A Comparative Exergoeconomic Evaluation of the Synthesis Routes for Methanol Production from Natural Gas
Abstract
:1. Introduction
2. State of the Art
2.1. Reforming Technologies
2.2. Methanol Synthesis
3. Methodology and Assumptions
3.1. Exergy Analysis
3.2. Economic Analysis
3.3. Exergoeconomic Analysis
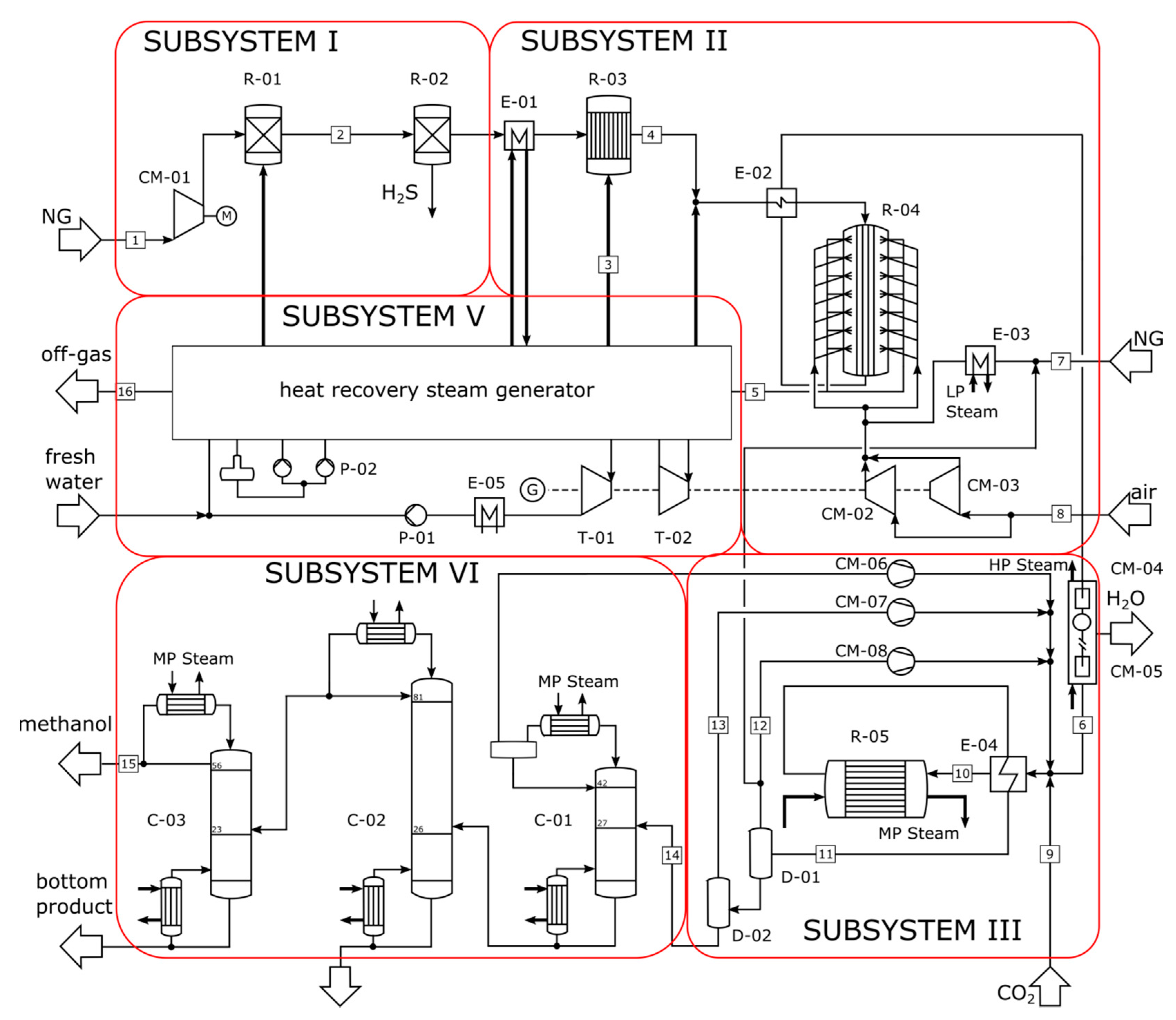
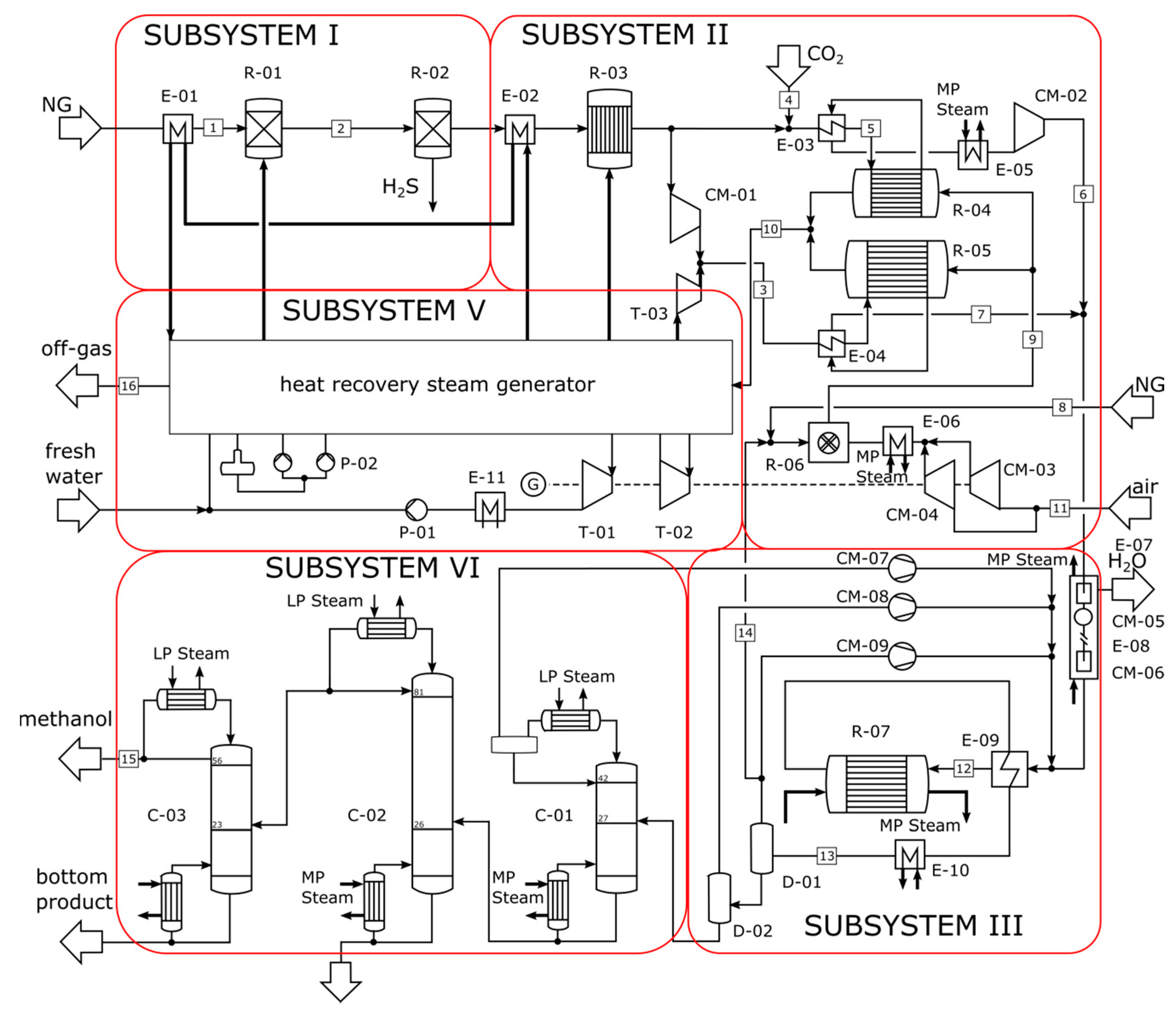
4. Case Studies and Process Description
- conventional SMR with low-pressure methanol synthesis for a production capacity of 2200 MTPD,
- a serial two-step configuration of SMR and ATR with low-pressure methanol synthesis for a production capacity of 4750 MTPD, and
- a parallel configuration of SMR and DMR with low-pressure methanol synthesis for a production capacity of 5000 MTPD.
5. Results
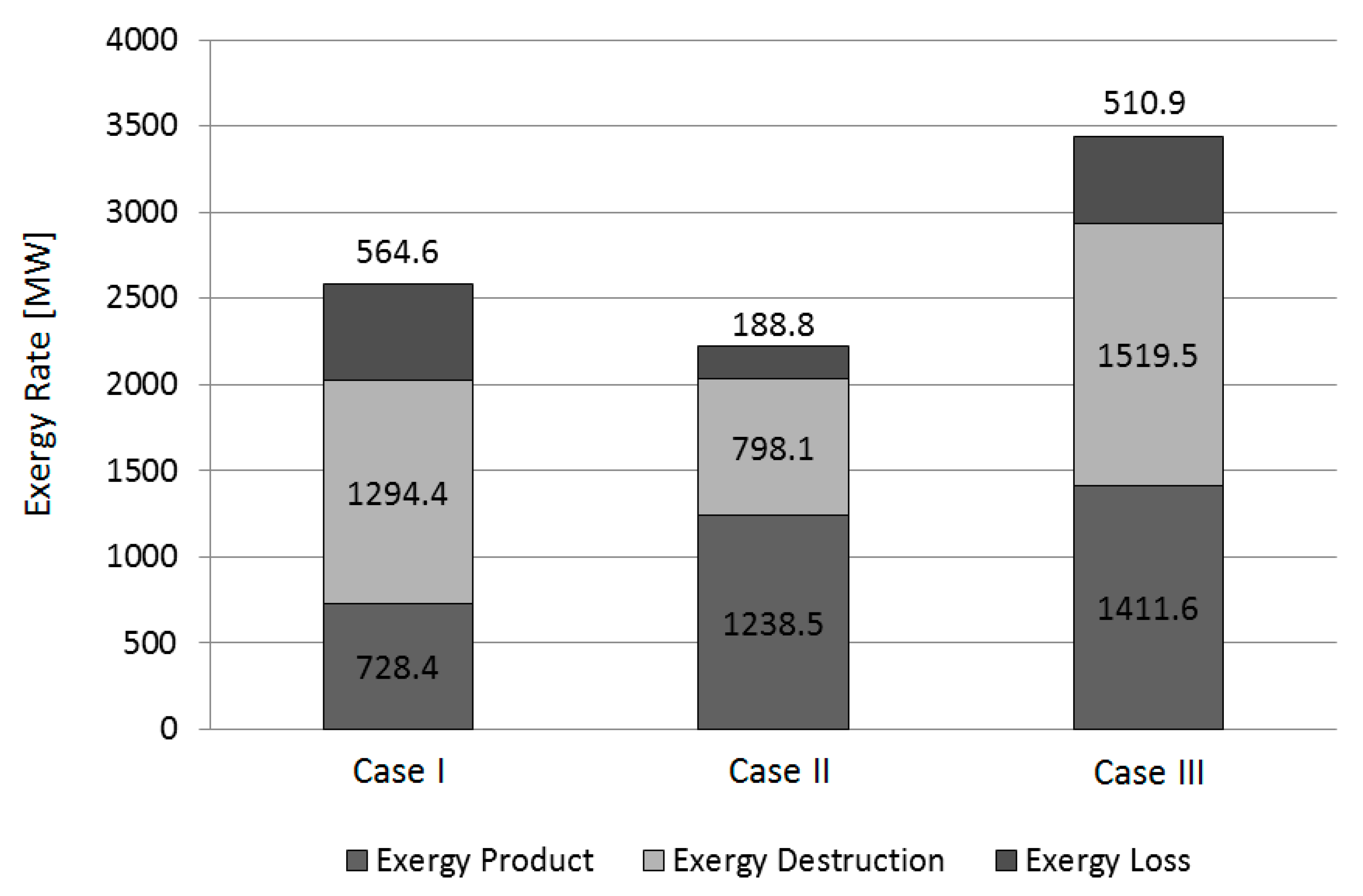
5.1. Exergy Analysis
5.2. Economic Analysis
5.3. Exergoeconomic Analysis
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Methanol Market Services Asia (MMSA). Methanol and Derivates Analysis (MDA); Methanol Market Services Asia (MMSA): Singapore, 2016; Volume 11. [Google Scholar]
- Alvarado, M. The changing face of the global methanol industry. IHS Chem. Bull. Insights 2016, 3, 10–11. Available online: https://cdn.ihs.com/www/pdf/IHS-Chemical-Bulletin-2016-Issue-3.pdf (accessed on 21 November 2017).
- Olah, G.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy, 2nd ed.; Wiley VCH Verlag GmbH & Co. KGaA: Los Angeles, CA, USA, 2009. [Google Scholar]
- Cheng, W.H.; Kung, H. Methanol production and use. In Chemical Industries; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Jörg, O.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Aasberg-Petersen, K.; Stub Nielsen, K.; Dybkjær, I.; Perregaard, J. Large Scale Methanol Production from Natural Gas; Research Paper; Haldor Topsøe: Lyngby, Denmark, 2014. [Google Scholar]
