Improved Imaging of Magnetically Labeled Cells Using Rotational Magnetomotive Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
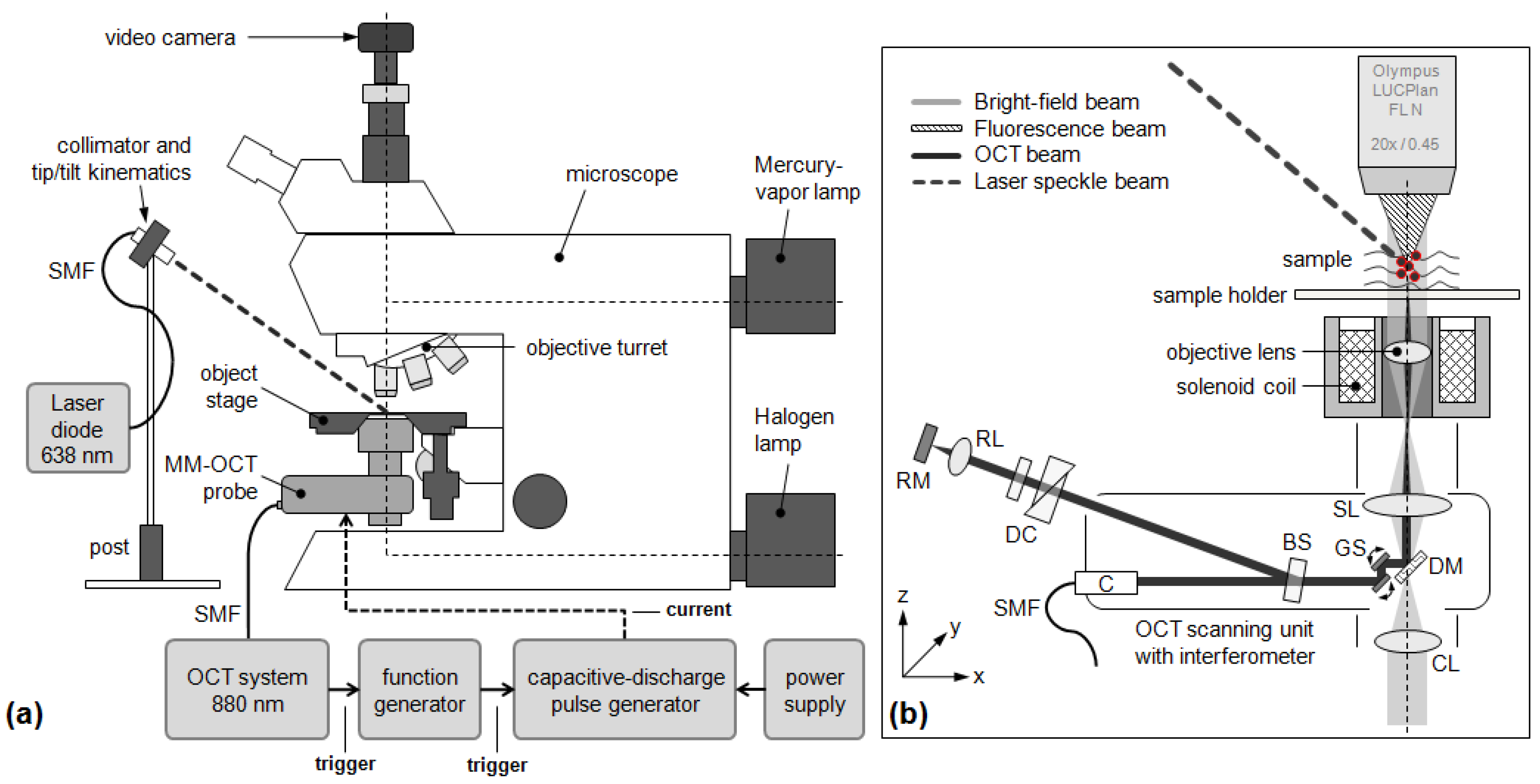
2.1. Experimental Setup for MM-OCT
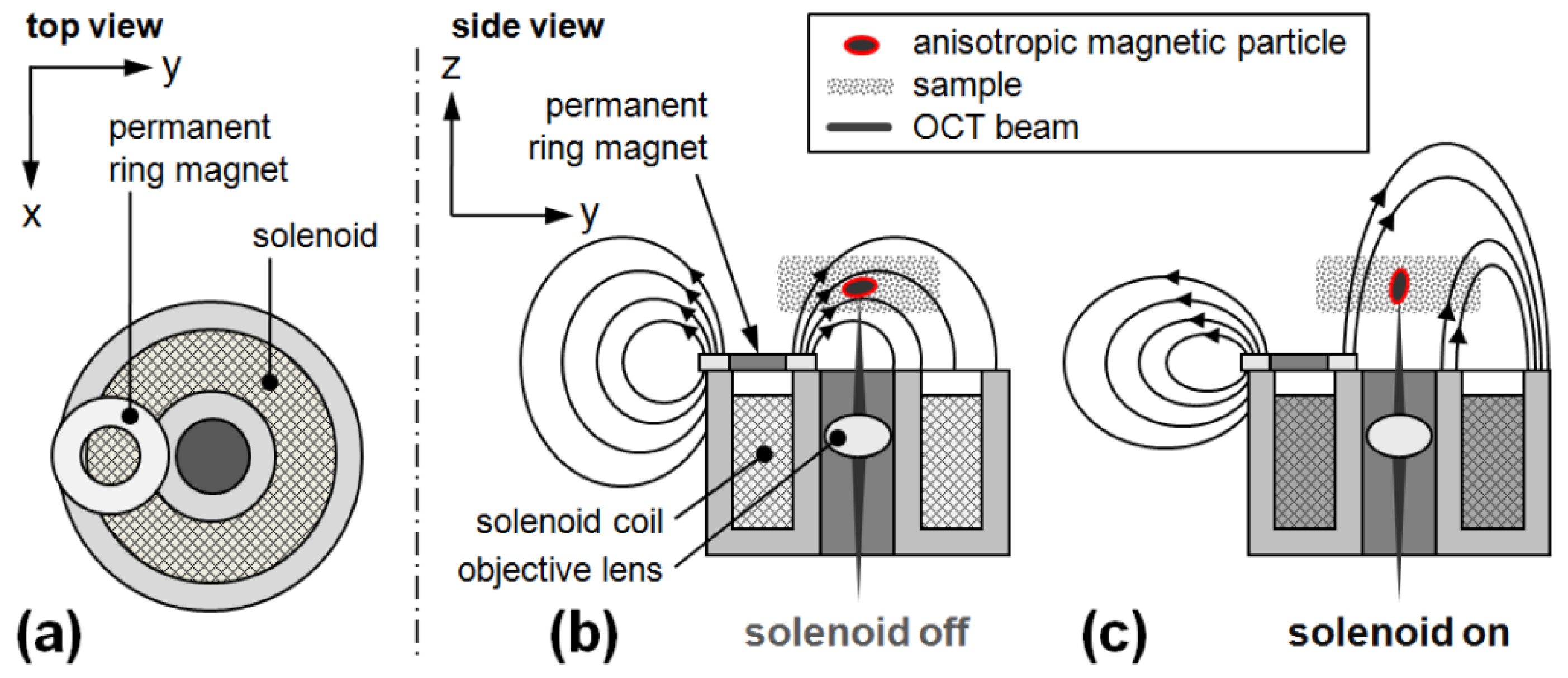
2.2. Additional Experimental Setup for Rotational MM-OCT
3. Results
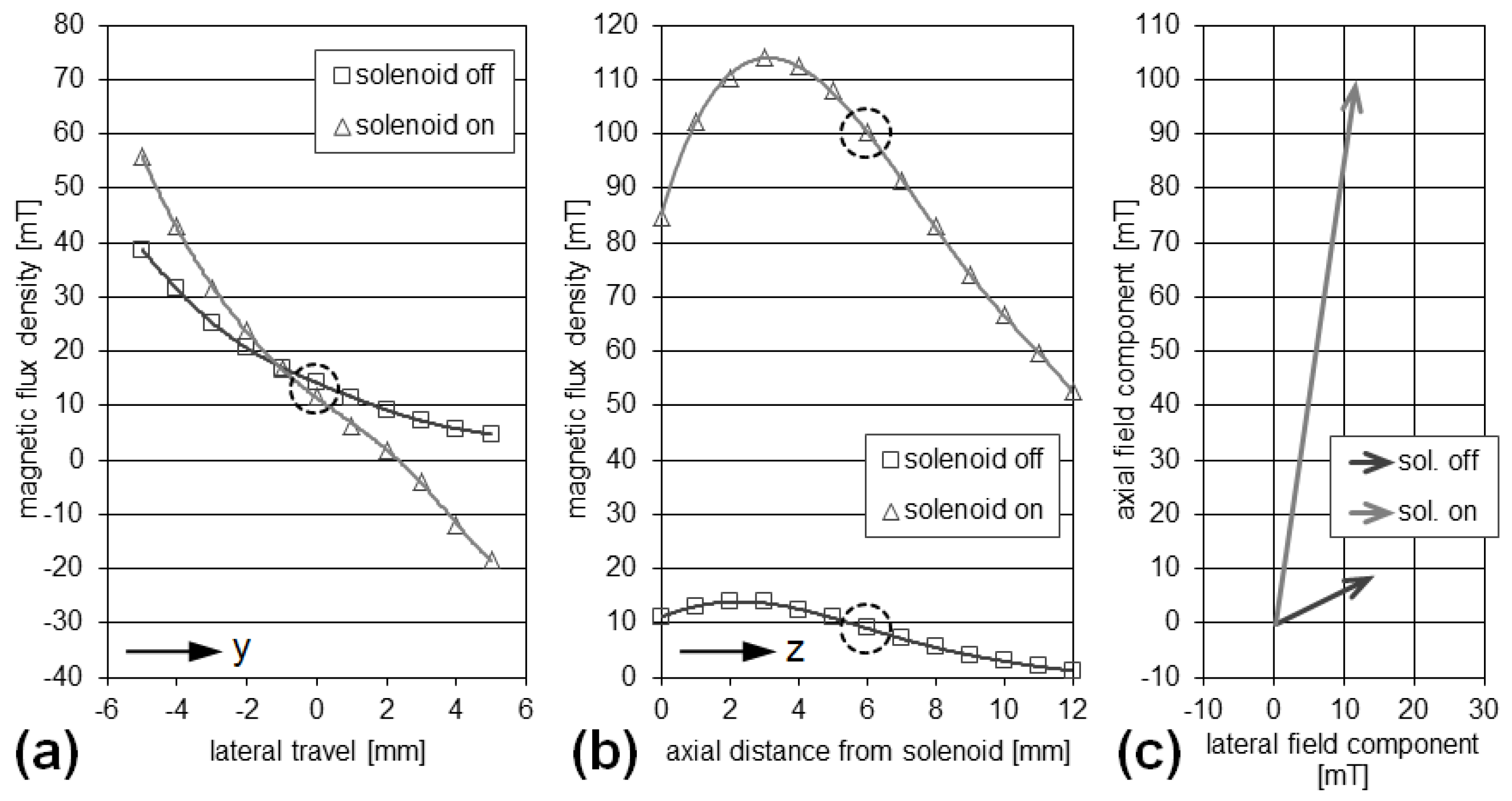
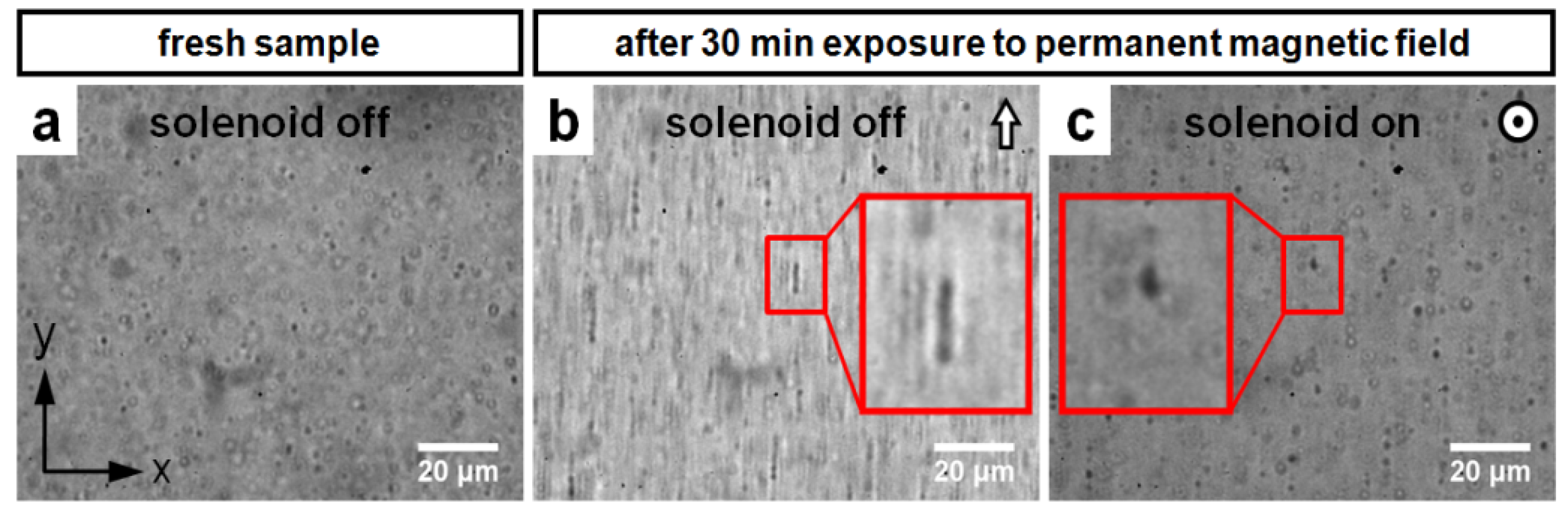
3.1. Validation of the Rotational Magnetomotive Concept
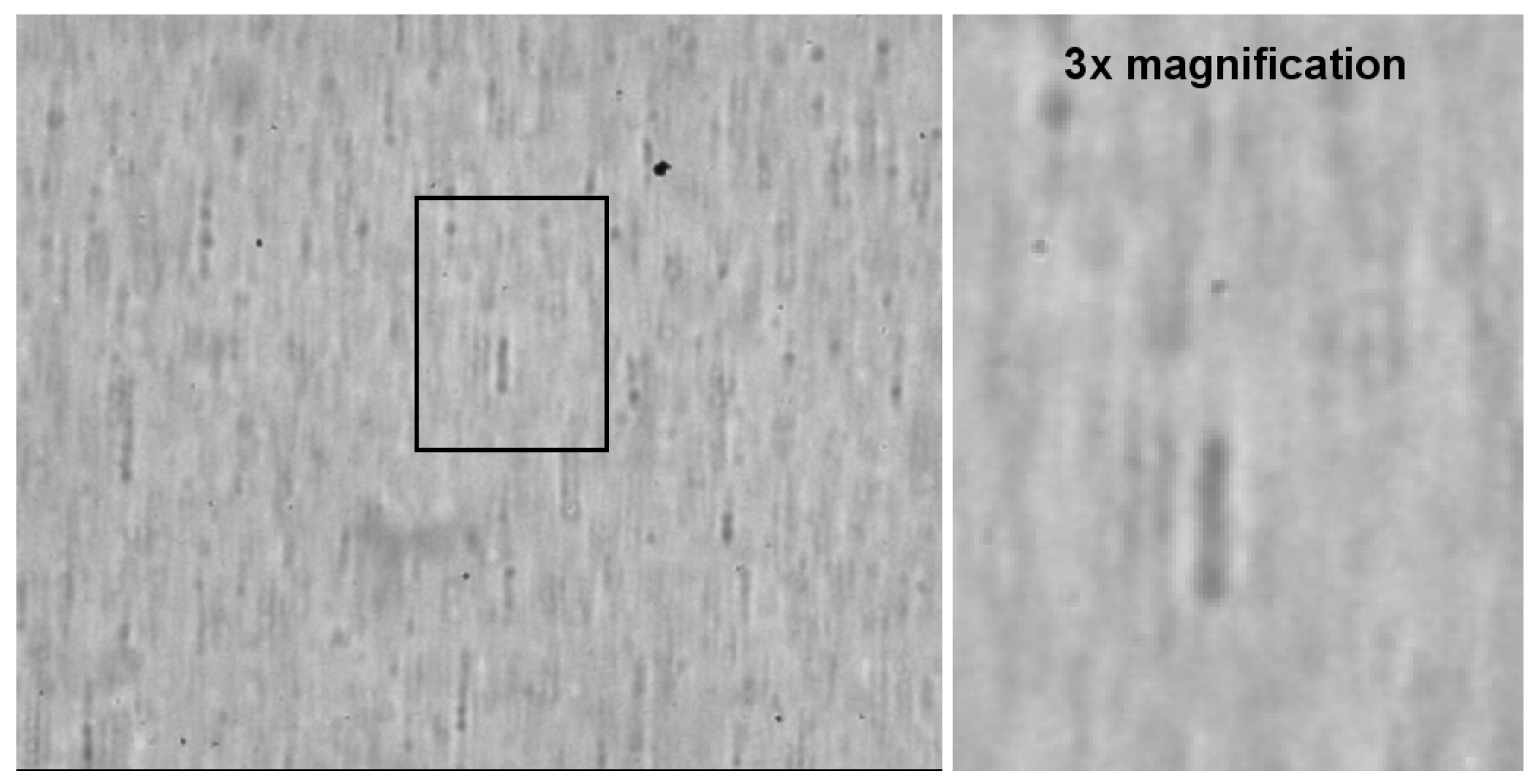
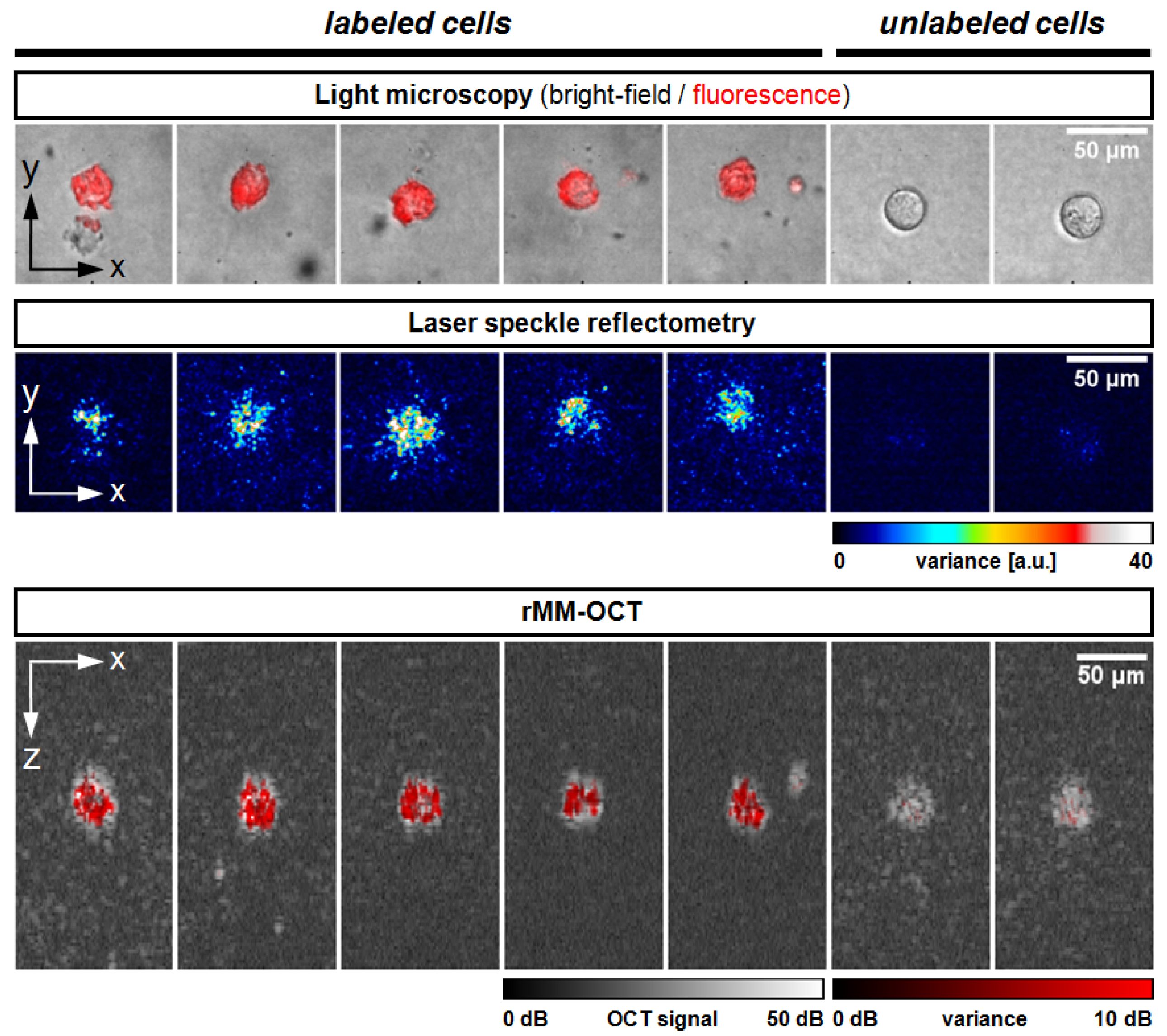
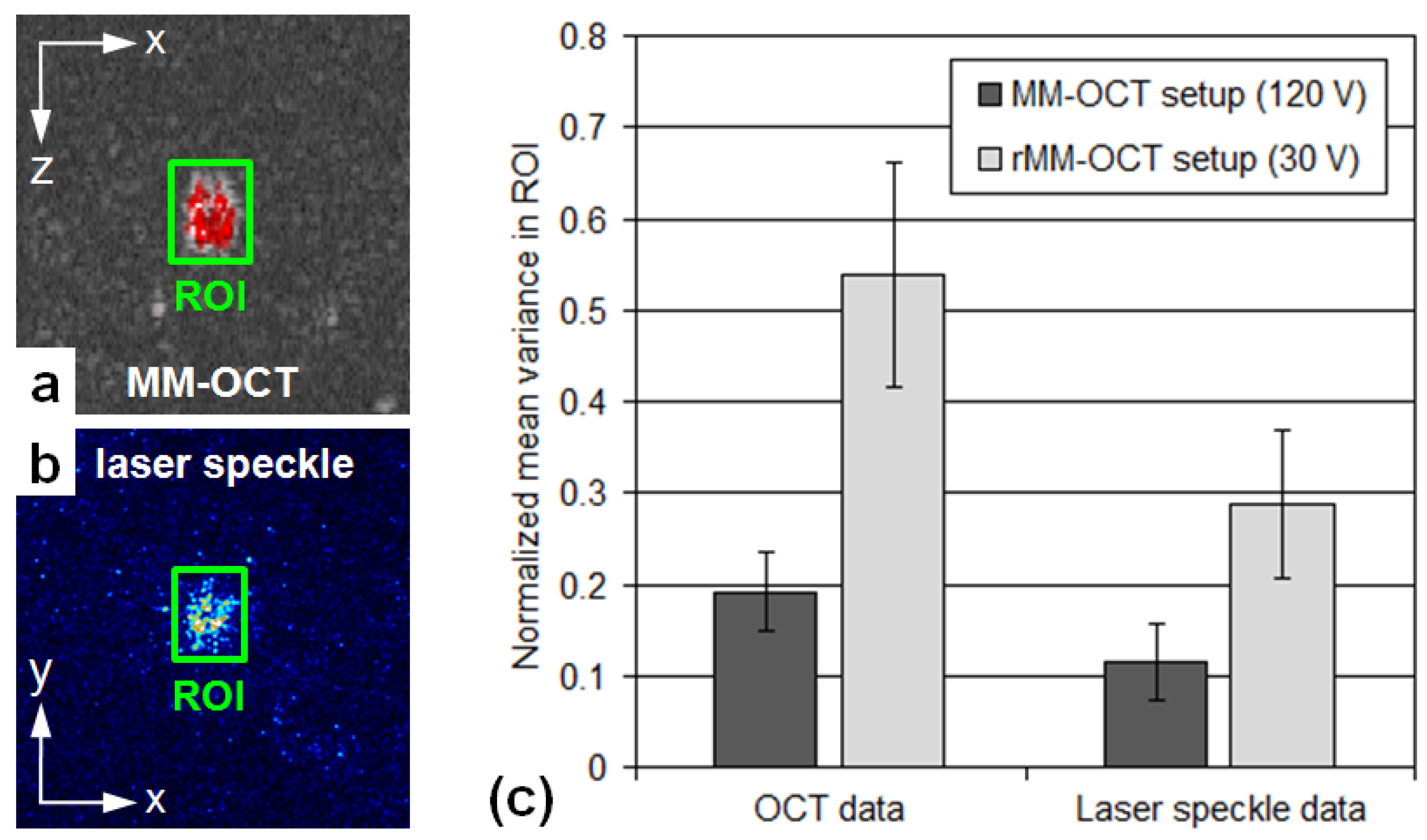
3.2. Cell Imaging
