Gas Sensor Design Based on a Line Locked Tunable Fiber Laser and the Dual Path Correlation Spectroscopy Method
Abstract
:1. Introduction
2. Gas Sensor Mathematical Model
3. Simulation Results
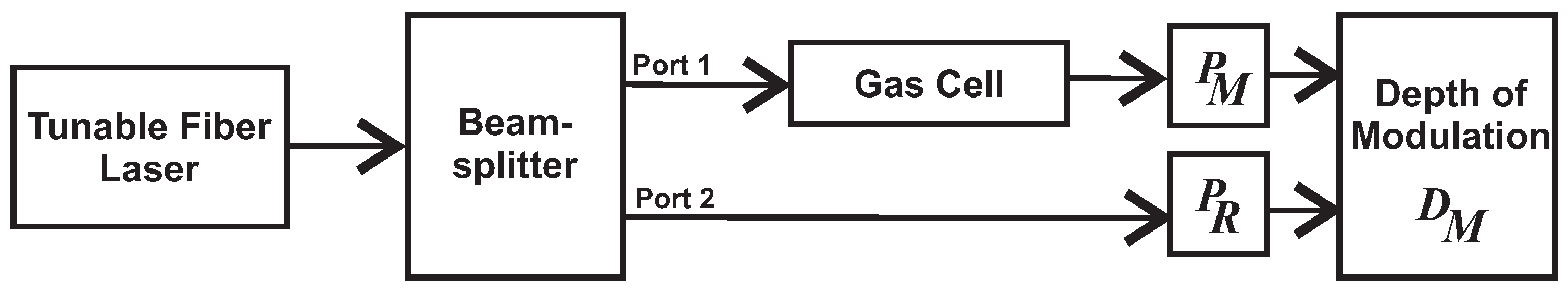
4. Experimental Sensing Setup
4.1. Tunable Fiber Laser
4.2. Dual Path Correlation Spectroscopy
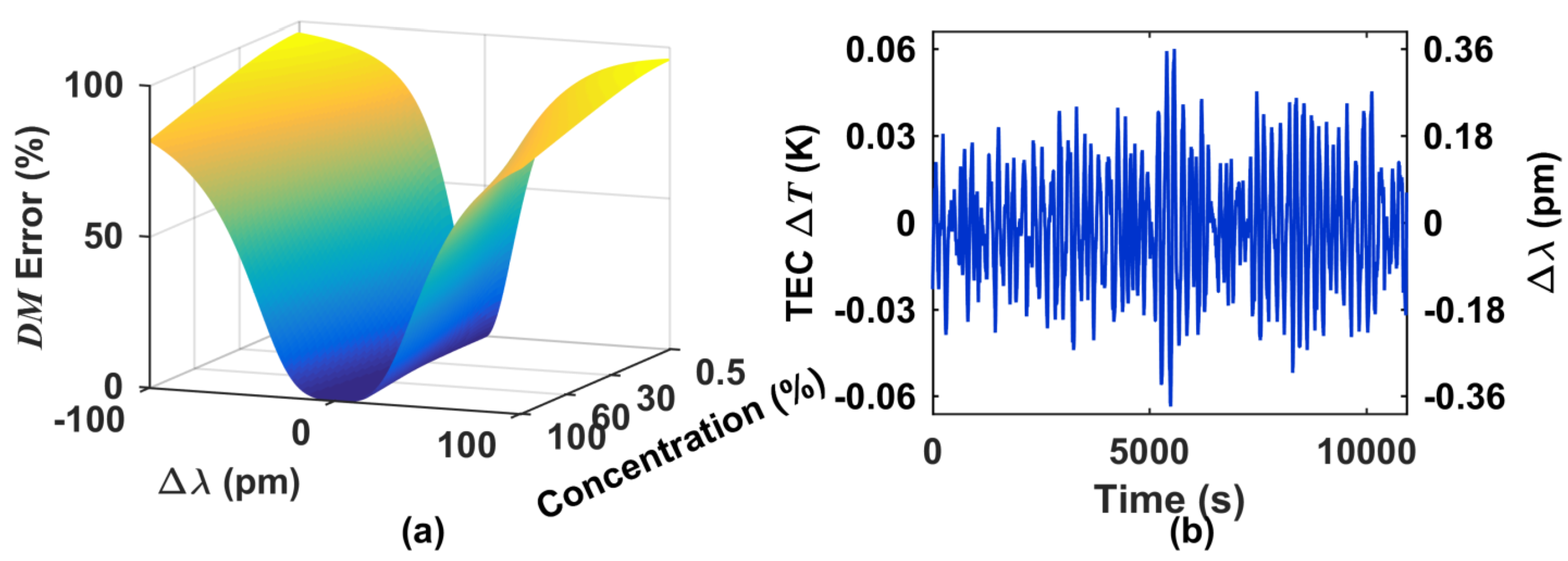
5. Minimization of Errors Due to Laser Intensity Fluctuations
6. Sensor Sensitivity
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goody, R. Cross-Correlating Spectrometer. J. Opt. Soc. Am. 1968, 58, 900–908. [Google Scholar] [CrossRef]
- Johnston, S.F. Gas monitors employing infrared LEDs. Meas. Sci. Technol. 1992, 3, 191–195. [Google Scholar] [CrossRef]
- Dakin, J.P.; Edwards, H.O.; Weigl, B.H. Latest developments in gas sensing using correlation spectroscopy. Proc. SPIE 1995, 2508, 2–17. [Google Scholar]
- Shemshad, J.; Aminossadati, S.M.; Kizil, M.S. A review of developments in near infrared methane detection based on tunable diode laser. Sens. Actuators B 2012, 171–172, 77–92. [Google Scholar] [CrossRef]
- Fetzer, G.J.; Pittner, A.S.; Ryder, W.L.; Brown, D.A. Tunable diode laser absorption spectroscopy in coiled hollow optical waveguides. Appl. Opt. 2002, 41, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, R.M.; Baer, D.S.; Hanson, R.K. Diode laser sensor for measurements of CO, CO2, and CH4 in combustion flows. Appl. Opt. 1997, 36, 8745–8752. [Google Scholar] [CrossRef] [PubMed]
- Eng, R.S.; Mantz, A.W. Tunable Diode Laser Spectroscopy of CO2 in the 10 to 15 μm Spectral Region-Lineshape and Q-Branch Head Absorption Profile. J. Mol. Spectrosc. 1979, 74, 331–344. [Google Scholar] [CrossRef]