- Aasberg-Petersen, K.; Bak Hansen, J.-H.; Christensen, T.S.; Dybkjaer, I.; Seier Christensen, P.; Stub Nielsen, C.; Winter Madsen, S.E.L.; Rostrup-Nielsen, J.R. Technologies for large-scale gas conversion. Appl. Catal. A Gen. 2001, 221, 379–387. [Google Scholar] [CrossRef]
- Dahl, P.J.; Christensen, T.S.; Winter-Madsen, S.; King, S.M. Proven autothermal reforming technology for modern large-scale methanol plants. In Proceedings of the International Conference on Nitrogen+ Syngas, Paris, France, 24–27 February 2014. [Google Scholar]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming; Springer Verlag: Berlin/Heidelberg, Germany; Tokyo, Japan; New York, NY, USA, 1984. [Google Scholar]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and Synthesis Gas by Steam and CO2 Reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Holm-Larsen, H. CO2-reforming for large scale methanol plants—An actual case. In Natural Gas Conversion VI; Studies in Surface Science Catalysis Book Series; Elsevier: Amsterdam, The Netherlands, 2001; pp. 441–446. [Google Scholar]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Khoshtinat Nikoo, M.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [Green Version]
- Baltrusaitis, J.; Luyben, W.L. Methane Conversion to Syngas for Gas-to-Liquids (GTL): Is Sustainable CO2 Reuse via Dry Methane Reforming (DMR) Cost Competitive with SMR and ATR Processes. ACS Sustain. Chem. Eng. 2015, 3, 2100–2111. [Google Scholar] [CrossRef]
