1. Introduction
Global environmental pollution and destruction are issues of increasing concern in today’s society, thus necessitating effective catalysts to degrade harmful pollutants. In recent years, the utilization of semiconductors has garnered widespread interest, as a means to induce and improve photocatalytic reactions. A prime example would be metal oxide semiconductor powders, which have exhibited activity when irradiated with ultraviolet (UV) light, and have been widely used as photocatalysts for the degradation of pollutants [
1,
2]. Titania (TiO
2) has been widely used as a catalyst, due to its many advantageous qualities, namely optical and electronic properties, low cost, high level of photocatalytic activity, chemical stability and non-toxicity [
3,
4,
5].
Although the most widely used system, TiO
2, is usually in nanopowdered-form, as it provides high surface area, filtration is required at the end of a reaction, which complicates the whole process. The other popular form of TiO
2, supported TiO
2 thin films, on the other hand, mitigates the need for any further post-treatment filtering of the decontaminated fluid, in spite of the limited surface area compared to the dispersed nanoparticle form [
6]. Film coatings induce the catalytic properties of TiO
2 while taking advantage of the substrate’s mechanical properties. There are a number of ways by which TiO
2 films are fabricated onto supporting materials (substrates), such as the sol gel method, sputtering technique, electrochemical process, chemical vapour deposition (CVD) and spray pyrolysis [
7].
Spray-ILGAR is an extension of the ion layer gas reaction (ILGAR) technique, which was developed and patented by the group of Professor Dr. Christian-Herbert Fischer at the Helmholtz-Zentrum Berlin for Materials and Energy, in Berlin, Germany [
8], and which is equivalent to the aerosol assisted chemical vapour depostition (AACVD) method [
9]. ILGAR is a non-vacuum, thin-film deposition technique, involving a sequential and cyclic process that enables the deposition of semiconductor thin films, especially chalcopyrite absorber layers and buffer layers. This chemical process permits deposition of layers in a homogenous, adherent and mechanically stable form with the absence of vacuum or high temperatures. This whole process is able to be automated and scaled up. For the case of metal oxides, it usually involves the deposition of an intermediate precursor film (alkoxide), followed by the addition of energy (heating) to chemically convert the precursor to the final product (metal oxide).
In the present work, titania on stainless steel with different concentrations, thicknesses and preparation temperatures was obtained by the aforementioned Spray-ILGAR technique. The photocatalytic activity of the prepared samples was measured in the photocatalytic degradation of gaseous acetaldehyde under UV light irradiation.
3. Results and Discussion
For titania prepared on stainless steel using the Spray-ILGAR technique, the standard procedure is to use titania (TiO
2) precursor of titanium diisopropoxide bis(acetylacetone) in ethanol with a concentration of 6.83 mM, sprayed for 12 min at a temperature of 460 °C. Using these procedures, the thickness of studied films was determined to be 101 nm. However, this method is usually used for the preparation of solar cells, and not as a photocatalyst. So in order to determine the best condition to prepare a sample with the best photocatalytic activity in the photocatalytic decomposition of air pollutants, the TiO
2 on stainless steel was prepared with different concentrations of TiO
2-precursor, different heating temperatures, and different durations of spraying time. The thickness of all the samples prepared is presented in
Table 1. All of the samples showed similar thickness, in the range of 94–107 nm. Only the sample that had been prepared with 6 min of spraying time showed a lower thickness of only 75 nm, indicating that the spraying time may have been a bit too short for a thicker layer to form. This shows that the thickness of the TiO
2 film on stainless steel was only influenced by the spraying time, and not so much by the concentration of the TiO
2 precursor and heating temperature. This is in accordance with the findings of Fischer et al. [
8] where they reported that a reasonable time of deposition by ILGAR is needed in order to obtain layers with a maximum thickness of about 100 nm.
