N-Type Semiconducting Behavior of Copper Octafluorophthalocyanine in an Organic Field-Effect Transistor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic and Crystallisation Procedures
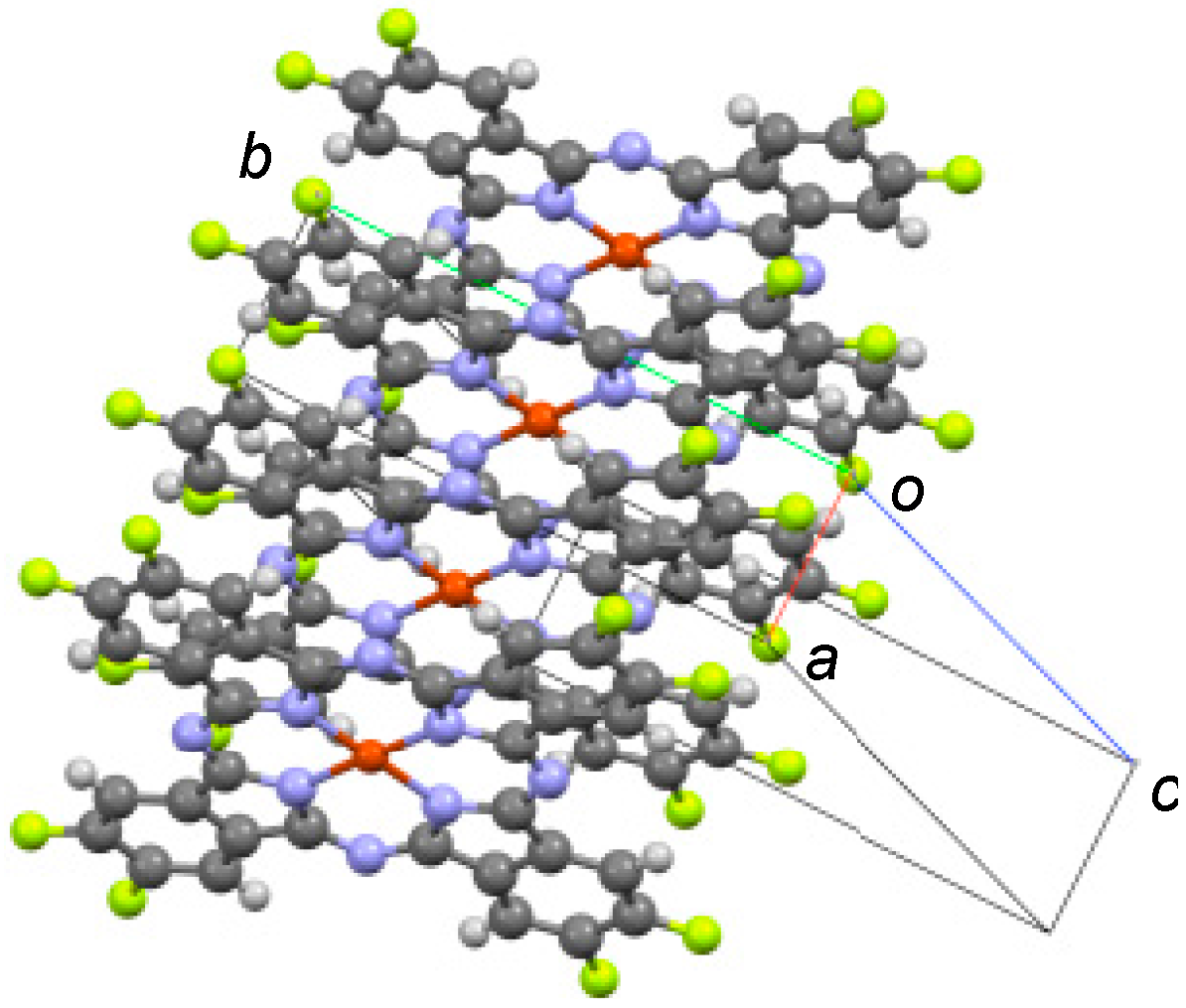
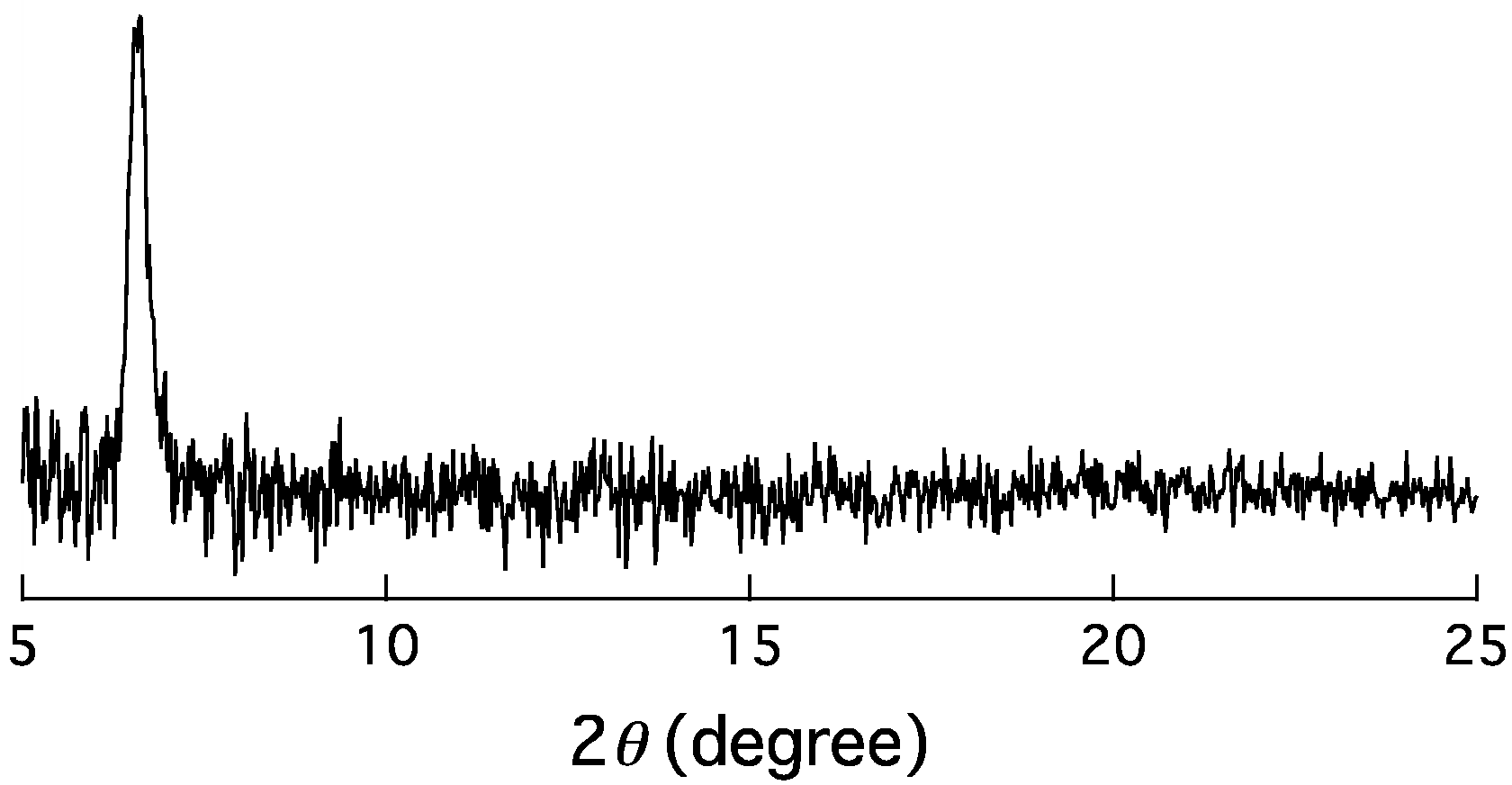
2.2. X-ray Diffraction (XRD) Measurements
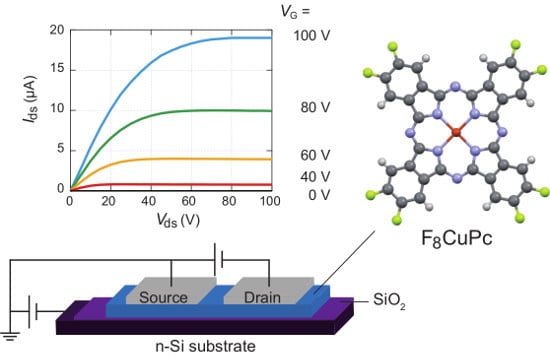
2.3. Fabrication of OFET and Measurement of the Transport Properties
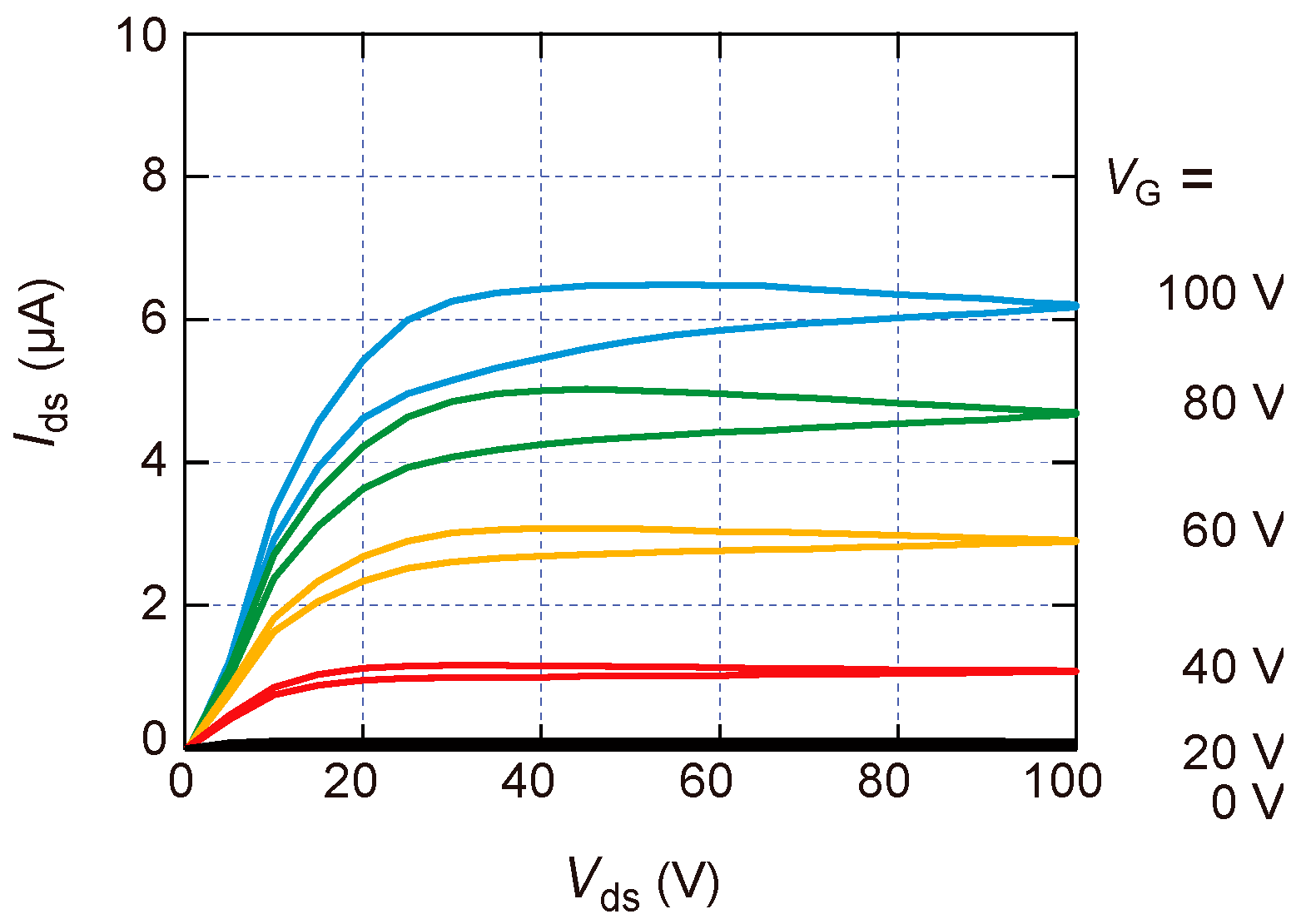
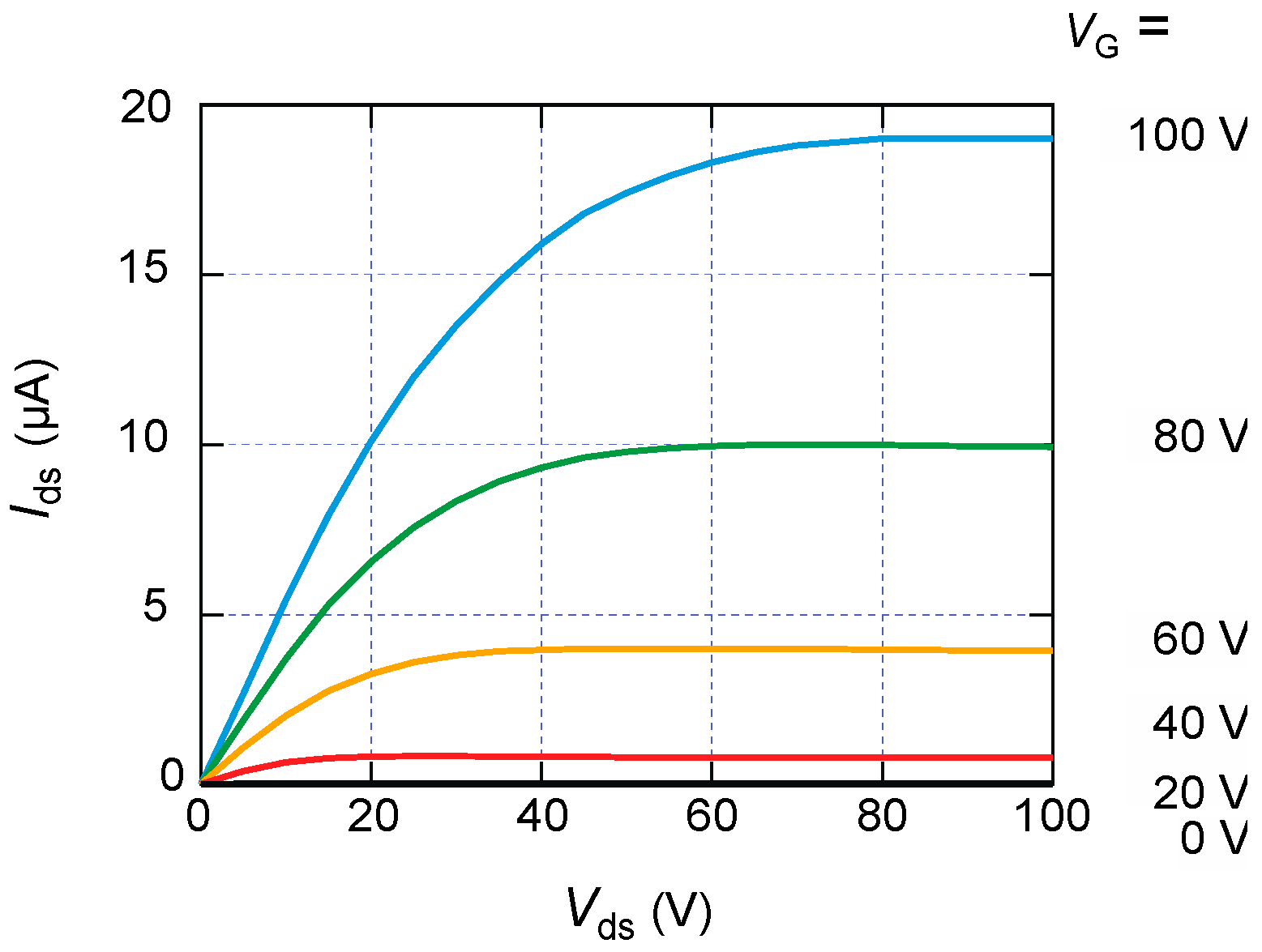
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bao, Z.; Lovinger, A.J.; Dodabalapur, A. Organic field-effect transistors with high mobility based on copper phthalocyanine. Appl. Phys. Lett. 1996, 69, 3066–3068. [Google Scholar] [CrossRef]
- Tang, C.W.; van Slyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhang, Y.; Jiang, J. Theoretical investigation of the molecular, electronic structures and vibrational spectra of a series of first transition metal phthalocyanines. Spectrochim. Acta A 2007, 67, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.A. Work function of gold. Phys. Rev. 1959, 115, 553–554. [Google Scholar] [CrossRef]