- Noureldin, M.M.B.; Elbashir, N.O.; Gabriel, K.J.; El-Halwagi, M.M. A Process Integration Approach to the Assessment of CO2 Fixation through Dry Reforming. ACS Sustain. Chem. Eng. 2015, 3, 625–636. [Google Scholar] [CrossRef]
- Luyben, W.L. Control of parallel dry methane and steam methane reforming processes for Fischer–Tropsch syngas. J. Process Control 2016, 39, 77–87. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.; Gao, R.; Kwak, G.; Park, H. Carbon dioxide utilization in a gas-to-methanol process combined with CO2/Steam-mixed reforming: Techno-economic analysis. Fuel 2017, 190, 303–311. [Google Scholar] [CrossRef]
- Machado, C.F.R.; de Medeiros, J.L.; Araújo, O.F.Q. A comparative analysis of methanol production routes: Synthesis gas versus CO2 hydrogenation. In Proceedings of the 2014 International Conference on Industrial Engineering and Operations Management, Bali, Indonesia, 7–9 January 2014. [Google Scholar]
- Luu, M.; Milani, D.; Bahadori, A.; Abbas, A. A comparative study of CO2 utilization in methanol synthesis with various syngas production technologies. J. CO2 Util. 2015, 12, 62–76. [Google Scholar] [CrossRef]
- Rosen, A.; Scott, D. Energy and Exergy Analyses of a Production Process for Methanol from Natural Gas. Int. J. Hydrogen Energy 1988, 13, 617–623. [Google Scholar] [CrossRef]
- Wender, I. Reactions of Synthesis Gas. Fuel Process. Technol. 1996, 48, 189–297. [Google Scholar] [CrossRef]
- Dybkjaer, I.; Christensen, T.S. Syngas for large scale conversion of natural gas to liquid fuels. Stud. Surf. Sci. Catal. 2001, 136, 435–440. [Google Scholar]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas; Technical Report NREL/TP-510-34929; National Renewable Energy Laboratory: Golden, CO, USA, 2003.