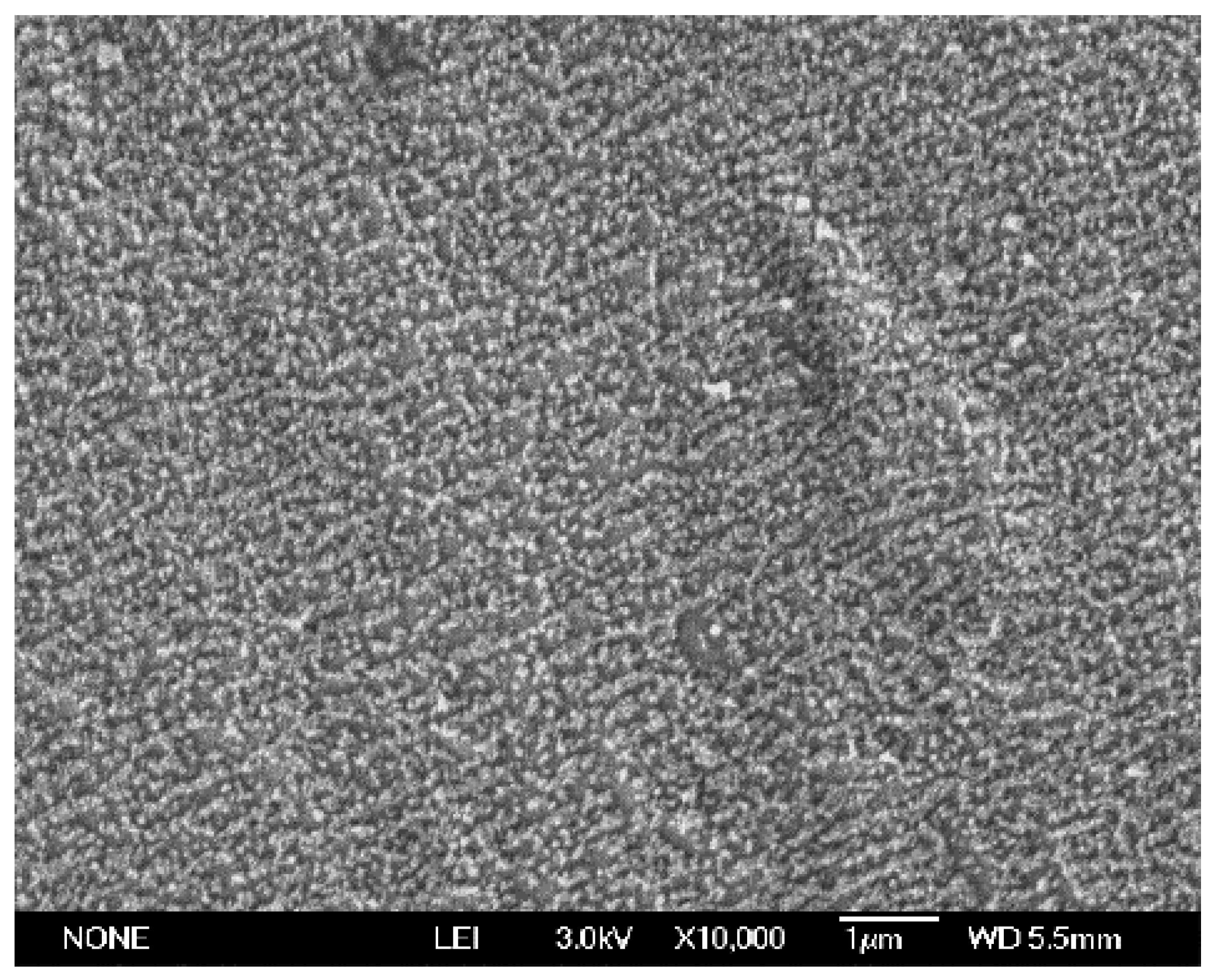
The SEM image in
Figure 1 shows that the TiO
2 prepared by Spray-ILGAR technique using 6.83 mM of TiO
2 precursor, heated at 460 °C and 12 min of spraying time. The SEM image shows that the TiO
2 was dispersed uniformly on top of the stainless steel. Similar morphology has also been reported for TiO
2 coated on the surface of glass by the same method [
10]. The layer of TiO
2 was also very durable, as the attachment was very strong. This was proven by the Scotch Tape test, where the layer of TiO
2 remained intact even after removal of the adhesive tapes from the coatings.