- Park, Y.; Choong, V.; Gao, Y. Work function of indium tin oxide transparent conductor measured by photoelectron spectroscopy. Appl. Phys. Lett. 1996, 68, 2699–2701. [Google Scholar] [CrossRef]
- Reese, C.; Bao, Z. Organic single-crystal field effect transistors. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J.; Brown, J. Air-stable n-channel organic thin film transistors. J. Am. Chem. Soc. 1998, 120, 207–208. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, J.; Hu, P.; Wei, F.; Du, K.; Wang, N.; Ba, T.; Feng, S.; Kloc, C. Fluorinaion of metal phthalocyanines: Single-crystal growth, efficient n-channel organic field-effect transistors, and structure-property relationships. Sci. Rep. 2014, 4, 7573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, H.; Geng, Y.; Yan, D. Organic photovoltaic cells using hexadecafluorophthlaocyaninatocopper (F16CuPc) as electron acceptor material. Chem. Phys. Lett. 2007, 446, 329–332. [Google Scholar] [CrossRef]
- Jea, M.; Kumar, A.; Cho, H.; Yang, D.; Shim, H.; Palai, A.; Pyo, S. An organic microcrystal array-embedded layer: Highly directional alternating p- and n-channels for ambipolar transistors and inverters. J. Mater. Chem. C 2014, 2, 3980–3987. [Google Scholar] [CrossRef]