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oldenburg, A.L.; Gunther, J.R.; Boppart, S.A. Imaging magnetically labeled cells with magnetomotive optical coherence tomography. Opt. Lett. 2005, 30, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, A.L.; Gallippi, C.M.; Tsui, F.; Nichols, T.C.; Beicker, K.N.; Chhetri, R.K.; Spivak, D.; Richardson, A.; Fischer, T.H. Magnetic and contrast properties of labeled platelets for magnetomotive optical coherence tomography. Biophys. J. 2010, 99, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, A.L.; Wu, G.; Spivak, D.; Tsui, F.; Wolberg, A.S.; Fischer, T.H. Imaging and elastometry of blood clots using magnetomotive optical coherence tomography and labeled platelets. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahmad, A.; Boppart, S.A. Dual-coil magnetomotive optical coherence tomography for contrast enhancement in liquids. Opt. Express 2013, 21, 7139–7147. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Sanchez, P.; Bodensiek, K.; Li, S.; Simons, M.; Schaap, I.A.T. Cellvisco-elasticity measured with AFM and optical trapping at sub-micrometer deformations. PLoS ONE 2012, 7, e45297. [Google Scholar] [CrossRef] [PubMed]
- Cimalla, P.; Werner, T.; Winkler, K.; Mueller, C.; Wicht, S.; Gaertern, M.; Mehner, M.; Walther, J.; Rellinghaus, B.; Wittig, D.; et al. Imaging of nanoparticle-labeled