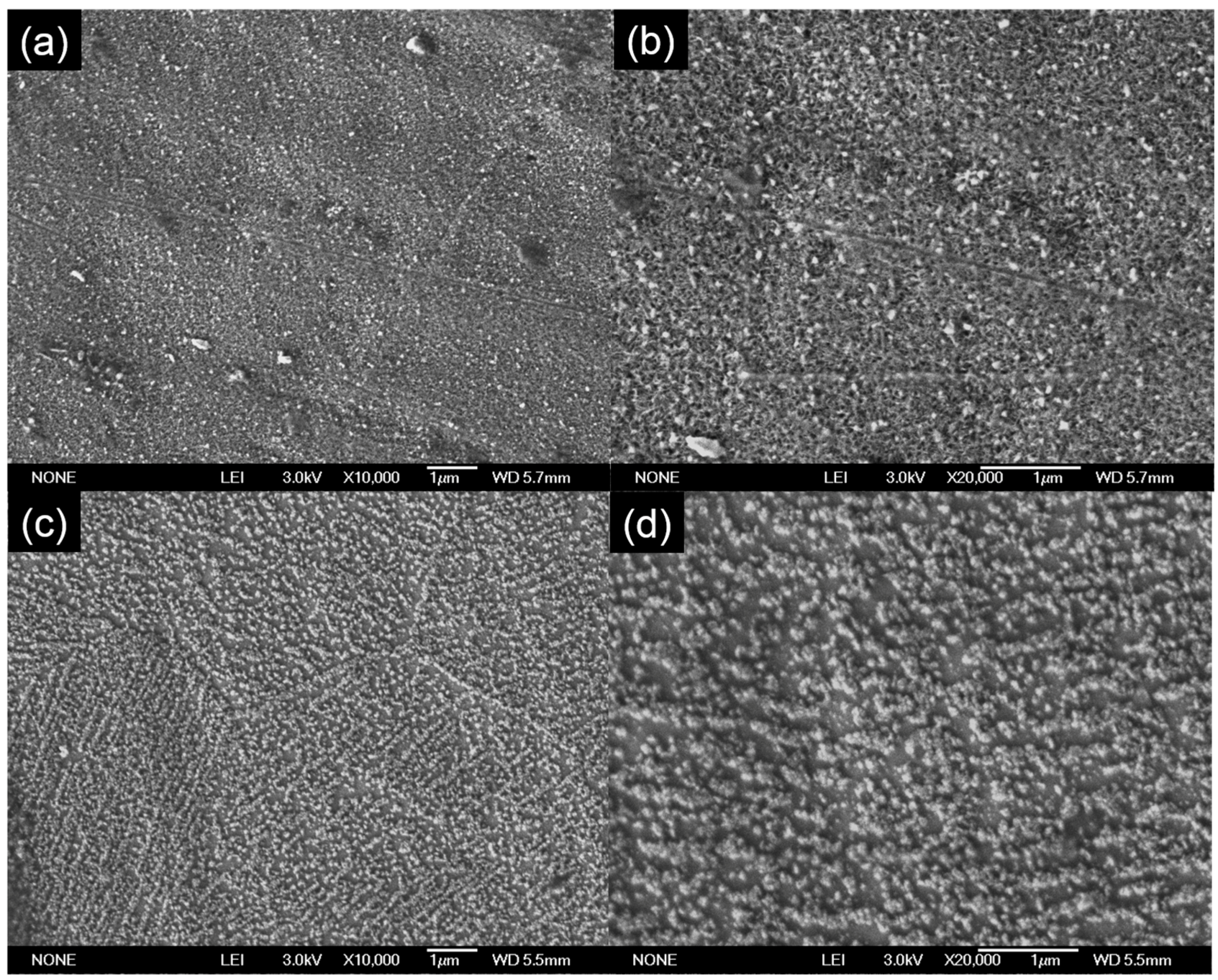
The concentration of the TiO
2 on the stainless steel can easily be changed by changing the concentration of the TiO
2-precursor solution (titanium diisopropoxide bis(acetylacetone) solution). Higher concentrations will result in a more compact and dense layer (
Figure 2a). This was further proven by the SEM image was taken at a higher magnification value (20,000), as shown in
Figure 2b. By lowering the concentration of the precursor solution, a less dense layer of TiO
2 was produced (
Figure 2c). Some spaces can be seen in between the TiO
2 particles when lower concentrations of TiO
2 precursor were used, as can be clearly seen in the SEM image with higher magnification (
Figure 2d). Using different temperatures did not change the distribution of the samples much.
