The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Design of Experiment and Data Analysis
2.4. Supercritical Fluid Extraction
2.5. Extraction with Organic Solvents
2.5.1. Comparison of the Extraction Methods
2.5.2. Determination of Amount of Phenolic Compounds in the Pomace
2.6. Chemical Analysis of the Samples
3. Results and Discussion
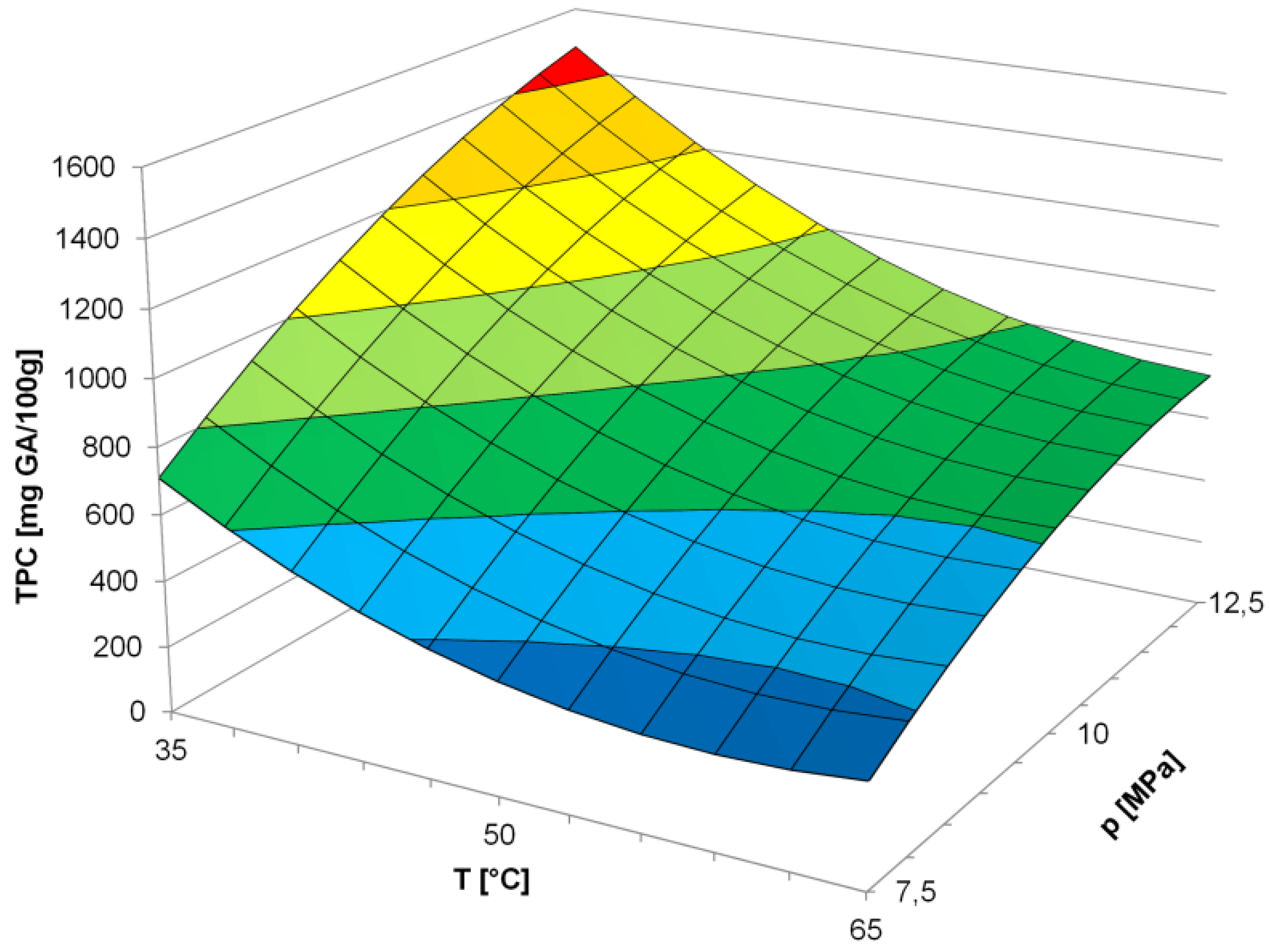
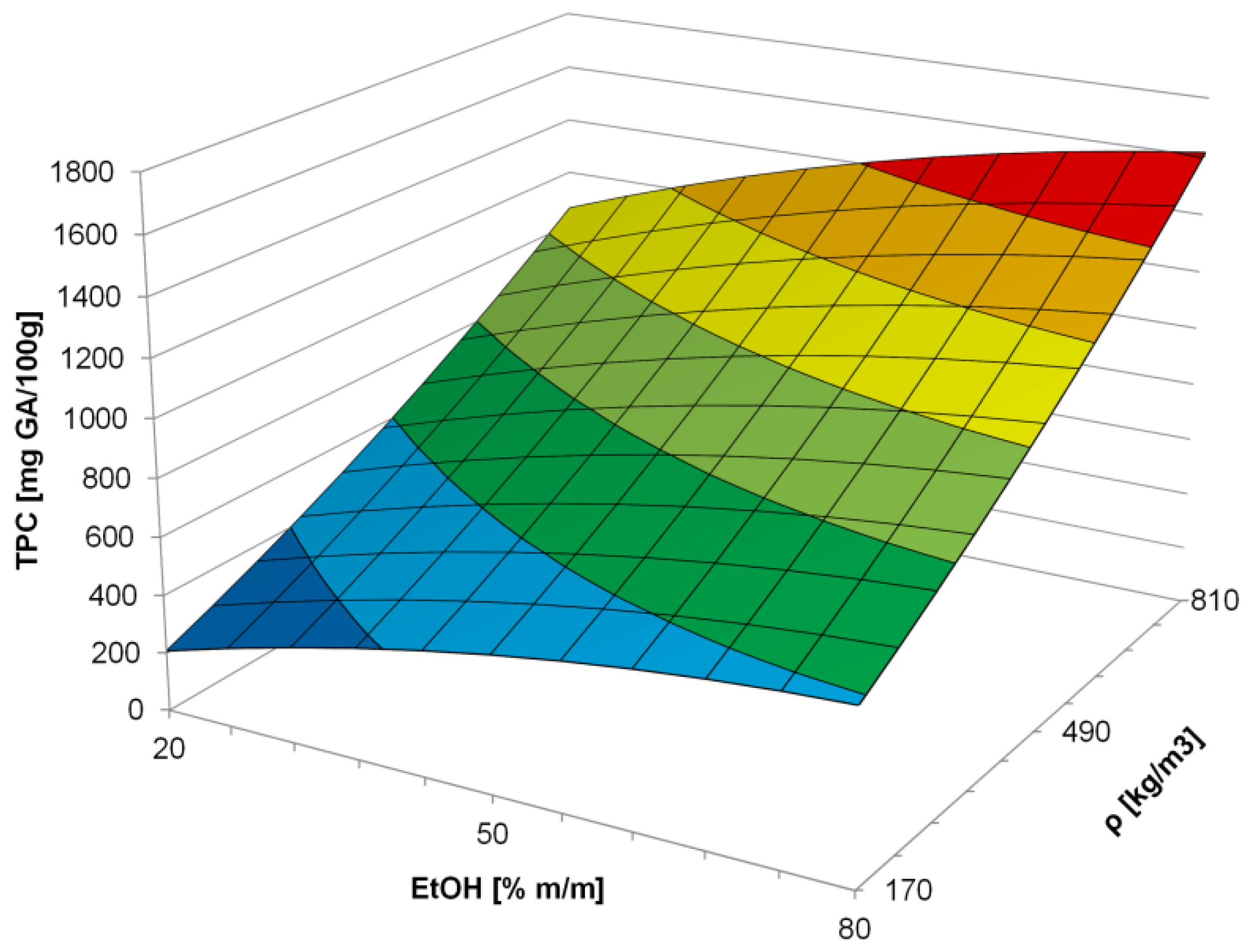
3.1. Effect of Process Conditions on TPC
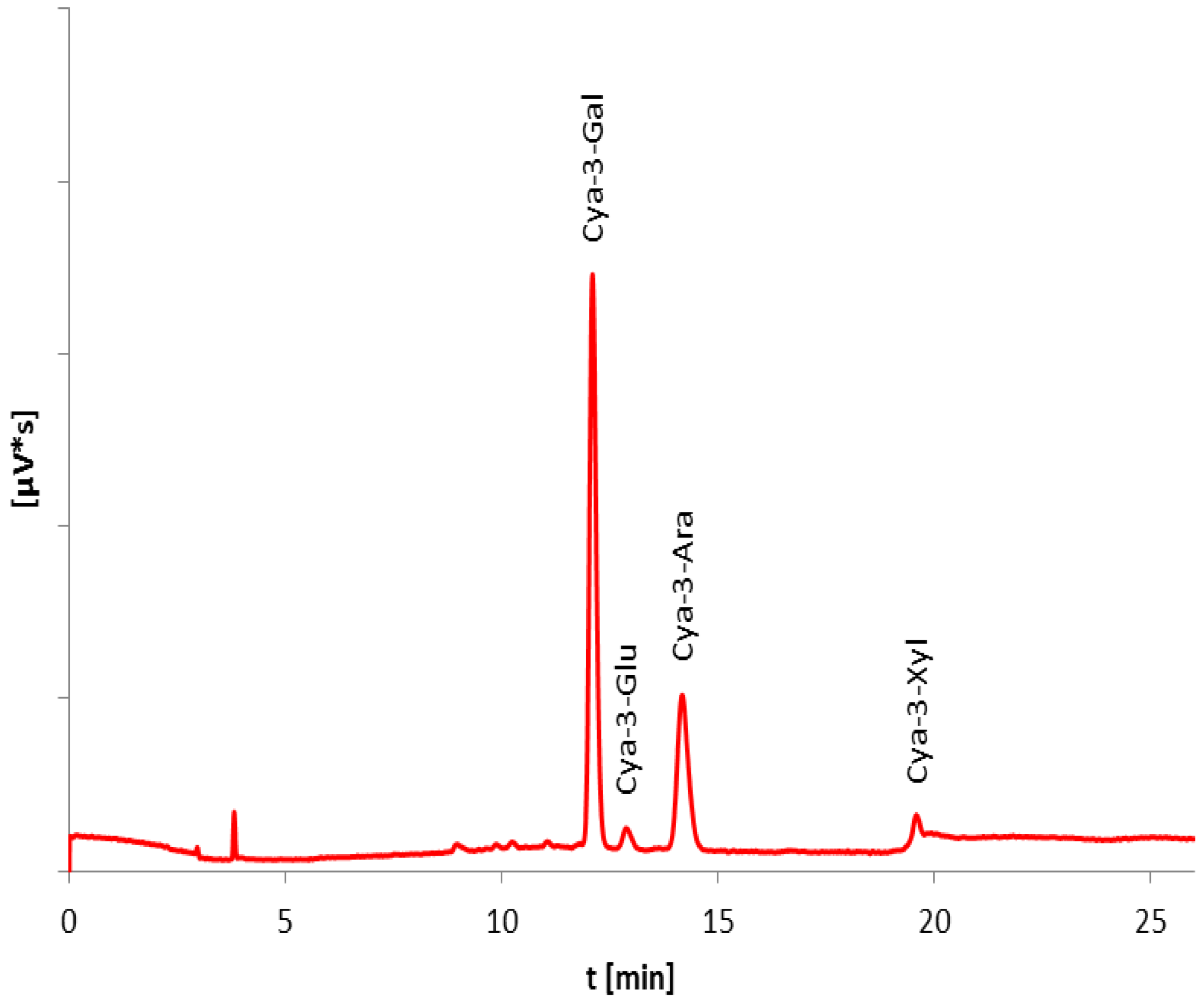
3.2. Anthocyanin Content
3.3. Free Radical Scavenging
3.4. Comparison of SFE and Solvent Extraction
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, L.R.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crops Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Gervasi, T.; Oliveri, F.; Gottuso, V.; Squadrito, M.; Bartolomeo, G.; Cicero, N.; Dugo, G. Nero d’Avola and Perricone cultivars: Determination of polyphenols, flavonoids and anthocyanins in grapes and wines. Nat. Prod. Res. 2016, 30, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Lo Bianco, R. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2016, 30. [Google Scholar] [CrossRef]
- Alesci, A.; Cicero, N.; Salvo, A.; Palombieri, D.; Zaccone, D.; Dugo, G.; Bruno, M.; Vadalá, R.; Lauriano, E.R.; Pergolizzi, S. Extracts deriving from olive mill waste waer and their effects on the liver of the goldfish Carassius aureus fed with hypercholesterolemic diet. Nat. Prod. Res. 2014, 28, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifakhr, M.; Parjikolaei, B.R.; Roda-Serrat, M.C.; Norddahl, B. Production of anthocyanin from chokeberry (Aronia melanocarpa) pomace by extraction, enzymatic treatment, and membrane filtration. In Proceedings of the Euromembrane 2015, Aachen, Germany, 6–10 September 2015. [Google Scholar]