- Dybkjær, I.; Aasberg-Petersen, K. Synthesis gas technology large-scale applications. Can. J. Chem. Eng. 2016, 94, 607–612. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Rostrup-Nielsen, T. Large-scale Hydrogen Production. In Proceedings of the 6th World Congress of Chemical Engineering, Melbourne, Australia, 23–27 September 2001. [Google Scholar]
- Zahedi, N.M.; Rowshanzamir, S.; Eikani, M.H. Autothermal reforming of methane to synthesis gas: Modeling and simulation. Int. J. Hydrogen Energy 2009, 34, 1292–1300. [Google Scholar]
- Molburg, J.; Doctor, R. Hydrogen from Steam-Methane Reforming with CO2-capture. In Proceedings of the 20th Annual International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 15–19 September 2003. [Google Scholar]
- Hansen, J.B. Methanol Production Technology: Todays and Future Renewable Solutions; Methanol Workshop, Lund University; Haldor Topsøe: Lyngby, Denmark, 2015. [Google Scholar]
- Wurzel, T. Lurgi MegaMethanol Technology. In Proceedings of the DGMK-Conference, Dresden, Germany, 4–6 October 2006. [Google Scholar]
- Wesenberg, M.H. Gas Heated Steam Reformer Modelling. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2006. [Google Scholar]
- Chen, L.; Jiang, Q.; Zhaozheng, S.; Posarac, D. Optimization of Methanol Yield from a Lurgi Reactor. Chem. Eng. Technol. 2011, 34, 817–822. [Google Scholar] [CrossRef]
- Sinadinović-Frišer, S.V.; Janković, M.R.; Radicević, R.Ž. Simulation of the fixed bed Reactor for methanol synthesis. Pet. Coal 2001, 43, 31–34. [Google Scholar]
- Vanden Bussche, K.M.; Froment, G.F. A steady-state kinetic model for methanol synthesis and the water-gas shift reaction on a commercial Cu/ZnO/Al2O3 catalyst. J. Catal. 1996, 161, 1–10. [Google Scholar] [CrossRef]
- Luyben, W.L. Chemical Reactor Design and Control; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Brasington, R.D.; Haslbeck, J.L.; Kuehn, N.J.; Lewis, E.G.; Pinkerton, L.L.; Turner, M.; Varghese, E.; Woods, M. Cost and Performance Baseline for Fossil Energy Plants—Volume 2: Coal to Synthetic Natural Gas and Ammonia; DOE/NETL-2010/1402; National Energy Laboratory: Lakewood, CO, USA, 2011. [Google Scholar]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design & Optimization; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; ISBN 978-0471584674. [Google Scholar]
- Szargut, J.; Morris, D.R.; Steward, F.R. Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere: Bay Shore, NY, USA, 1988. [Google Scholar]
- Lazzaretto, A.; Tsatsaronis, G. SPECO: A systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 2006, 31, 1257–1289. [Google Scholar] [CrossRef]
- Ulrich, G.D.; Vasudevan, P.T. Chemical Engineering Process Design and Economics: A Practical Guide; Process Publishing: Durham, UK, 2002; ISBN 978-0849320330. [Google Scholar]
- Loh, H.P. Process Equipment Cost Estimation Final Report; DOE/NETL-2002/1169; National Energy Laboratory: Lakewood, CO, USA, 2002. [Google Scholar]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2007; ISBN 978-0080966595. [Google Scholar]
- De María, R.; Díaz, I.; Rodríguez, M.; Sáiz, A. Kinetic study and process simulation. Int. J. Chem. React. Eng. 2013, 11, 469–477. [Google Scholar]
- Luyben, W.L. Design and Control of a methanol reactor/column process. Ind. Eng. Chem. Res. 2010, 49, 6150–6163. [Google Scholar] [CrossRef]
- Hawkins, G. Methanol Plant, Theory of Distillation. Available online: https://de.slideshare.net/GerardBHawkins/methanol-plant-theory-of-distillation (accessed on 10 November 2017).