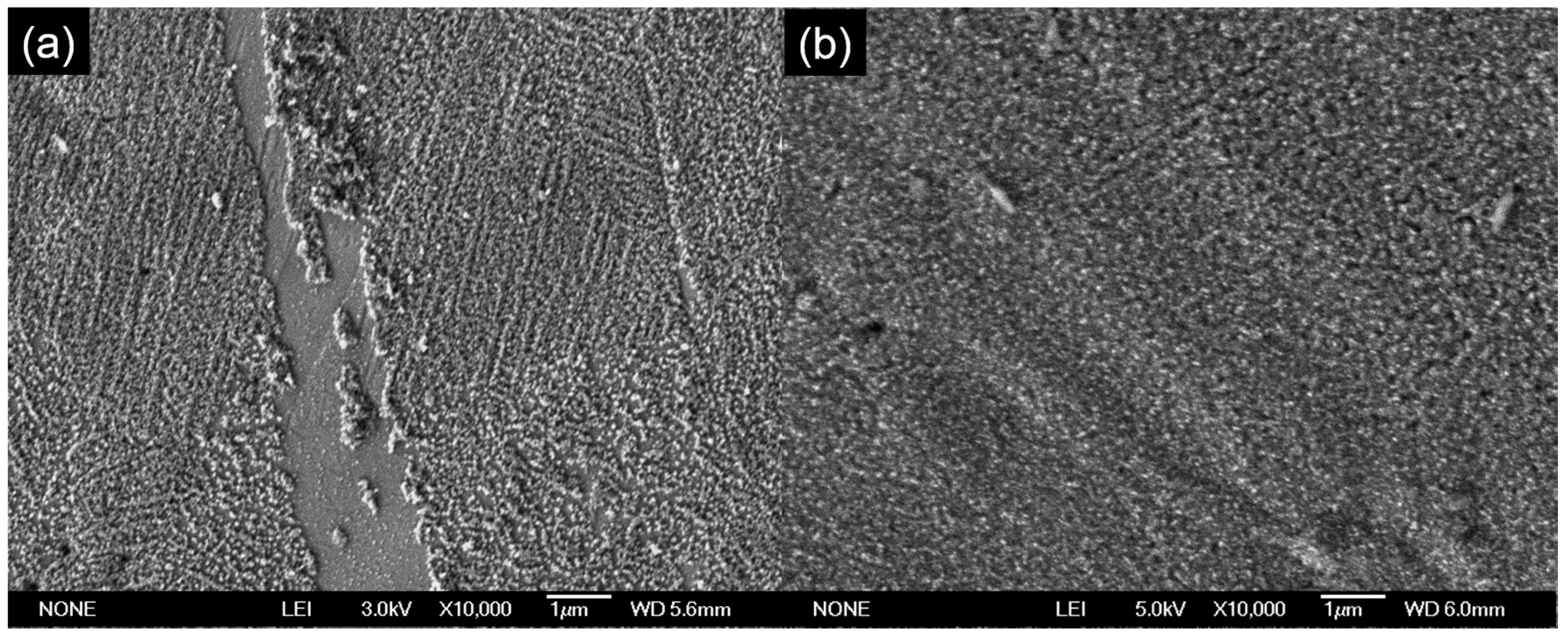
When the spraying duration was shortened to 6 min, some parts of the stainless steel were uncoated by the TiO
2 (
Figure 3a). This is most probably caused by insufficient spraying time for the layer to form [
8]. The thickness measurement also supports this hypothesis. As for the sample prepared by using 24 min of spraying time, a nice dense and compact layer of TiO
2 was formed (
Figure 3b).