- Baranowski, K.; Baca, E.; Salamon, A.; Michałowska, D.; Meller, D.; Karaś, M. Possibilities of retrieving and making a practical use of phenolic compounds from the waste products: Black currant and chokeberry pomace and spent hops. Zywnosc Nauka Technol. Jakosc 2009, 65, 100–109. [Google Scholar]
- Nawirska, A.; Kwaśniewska, M. Dietary fiber fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Bartosz, G. Druga Twarz Tlenu: Wolne Rodniki w Przyrodzie; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2006. [Google Scholar]
- Taylor, L.T. Supercritical Fluid Extraction; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Sep. Sci. Technol. 2016, 51, 1472–1479. [Google Scholar] [CrossRef]
- Murga, R.; Sanz, M.T.; Beltrán, S.; Cabezas, J.L. Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide. J. Supercrit. Fluids 2002, 23, 113–121. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. High pressure carbon dioxide pasteurization of solid foods: Current knowledge and future outlooks. Trends Food Sci. Technol. 2011, 22, 427–441. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Pinelo, M.; Ruiz-Rodriguez, A.; Sineiro, J.; Señoráns, F.J.; Reglero, G.; Nuñez, M.J. Supercritical fluid and solid-liquid extraction of phenolic antioxidants from grape pomace: A comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niu, H.; Li, D.; Zhang, J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J. Food Eng. 2008, 89, 298–302. [Google Scholar] [CrossRef]