- Amirkhas, E.; Bedi, R.; Harley, S.; Lango, T. Methanol Production in Trinidad & Tobago; Final Report; University of California: Oakland, CA, USA, 2006. [Google Scholar]
- Tesch, S.; Morosuk, T.; Tsatsaronis, G. Exergoeconomic analysis applied to the process of regasification of LNG integrated into an air separation process. In Proceedings of the ECOS 2015—The 29th Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Pau, France, 29 June–3 July 2015. [Google Scholar]
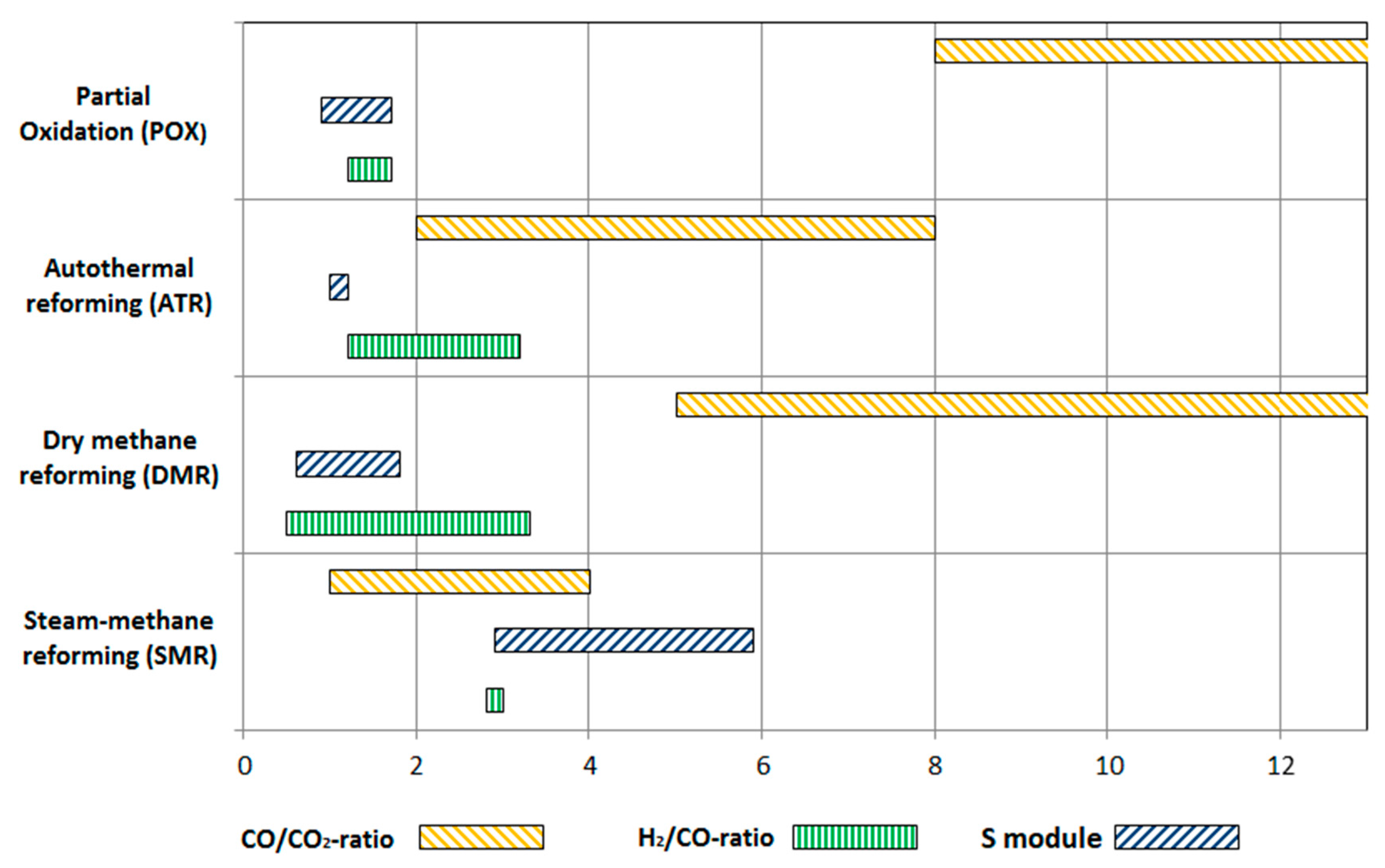
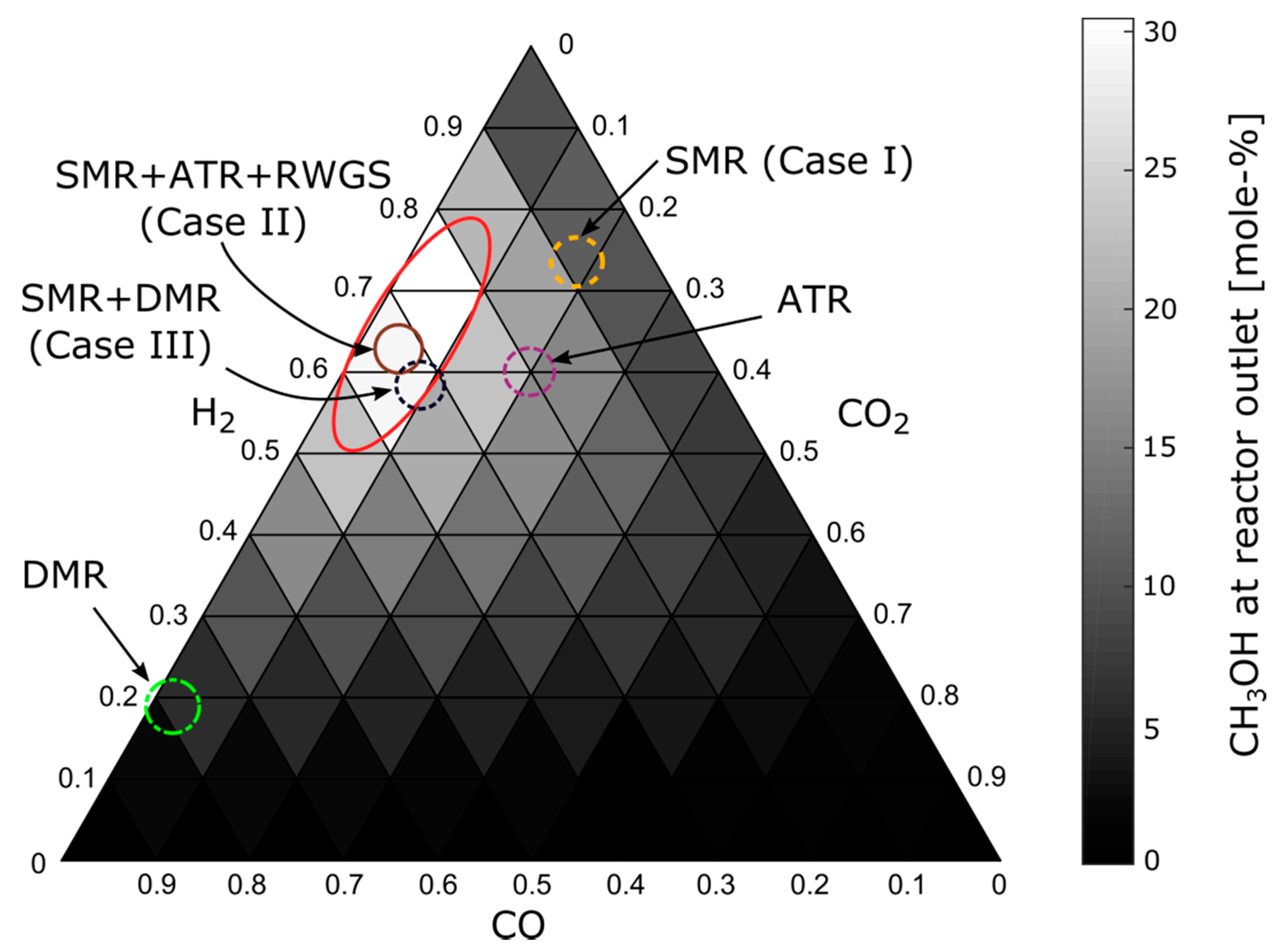


| Reforming Type | Main Reactions | Δh0 |
|---|---|---|
| SMR [25] | CH4 + H2O → CO + 3H2 | 206 kJ/mol |
| CO + H2O → CO2 + H2 | −41 kJ/mol | |
| ATR [10] | CH4 + 1.5 O2 → CO + 2H2O | −520 kJ/mol |
| CO + H2O → CO2 + H2 | −41 kJ/mol | |
| CH4 + H2O → CO + 3H2 | 206 kJ/mol | |
| DMR [10,26] | CH4 + CO2 → 2CO + 2H2 | 247 kJ/mol |
| Component/System | Unit | Case I | Case II | Case III |
|---|---|---|---|---|
| General | ||||
| Property Model | Redlich–Kwong–Soave | |||
| Ambient Temperature | °C | 15 | 15 | 15 |
| Ambient Pressure | bar | 1.013 | 1.013 | 1.013 |
| Mechanical Efficiency | % | 99 | 99 | 99 |
| Compressor isentropic efficiency | % | 85 | 85 | 85 |
| Pretreatment (sub-system I) | ||||
| Hydrogenolysis | ||||
| Conversion rate of COS [35] | % | 99.5 | 99.5 | 99.5 |
| Zinc Oxide Guard Bed | ||||
| H2S absorption coefficient [35] | % | 99.9 | 99.9 | 99.9 |
| Reforming Unit (sub-system II) | ||||
| Steam reformer (SMR and GHR design) | ||||
| Catalyst Density | kg/m3 | 2000 | 2000 | 2000 |
| Void Fraction | - | 0.5 | 0.5 | 0.5 |
| Number of Tubes | - | 3500 | 6000 | 1600 |
| Length | m | 12 | 10 | 15 |
| Diameter | m | 0.1 | 0.01 | 0.1 |
| Heat Transfer Coefficient | kW/m2K | 0.5 | 0.5 | 0.5 |
| S/C-ratio | - | 3.5 | 0.5 | 1.0 |
| Autothermal Reformer (The information refers to the catalytic bed of the ATR) and Dry Methane Reformer | ||||
| Catalyst Density | kg/m3 | - | 2500 | 2000 |
| Void Fraction | - | - | 0.5 | 0.5 |
| Number of Tubes | - | - | - | 550 |
| Length | m | - | 7 | 13.5 |
| Diameter | m | - | 3 | 0.1 |
| Heat Transfer Coefficient | kW/m2K | - | - | 0.5 |
| O2/C-ratio, CO2/C-ratio | - | 0.55 | 0.5 | |
| Methanol Synthesis Unit (sub-system III) | ||||
| Catalyst Density | kg/m3 | 1775 | 1775 | 1775 |
| Void Fraction | - | 0.5 | 0.5 | 0.5 |
| Number of Tubes | - | 10,000 | 10,000 | 10,000 |
| Length | m | 18 | 18 | 18 |