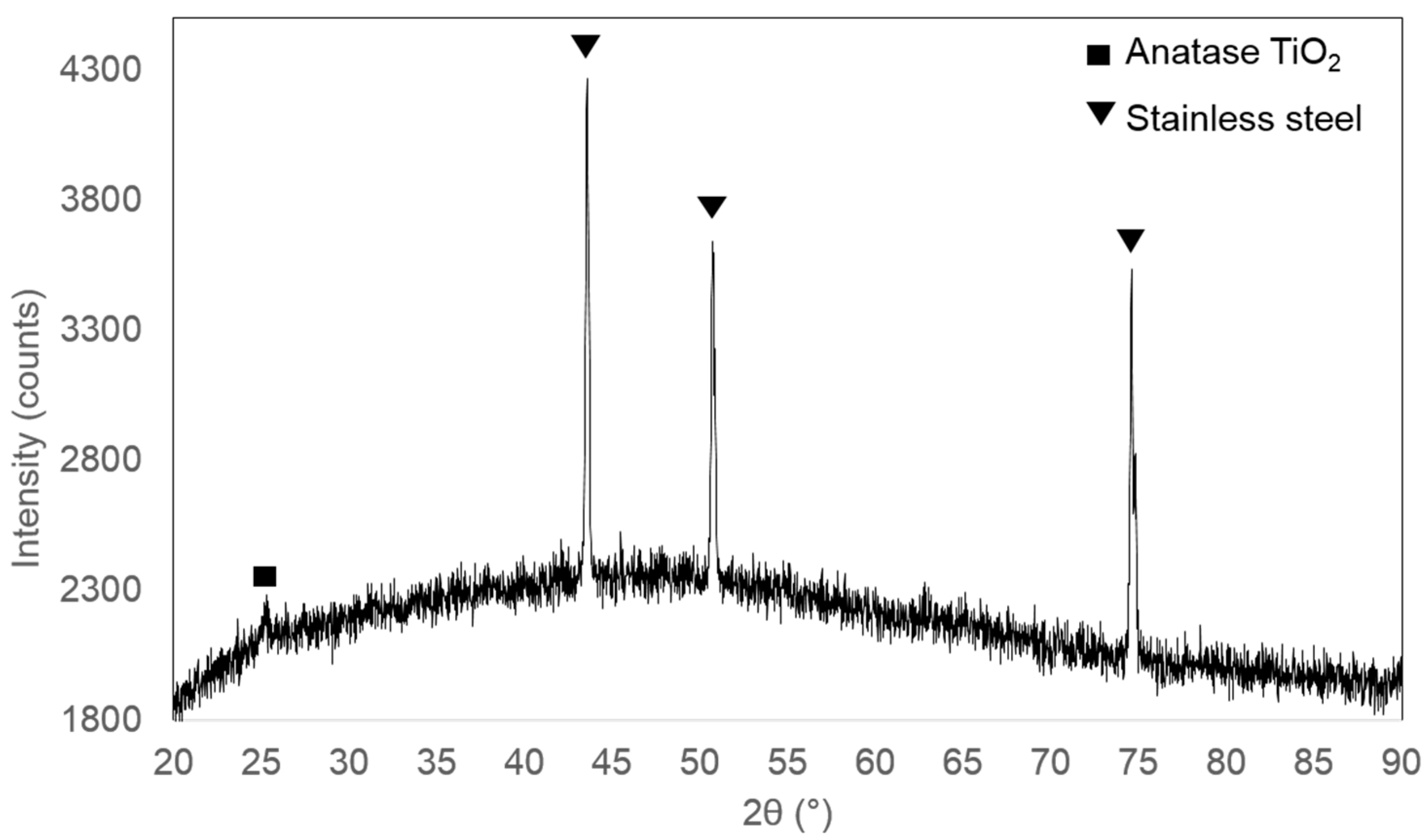
Figure 4 shows the XRD pattern for the prepared titania on stainless steel by Spray-ILGAR method. However, only a small peak corresponding to the (101) reflection of anatase TiO
2 at 2
θ value of around 25° can be detected. The rest of the diffraction peaks are attributed to the stainless steel substrate [
11]. This could be caused by the small amount and thin layer of TiO
2 present in the sample. As no further analysis using XRD was carried out, no conclusive results can be drawn from the XRD analysis.