- Stavroulias, S.; Panayiotou, C. Determination of optimal conditions for the extraction of squalene from olive pomace with supercritical CO2. Chem. Biochem. Eng. Q. 2005, 19, 373–381. [Google Scholar]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 112, 99–108. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Ouyang, L.B. New correlations for predicting the density and viscosity of supercritical carbon dioxide under conditions expected in carbon capture and sequestration operations. Open Pet. Eng. J. 2011, 4, 13–21. [Google Scholar] [CrossRef]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press Inc.: New York, NY, USA, 1982. [Google Scholar]
- Gao, X.; Ohlander, M.; Jeppson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophaerhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J. Stabilization and application of anthocyanin chokeberry dye to colouring of beverages. Acta Sci. Pol. Technol. Aliment. 2002, 1, 37–45. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.L.; Droy-Lefaix, M.T.; Packer, L. The nitric oxide scavenging properties of Gingko biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Rad. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radical, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Food Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
| Sample | Carbon Dioxide Properties | Extract Composition | Activity Against | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T (X1) | p (X2) | c (X3) | ρ (X4) | ACNs | TPC | DPPH• | ABTS•+ | NO• | O2•− | •OH | |
| (°C) | (MPa) | (% m/m) | (kg m−3) | (mg C3G per 100 g) | (mg GA per 100 g) | (µmol Trolox per 100 g) | (µmol Trolox per 100 g) | (µmol curc per 100 g) | (µmol querc per 100 g) | (mmol sorb per 100 g) | |
| 1 | 50 (0) | 10.0 (0) | 50 (0) | 398 (−0.28) | 410.3 | 711.4 | 1309.2 | 1247.8 | 24.7 | 648.8 | 41.15 |