| Diameter | m | 0.037 | 0.037 | 0.037 |
| Heat Transfer Coefficient | kW/m2K | 0.28 | 0.28 | 0.28 |
| Purge Ratio | % | 5 | 10 | 20 |
| Purification (sub-system IV) | ||||
| Topping Column | ||||
| Reflux Ratio | - | 0.6 | 0.6 | 0.6 |
| Distillate to Feed Mole Ratio | - | 0.3 | 0.1 | 0.1 |
| Pressure Column | ||||
| Reflux Ratio | - | 0.75 | 0.6 | 0.75 |
| Distillate to Feed Mole Ratio | - | 0.9 | 0.95 | 0.95 |
| Atmospheric Column | ||||
| Reflux Ratio | - | 0.9 | 0.8 | 0.8 |
| Distillate to Feed Mole Ratio | - | 0.77 | 0.95 | 0.95 |
| Steam Cycle (sub-system V) | ||||
| steam turbine (driver) isentropic efficiency | - | 0.92 | 0.92 | 0.92 |
| condenser pressure | bar | 0.05 | 0.05 | 0.05 |
| Stream | Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| kg/s | 16.88 | 16.90 | 1.31 | 18.20 | 1634.26 | 40.22 | 26.19 | 1586.00 | |
| t | °C | 15.00 | 124.57 | 550.00 | 509.55 | 884.75 | 117.10 | 15.00 | 15.00 |
| p | bar | 10.00 | 29.00 | 29.50 | 26.70 | 6.50 | 50.00 | 10.00 | 1.01 |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 75.74 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.96 | 0.00 | 15.59 | 0.00 | 0.00 | |
| % (mole) | 0.70 | 0.72 | 0.00 | 0.68 | 3.68 | 7.60 | 0.70 | 0.00 | |
| % (mole) | 94.90 | 94.81 | 0.00 | 91.17 | 0.00 | 0.69 | 94.90 | 0.00 | |
| % (mole) | 0.00 | 0.08 | 100.00 | 5.74 | 7.62 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| e | MJ/kg | 49.66 | 49.79 | 1.49 | 46.85 | 0.73 | 25.07 | 49.66 | 0.00 |
| Stream | Units | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| kg/s | 13.50 | 351.62 | 351.62 | 276.06 | 2.40 | 58.62 | 31.32 | 1630.63 | |
| t | °C | 45.00 | 250.00 | 38.00 | 38.52 | 38.00 | 38.00 | 70.97 | 100.00 |
| p | bar | 50.00 | 50.00 | 49.30 | 48.50 | 2.00 | 2.00 | 1.30 | 4.80 |
| % (mole) | 0.00 | 67.99 | 63.84 | 69.46 | 3.03 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 10.18 | 8.81 | 9.58 | 0.99 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 100.00 | 15.88 | 15.40 | 16.50 | 81.24 | 0.65 | 0.00 | 3.67 | |
| % (mole) | 0.00 | 2.09 | 2.25 | 2.45 | 1.33 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 0.05 | 1.75 | 0.05 | 0.95 | 21.65 | 0.08 | 7.55 | |
| % (mole) | 0.00 | 2.65 | 6.70 | 0.62 | 12.08 | 77.69 | 99.92 | 0.00 | |
| e | MJ/kg | 0.65 | 18.64 | 18.34 | 18.30 | 3.10 | 19.20 | 22.42 | 0.17 |
| Stream | Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| kg/s | 43.89 | 43.91 | 71.35 | 56.75 | 45.39 | 116.75 | 92.59 | 403.90 | |
