mock, N. The Micronutrient Report: Current Progress and Trends in the Control of Vitamin A, Iodine, and Iron Deficiencies; The Micronutrient Initiative/International Development Research Centre: Ottawa, ON, Canada, 2001. [Google Scholar]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Selinus, O.; Fordyce, F. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Springer: Heidelberg, Germany, 2013; pp. 375–416. [Google Scholar]
- Yasin, M.; El-Mehdawi, A.F.; Pilon-Smits, E.A.; Faisal, M. Selenium-fortified wheat: Potential of microbes for biofortification of selenium and other essential nutrients. Int. J. Phytoremediumtion 2015, 17, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.; Faisal, M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)—Applications in phytoremediumtion and biofortification. Int. J. Phytoremediumtion 2015, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Zhan, C.Y.; Lu, Y.; Cui, H.R.; Wang, X.Y. The conservative cysteines in transmembrane domain of AtVKOR/LTO1 are critical for photosynthetic growth and photosystem II activity in Arabidopsis. Front. Plant. Sci. 2015, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Zhu, X.F.; Wang, Z.W.; Dong, F.; Dong, N.Y.; Zheng, S.J. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ. 2014, 37, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Cassin, G.; Mari, S.; Curie, C.; Briat, J.F.; Czernic, P. Increased sensitivity to iron deficiency in Arabidopsis thaliana over accumulating nicotianamine. J. Exp. Bot. 2009, 60, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, S.D.; Fakra, S.C.; Landon, J.; Schulz, P.; Tracy, B.; Pilon-Smits, E.A.H. Inoculation of Astragalus racemosus and Astragalus convallarius with selenium-hyperaccumulator rhizosphere fungi: Effects on growth and accumulation of selenium and other elements. Planta 2012, 237, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol. 2016, 170, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; He, X.; Gao, J.; Ma, H.; Zhang, Z.; Shen, Y.; Pan, G.; Lin, H. Transcriptomic changes during maize roots development responsive to Cadmium (Cd) pollution using comparative RNAseq-based approach. Biochem. Biophys. Res. Commun. 2015, 464, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, J.; Zhu, J.; Zhou, C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 75, 24–35. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Shinmachi, F.; Buchner, P.; Stroud, J.L.; Parmar, S.; Zhao, F.J.; McGrath, S.P.; Hawkesford, M.J. Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol. 2010, 153, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulphur metabolism. Front. Plant Sci. 2014, 5, 422. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M. Nitric oxide regulation of bacterial biofilms. Biochemistry 2015, 54, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, P.; Silva, L.; Catrice, O.; Bruand, C.; Carvalho, H.; Meilhoc, E. Sinorhizobium meliloti controls nitric oxide-mediated post-translational modification of a Medicago truncatula nodule protein. Mol. Plant Microbe Interact. 2015, 28, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Boscari, A.; Castella, C.; Rovere, M.; Puppo, A.; Brouquisse, R. Nitric oxide: A multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 2015, 66, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.; Lamattina, L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 2007, 52, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Simontacchi, M.; Buet, A.; Lamattina, L.; Puntarulo, S. Exposure to nitric oxide increases the nitrosyl-iron complexes content in sorghum embryonic axes. Plant Sci. 2012, 183, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Yang, H.J.; Oh, B.J.; Anderson, A.J.; Kim, Y.C. Biological control potential of Bacillus amyloliquefaciens KB3 isolated from the feces of Allomyrina dichotoma Larvae. Plant Pathol. J. 2016, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.M.; Yun, B.W.; Lee, I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2R,3R-butanediol in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Berthomieu, P.; Clairotte, M.; Shibagaki, N.; Davidian, J.C.; Gosti, F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol. 2008, 180, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Alberdi, K.; Godoy, Y.; Rojas-Lillo, P.; Cartes, P.; Mora, M.L. Influence of selenite on selenium uptake, differential antioxidant performance and gene expression of sulfate transporters in wheat genotypes. Plant Soil 2013, 369, 47–59. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon, M.; Malagoli, M.; Pilon-Smits, E.A.H. Exploring the importance of sulphate transporters and ATPsulphurylases for selenium hyperaccumulation—A comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front. Plant Sci. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulphur ratios define ‘Se-accumulator’ plants. Ann. Bot. 2007, 100, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Uraji, M.; Shimoishi, Y.; Mori, I.C.; Nakamura, Y.; Murata, Y. Mechanisms of the selenium tolerance of the Arabidopsis thaliana knockout mutant of sulfate transporter SULTR1;2. Biosci. Biotechnol. Biochem. 2012, 76, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Parmar, S.; Kriegel, A.; Carpentier, M.; Hawkesford, M.J. The sulfate transporter family in wheat: Tissue-specific gene expression in relation to nutrition. Mol. Plant 2010, 3, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Cabannes, E.; Buchner, P.; Broadley, M.R.; Hawkesford, M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011, 157, 2227–2239. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, C.; Xiao, X.; Xie, Y.; Zhu, L.; Ma, Z. Enhanced Iron and Selenium Uptake in Plants by Volatile Emissions of Bacillus amyloliquefaciens (BF06). Appl. Sci. 2017, 7, 85. https://doi.org/10.3390/app7010085
Wang J, Zhou C, Xiao X, Xie Y, Zhu L, Ma Z. Enhanced Iron and Selenium Uptake in Plants by Volatile Emissions of Bacillus amyloliquefaciens (BF06). Applied Sciences. 2017; 7(1):85. https://doi.org/10.3390/app7010085
Chicago/Turabian StyleWang, Jianfei, Cheng Zhou, Xin Xiao, Yue Xie, Lin Zhu, and Zhongyou Ma. 2017. "Enhanced Iron and Selenium Uptake in Plants by Volatile Emissions of Bacillus amyloliquefaciens (BF06)" Applied Sciences 7, no. 1: 85. https://doi.org/10.3390/app7010085