| 2 | 50 (0) | 12.5 (+1) | 80 (+1) | 596 (+0.33) | 749.1 | 1197.7 | 2017.2 | 2007.3 | 20.3 | 848.0 | 49.00 |
| 3 | 50 (0) | 12.5 (+1) | 20 (−1) | 596 (+0.33) | 341.3 | 680.1 | 987.1 | 1214.1 | 16.2 | 621.8 | 28.41 |
| 4 | 50 (0) | 7.5 (−1) | 80 (+1) | 177 (−0.98) | 324.0 | 545.4 | - | - | - | - | - |
| 5 | 50 (0) | 7.5 (−1) | 20 (−1) | 177 (−0.98) | 116.8 | 187.2 | 459.7 | 411.0 | 13.3 | 249.4 | 16.44 |
| 6 | 50 (0) | 10.0 (0) | 50 (0) | 398 (−0.28) | 430.3 | 802.0 | - | - | - | - | - |
| 7 | 65 (+1) | 10.0 (0) | 80 (+1) | 266 (−0.70) | 455.5 | 787.2 | - | - | - | - | - |
| 8 | 65 (+1) | 10.0 (0) | 20 (−1) | 266 (−0.70) | 197.9 | 367.4 | - | - | - | - | - |
| 9 | 35 (−1) | 10.0 (0) | 80 (+1) | 766 (+0.86) | 1002.4 | 1520.7 | 2907.0 | 3384.1 | 29.0 | 1138.4 | 72.24 |
| 10 | 35 (−1) | 10.0 (0) | 20 (−1) | 766 (+0.86) | 574.8 | 1002.2 | - | - | - | - | - |
| 11 | 50 (0) | 10.0 (0) | 50 (0) | 398 (−0.28) | 422.4 | 681.4 | - | - | - | - | - |
| 12 | 65 (+1) | 12.5 (+1) | 50 (0) | 419 (−0.22) | 437.5 | 801.4 | - | - | - | - | - |
| 13 | 65 (+1) | 7.5 (−1) | 50 (0) | 169 (−1.00) | 201.6 | 356.4 | 671.0 | 702.4 | 18.7 | 328.4 | 27.28 |
| 14 | 35 (−1) | 12.5 (+1) | 50 (0) | 812 (+1.00) | 967.7 | 1429.9 | - | - | - | - | - |
| 15 | 35 (−1) | 7.5 (−1) | 50 (0) | 207 (−0.88) | 406.5 | 641.1 | - | - | - | - | - |
| S | full extraction of polyphenols with methanol | 1812.4 | 3204.4 | 6418.3 | 6877.4 | 54.7 | 2590.7 | 135.77 | |||
| T | ethanol extract (ethanol-solid ratio 50% m/m) | 245.3 | 433.0 | 923.1 | 908.6 | 11.9 | 341.1 | 30.12 | |||
| Model Constant | Value | Significance (p-Value) |
|---|---|---|
| a0 | 731.60 | - |
| a1 | −285.19 | 0.0007 |
| a2 | 297.38 | 0.0006 |