stem cells using magnetomotive optical coherence tomography, laser speckle reflectometry, and light microscopy. J. Biomed. Opt. 2015, 20, 036018. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjemeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Carido, M.; Meinhardt, A.; Kurth, T.; Karl, M.O.; Ader, M.; Tanaka, E.M. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS ONE 2013, 8, e54552. [Google Scholar] [CrossRef] [PubMed]
- Carido, M.; Zhu, Y.; Postel, K.; Benkner, B.; Cimalla, P.; Karl, M.O.; Kurth, T.; Paquet-Durand, F.; Koch, E.; Münch, T.A.; et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.; Walther, J.; Cimalla, P.; Mehner, M.; Koch, E. Endoscopic optical coherence tomography device for forward imaging with broad field of view. J. Biomed. Opt. 2012, 17, 071302. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Gazeau, F.; Bacri, J.C. Rotational magnetic endosome microrheology: Viscoelastic architecture inside living cells. Phys. Rev. E 2003, 67, 061908. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; Häfeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Coffey, P.J.; Girman, S.; Wang, S.M.; Hetherington, L.; Adamson, P.; Greenwood, J.; Lund, R.D. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat. Neurosci. 2002, 5, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, B.; Lund, R.D. Morphological changes in the Royal College of Surgeons rat retina during photoreceptor degeneration and after cell-based therapy. J. Comp. Neurol. 2005, 491, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, I.; Cuenca, N.; Sauvé, Y.; Wang, S.; Lund, R.D. Preservation of outer retina and its synaptic connectivity following subretinal injections of human RPE cells in the Royal College of Surgeons rat. Exp. Eye Res. 2007, 85, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Song, H.M.; Leonov, A.P.; Hale, J.A.; Oh, D.; Ong, Q.K.; Ritchie, K.; Wei, A. Gyromagnetic imaging: Dynamic optical contrast using goldnanostars with magnetic cores. J. Am. Chem. Soc. 2009, 131, 9728–9734. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Wei, Q.; Ong, Q.K.; Wei, A. Plasmon-resonant nanoparticles and nanostars with magnetic cores: Synthesis and magnetomotive imaging. ACS Nano 2010, 4, 5163–5173. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimalla, P.; Walther, J.; Mueller, C.; Almedawar, S.; Rellinghaus, B.; Wittig, D.; Ader, M.; Karl, M.O.; Funk, R.H.W.; Brand, M.; et al. Improved Imaging of Magnetically Labeled Cells Using Rotational Magnetomotive Optical Coherence Tomography. Appl. Sci. 2017, 7, 444. https://doi.org/10.3390/app7050444
Cimalla P, Walther J, Mueller C, Almedawar S, Rellinghaus B, Wittig D, Ader M, Karl MO, Funk RHW, Brand M, et al. Improved Imaging of Magnetically Labeled Cells Using Rotational Magnetomotive Optical Coherence Tomography. Applied Sciences. 2017; 7(5):444. https://doi.org/10.3390/app7050444
Chicago/Turabian StyleCimalla, Peter, Julia Walther, Claudia Mueller, Seba Almedawar, Bernd Rellinghaus, Dierk Wittig, Marius Ader, Mike O. Karl, Richard H. W. Funk, Michael Brand, and et al. 2017. "Improved Imaging of Magnetically Labeled Cells Using Rotational Magnetomotive Optical Coherence Tomography" Applied Sciences 7, no. 5: 444. https://doi.org/10.3390/app7050444