The photocatalytic results of the samples using different concentrations of TiO
2 precursor, different heating temperature and different duration of spraying time are summarized in
Table 2. Based on the results, it was found that sample prepared with concentration of TiO
2 precursor of 6.83 mM, temperature of 550 °C and spraying time of 12 min took the shortest time (35 min) to completely degrade 500 ppm of acetaldehyde under UV irradiation. It would seem that out of the three parameters studied, the heating temperature played a slightly more important role. This could be caused by the crystallinity of the TiO
2 as the crystallinity of a photocatalyst influences the charge recombination rate and, consequently, on the photocatalytic activity [
12]. However, as the crystallinity could not be properly determined using the XRD analysis carried out, this hypothesis could not be confirmed.
Apart from the heating temperature, the concentration of the TiO
2 precursor also seems to play a crucial role, as the sample prepared with a concentration of TiO
2 precursor of 13.66 mM, temperature of 460 °C and spraying time of 12 min took the second shortest time (40 min) to completely degrade 500 ppm of acetaldehyde under UV irradiation. As discussed in the morphology analysis, samples prepared with higher concentration of the TiO
2 precursor showed a more compact and dense layer (
Figure 2a,b). This would indicate that more TiO
2 (active sites) are available to be used in the photodegradation of acetaldehyde, hence the shorter time required.
Figure 5 shows the graph for the photocatalytic decomposition of acetaldehyde by using TiO
2 on stainless steel prepared by the Spray-ILGAR technique using different concentrations of TiO
2 precursor (3.42, 6.83 and 13.66 mM), heated at 460 °C and spraying time of 12 min. As expected, the sample prepared with TiO
2 precursor with concentration of 3.42 mM showed the slowest decomposition rate. The highest photodecomposition rate was shown by the sample prepared with TiO
2 precursor with concentration of 6.83 mM, instead of that by the sample prepared using 13.66 mM of TiO
2 precursor. As shown in the SEM results (
Figure 2a,b), the sample prepared with 13.66 mM of TiO
2 precursor showed a very compact and dense layer. The compactness would have caused a decrease in the surface area, hence the lower photodecomposition rate.