| a3 | 226.76 | 0.0011 |
| a11 | 171.19 | 0.0587 |
| a22 | −85.59 | 0.1424 |
| a33 | 16.59 | 0.7920 |
| a12 | −95.95 | 0.0908 |
| a13 | −24.68 | 0.7144 |
| a23 | 39.85 | 0.6036 |
| Model F-Value | 15.229 | 0.0038 |
| Model Constant | Value | Significance (p-Value) |
|---|---|---|
| a0 | 882.60 | - |
| a3 | 232.08 | <0.0001 |
| a4 | 470.51 | <0.0001 |
| a33 | −70.79 | 0.1645 |
| a44 | 55.64 | 0.2467 |
| a34 | 43.47 | 0.2897 |
| Model F-value | 84.660 | <0.0001 |
| Parameter | DPPH• | ABTS•+ | NO• | O2−• | OH• |
|---|---|---|---|---|---|
| reference compound | Trolox | Trolox | curcumin | quercetin | sorbitol |
| equivalent of 100 g of pomace | 459.7–2907.0 µmol | 411.0–3384.1 µmol | 13.3–29.0 µmol | 249.4–1138.4 µmol | 16.44–72.24 mmol |
| equivalent of 1 g of GAE | 1.87 ± 0.33 mmol | 1.93 ± 0.23 mmol | 36.3 ± 21.4 µmol | 0.92 ± 0.22 mmol | 58.7 ± 19.5 mmol |
| IC50 of reference compound | 23.3 µmol/L (5.83 mg/L) | 10.4 µmol/L (2.60 mg/L) | 33.0 µmol/L (12.16 mg/L) | 3.89 µmol/L (1.18 mg/L) | 27.1 mmol/L (4.94 g/L) |
| GAE for 50% of inhibition | 12.46 mg/L | 5.39 mg/L | 909.09 mg/L | 1.63 mg/L | 461.67 mg/L |
| correlation with TPC | 0.9715 | 0.9490 | 0.6249 | 0.9803 | 0.9248 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Appl. Sci. 2017, 7, 322. https://doi.org/10.3390/app7040322
Woźniak Ł, Marszałek K, Skąpska S, Jędrzejczak R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Applied Sciences. 2017; 7(4):322. https://doi.org/10.3390/app7040322
Chicago/Turabian StyleWoźniak, Łukasz, Krystian Marszałek, Sylwia Skąpska, and Renata Jędrzejczak. 2017. "The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace" Applied Sciences 7, no. 4: 322. https://doi.org/10.3390/app7040322