| t | °C | 15.00 | 132.92 | 551.78 | 1157.35 | 550.00 | 1210.13 | 1168.38 | 231.80 |
| p | bar | 10.00 | 38.35 | 37.80 | 39.30 | 30.00 | 31.00 | 30.00 | 3.00 |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 55.26 | 62.10 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 0.66 | 0.00 | 0.00 | 25.49 | 32.16 | 0.00 | |
| % (mole) | 0.70 | 0.72 | 0.45 | 0.00 | 0.00 | 2.69 | 0.73 | 0.00 | |
| % (mole) | 94.90 | 94.86 | 62.08 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | |
| % (mole) | 0.00 | 0.02 | 35.83 | 1.00 | 0.00 | 16.10 | 4.49 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| e | MJ/kg | 49.66 | 49.87 | 31.62 | 2.60 | 0.61 | 18.68 | 22.87 | 0.16 |
| Stream | Units | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| kg/s | 429.16 | 320.66 | 320.66 | 25.26 | 71.35 | 2.86 | 55.08 | 429.16 | |
| t | °C | 1155.90 | 241.75 | 38.00 | 38.00 | 551.78 | 96.74 | 70.96 | 276.81 |
| p | bar | 2.50 | 49.00 | 45.00 | 45.00 | 37.80 | 3.00 | 1.30 | 1.20 |
| % (mole) | 0.00 | 60.54 | 53.50 | 59.35 | 0.00 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 34.55 | 31.88 | 35.35 | 0.66 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 4.99 | 1.87 | 2.14 | 2.30 | 0.45 | 0.00 | 0.00 | 4.99 | |
| % (mole) | 0.00 | 0.03 | 0.04 | 0.04 | 62.08 | 0.00 | 0.00 | 0.00 | |
| % (mole) | 7.90 | 0.00 | 0.07 | 0.00 | 35.83 | 14.11 | 0.00 | 7.90 | |
| % (mole) | 0.00 | 1.37 | 10.44 | 0.81 | 0.00 | 85.89 | 99.99 | 0.00 | |
| e | MJ/kg | 0.92 | 20.40 | 19.93 | 19.35 | 0.03 | 20.56 | 22.43 | 0.13 |
| Stream | Units | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| kg/s | 45.58 | 45.60 | 73.77 | 59.29 | 71.74 | 71.74 | 73.77 | 22.79 | |
| t | °C | 155 | 155.13 | 634.11 | 550.00 | 700.00 | 360.87 | 798.65 | 15.00 |
| p | bar | 9.90 | 9.19 | 30.00 | 9.00 | 8.64 | 29.50 | 29.70 | 5.00 |
| % (mole) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 25.60 | 68.82 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 50.01 | 0.00 | 0.34 | 52.11 | 22.42 | 0.00 | |
| % (mole) | 0.70 | 0.72 | 0.34 | 1.00 | 64.52 | 6.99 | 0.78 | 0.70 | |
| % (mole) | 94.90 | 94.87 | 47.37 | 0.00 | 32.15 | 0.31 | 3.08 | 94.90 | |
| % (mole) | 0.00 | 0.02 | 51.03 | 0.00 | 2.48 | 14.67 | 4.48 | 0.00 | |
| % (mole) | 0.20 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | |
| e | MJ/kg | 49.73 | 49.70 | 24.33 | 0.82 | 8.81 | 10.42 | 28.56 | 49.57 |