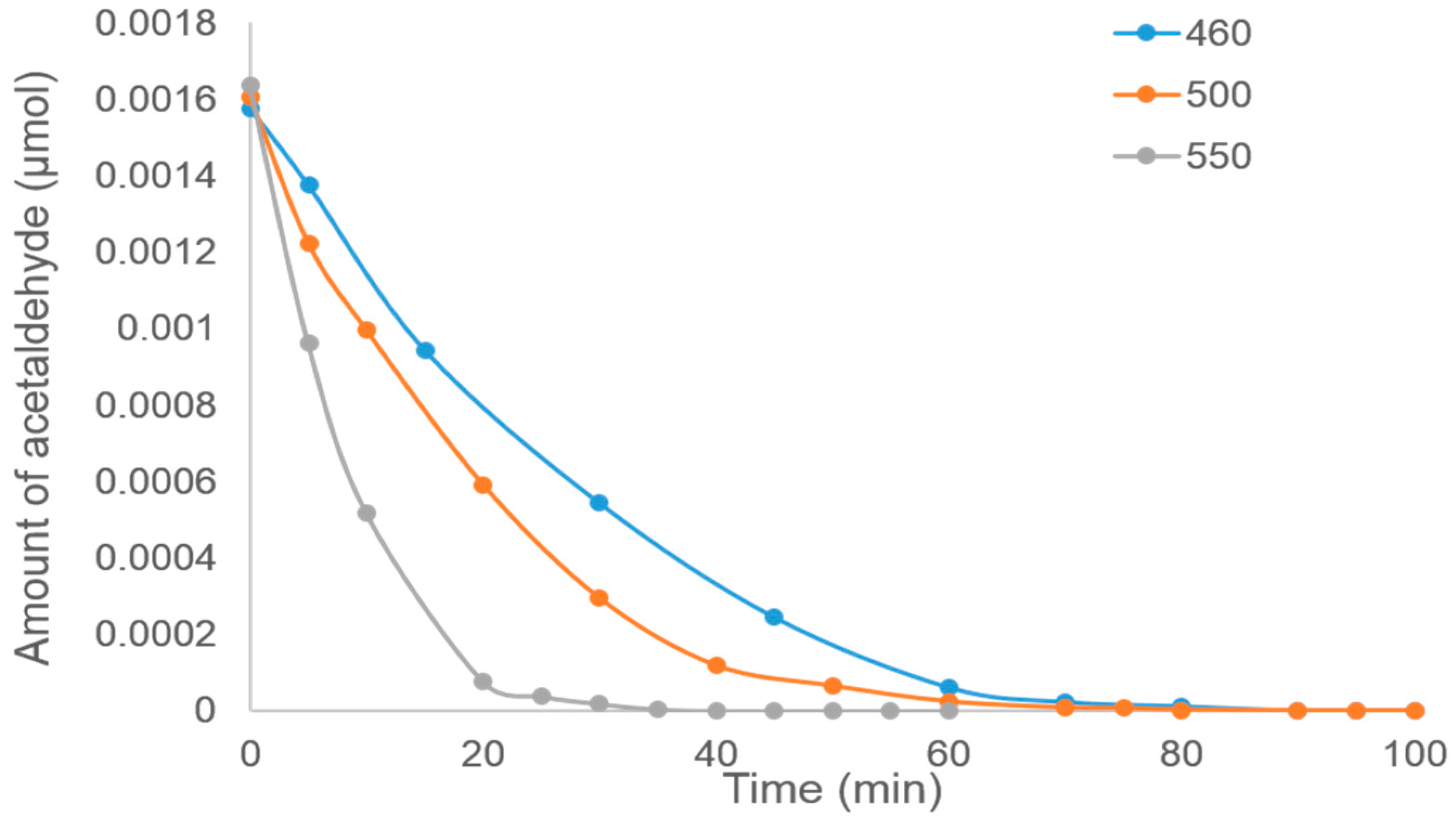
Figure 6 shows the graph for the photocatalytic decomposition of acetaldehyde by using TiO
2 on stainless steel prepared by the Spray-ILGAR technique using different heating temperatures (460, 500 and 550 °C), 6.83 mM of TiO
2 precursor and spraying time of 12 min. It was shown that the higher the heating temperature, the faster the decomposition rate. As discussed above, the possible cause for this could be caused by the crystallinity of the TiO
2. However, as the crystallinity could not be properly determined using XRD analysis carried out, the reason remains a hypothesis.