- Li, Q.; Ding, S.; Zhu, W.; Feng, L.; Dong, H.; Hu, W. Recent advances in one-dimensional organic p-n heterojunctions for optoelectronic device applications. J. Mater. Chem. C 2016, 4, 9388–9398. [Google Scholar] [CrossRef]
- Optiz, A.; Wilke, A.; Amsalem, P.; Oehzelt, M.; Blum, R.-P.; Rabe, J.P.; Mizokuro, T.; Hörmann, U.; Moons, E.; Koch, N. Organic heterojunctions: Contact-induced molecular reorientation, interface states, and charge re-distribution. Sci. Rep. 2016, 6, 21291. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.R.; Frisbie, C.D.; Filho, D.A.S.; Brédas, J.-L.; Ewbank, P.C.; Mann, K. Introduction to organic thin film transistors and design of n-channel organic semiconductors. Chem. Mater. 2004, 16, 4436–4451. [Google Scholar] [CrossRef]
- Murdey, R.; Sato, N.; Bouvet, M. Frontier electronic structures in fluorinated copper phthalocyanine thin films studied using ultraviolet and inverse photoemission spectroscopies. Mol. Cryst. Liq. Cryst. 2006, 455, 211–218. [Google Scholar] [CrossRef]
- Matsumoto, F.; Iwai, T.; Moriwaki, K.; Takao, Y.; Ito, T.; Mizuno, T.; Ohno, T. Design of fullerene derivatives for stabilizing LUMO energy using donor groups placed in spatial proximity to the C60 cage. J. Org. Chem. 2012, 77, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Z.; Tip, H.