| Stream | Units | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| kg/s | 1996.38 | 1996.38 | 1904.12 | 420.90 | 420.90 | 69.46 | 58.41 | 1996.38 | |
| t | °C | 1151.02 | 893.41 | 15.00 | 250.00 | 38.00 | 38.01 | 70.96 | 320.08 |
| p | bar | 4.50 | 4.00 | 1.01 | 50.00 | 47.00 | 48.50 | 1.30 | 2.25 |
| % (mole) | 0.00 | 0.00 | 0.00 | 54.35 | 47.55 | 52.35 | 0.00 | 0.00 | |
| % (mole) | 0.00 | 0.00 | 0.00 | 31.71 | 28.33 | 31.17 | 0.00 | 0.00 | |
| % (mole) | 4.99 | 4.99 | 0.00 | 7.01 | 8.61 | 9.21 | 0.00 | 4.99 | |
| % (mole) | 0.00 | 0.00 | 0.00 | 4.34 | 5.03 | 5.52 | 0.00 | 0.00 | |
| % (mole) | 8.05 | 8.05 | 0.00 | 0.62 | 0.24 | 0.00 | 0.00 | 8.05 | |
| % (mole) | 0.61 | 0.00 | 0.00 | 1.23 | 9.39 | 0.79 | 1.00 | 0.00 | |
| e | MJ/kg | 25.22 | 0.71 | 0.01 | 18.70 | 18.29 | 17.67 | 22.43 | 0.22 |
| Nr. | Case I | Case II | Case III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Comp. | [-] | ()/ [$/MWh] | Comp. | [-] | ()/ [$/MWh] | Comp. | [-] | ()/ [$/MWh] | |
| 1 | R-04 | 0.09 | 16.42 | R-09 | 0.01 | 13.46 | R-06 | 0.10 | 8.62 |
| 2 | CM-01 | 0.92 | 5.95 | MP-EVA | 0.00 | 9.95 | LP-ECO | 0.00 | 3.58 |
| 3 | CM-02 | 0.92 | 5.95 | CM-02 | 0.83 | 4.63 | MP-ECO | 0.00 | 3.20 |
| 4 | T-01 | 0.83 | 1.30 | MP-ECO | 0.00 | 3.49 | T-01 | 0.37 | 2.73 |
| 5 | T-02 | 0.83 | 1.30 | C-02 | 0.13 | 1.62 | CM-03 | 0.82 | 2.49 |
| 6 | CM-06 | 0.95 | 1.11 | T-01 | 0.14 | 1.49 | CM-04 | 0.82 | 2.49 |
| 7 | CM-04 | 0.95 | 0.94 | CM-05 | 0.54 | 1.43 | CM-01 | 0.89 | 1.28 |
| 8 | C-02 | 0.38 | 0.92 | R-05 | 0.24 | 1.22 | CM-02 | 0.87 | 1.15 |
| 9 | C-01 | 0.22 | 0.70 | CM-06 | 0.48 | 0.97 | T-02 | 0.70 | 0.99 |
| 10 | R-05 | 0.50 | 0.52 | CM-01 | 0.80 | 0.89 | R-05 | 0.06 | 0.86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumberg, T.; Morosuk, T.; Tsatsaronis, G. A Comparative Exergoeconomic Evaluation of the Synthesis Routes for Methanol Production from Natural Gas. Appl. Sci. 2017, 7, 1213. https://doi.org/10.3390/app7121213
Blumberg T, Morosuk T, Tsatsaronis G. A Comparative Exergoeconomic Evaluation of the Synthesis Routes for Methanol Production from Natural Gas. Applied Sciences. 2017; 7(12):1213. https://doi.org/10.3390/app7121213
Chicago/Turabian StyleBlumberg, Timo, Tatiana Morosuk, and George Tsatsaronis. 2017. "A Comparative Exergoeconomic Evaluation of the Synthesis Routes for Methanol Production from Natural Gas" Applied Sciences 7, no. 12: 1213. https://doi.org/10.3390/app7121213