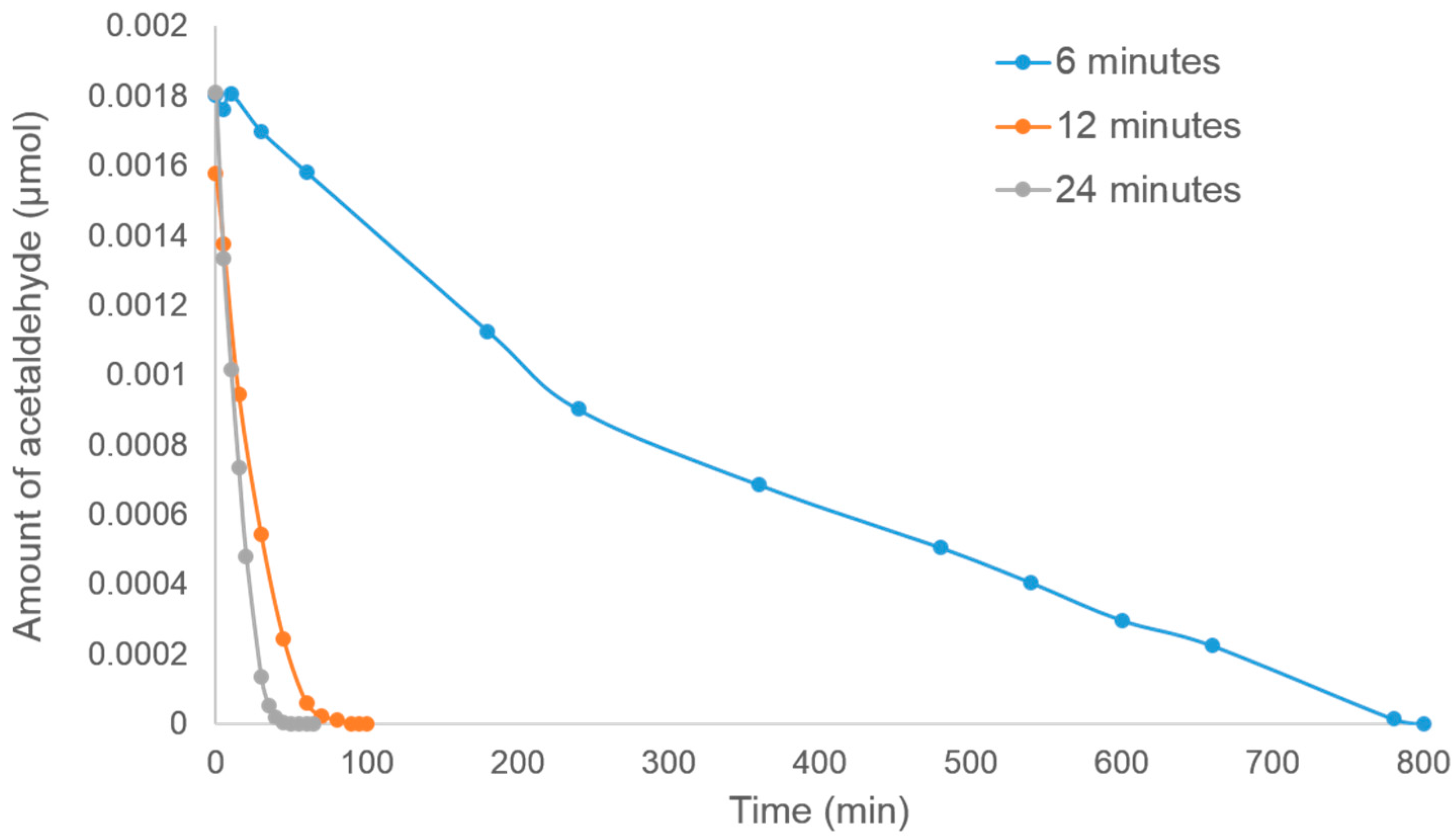
The photocatalytic decomposition of acetaldehyde by using TiO
2 on stainless steel prepared by the Spray-ILGAR technique using different spraying durations (6, 12 and 24 min), 6.83 mM of TiO
2 precursor and heating temperature of 460 °C is shown in
Figure 7. Based on the graph, it can be seen that the sample prepared using 24 min of spraying time showed the highest photodecomposition rate, while the slowest was shown by the sample prepared using 6 min of spraying time. This pattern was expected, as when the sample was prepared using 24 min of spraying time, a dense and compact layer of TiO
2 was formed (
Figure 3b), hence providing more TiO
2 to act as the photocatalyst. However, when the spraying time was decreased to only 6 min, a very thin layer resulted (
Table 1), and some parts of the stainless steel were also uncoated (
Figure 3a).