-L.; Jen, A.K. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- Anderson, T.L.; Komplin, G.C.; Pietro, W.J. Rectifying junctions in peripherally-substituted metallophthalocyanine bilayer films. J. Phys. Chem. 1993, 97, 6577–6578. [Google Scholar] [CrossRef]
- Pietro, W.J. Rectifying junctions on metallophthalocyanine thin films. Adv. Mater. 1994, 6, 239–242. [Google Scholar] [CrossRef]
- Shao, X.; Wang, S.; Li, X.; Chen, T.; Xiao, Y. Single component p- ambipolar and n-type OTFTs based on fluorinated copper phthlaocyanines. Dyes Pigments 2016, 132, 378–386. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Li, Y.; Li, H.; Zhang, X.; Li, R.; Dong, H.; Hu, W.; Kloc, C. Molecular crystal engineering: Tuning organic semiconductor from p-type to n-type by adjusting their substitutional symmetry. Adv. Mater. 2017, 29, 1605053. [Google Scholar] [CrossRef] [PubMed]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Inabe, T.; Tajima, H. Phthlalocyanines–Versatile components of molecular conductors. Chem. Rev. 2004, 104, 5503–5533. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.; Hunger, R.; Klein, A.; Jaegermann, W. Engineering the line up of electronic energy levels at inorganic–organic semiconductor interfaces by variation of surface termination and by substitution. Phys. Status Solidi (b) 2008, 245, 1838–1848. [Google Scholar] [CrossRef]
- Yoon, S.M.; Song, H.J.; Hwang, I.-C.; Kim, S.; Choi, H.C. Single crystal structure of copper hexadecafluorophthalocyanine (F16CuPc) ribbon. Chem. Commun. 2010, 46, 231–233. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matumoto, A.; Hoshino, N.; Akutagawa, T.; Matsuda, M. N-Type Semiconducting Behavior of Copper Octafluorophthalocyanine in an Organic Field-Effect Transistor. Appl. Sci. 2017, 7, 1111. https://doi.org/10.3390/app7111111
Matumoto A, Hoshino N, Akutagawa T, Matsuda M. N-Type Semiconducting Behavior of Copper Octafluorophthalocyanine in an Organic Field-Effect Transistor. Applied Sciences. 2017; 7(11):1111. https://doi.org/10.3390/app7111111
Chicago/Turabian StyleMatumoto, Akane, Norihisa Hoshino, Tomoyuki Akutagawa, and Masaki Matsuda. 2017. "N-Type Semiconducting Behavior of Copper Octafluorophthalocyanine in an Organic Field-Effect Transistor" Applied Sciences 7, no. 11: 1111. https://doi.org/10.3390/app7111111