- Reid, J.; Garside, B.K.; Shewchun, J.; El-Sherbiny, M.; Ballik, E.A. High sensitivity point monitoring of atmospheric gases employing tunable diode lasers. Appl. Opt. 1978, 17, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Rosenmann, L.; Langlois, S.; Taine, J. Diode Laser Measurements of CO2 Hot Band Line Intensities at High Temperature near 4.3 mm. J. Mol. Spectrosc. 1993, 158, 263–269. [Google Scholar] [CrossRef]
- Weldon, V.; O’Gorman, J.; Phelan, P.; Hegarty, J.; Tanbun-Ek, T. H2S and CO2 gas sensing using DFB laser diodes emitting at 1.57 mm. Sens. Actuators B 1995, 29, 101–107. [Google Scholar] [CrossRef]
- Bremer, K.; Pal, A.; Yao, S.; Lewis, E.; Sen, R.; Sun, T.; Grattan, K.T.V. Sensitive detection of CO2 implementing tunable thulium-doped all-fiber laser. Appl. Opt. 2013, 52, 3957–3963. [Google Scholar] [CrossRef] [PubMed]
- Whitenett, G.; Stewart, G.; Yu, H.; Culshaw, B. Investigation of a tuneable mode-locked fiber laser for application to multipoint gas spectroscopy. J. Lightwave Technol. 2004, 22, 813–819. [Google Scholar] [CrossRef]
- Ball, G.A.; Morey, W.W. Continuously tunable single-mode erbium fiber laser. Opt. Lett. 1992, 17, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Paschotta, R.; Nilsson, J.; Reekie, L.; Trooper, A.C.; Hanna, D.C. Single-frequency ytterbium-doped fiber laser stabilized by spatial hole burning. Opt. Lett. 1997, 22, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ye, Q.; Cai, H.; Qu, R. Mode-hop-free electro-optically tuned external-cavity diode laser using volume Bragg grating and PLZT ceramic. Opt. Express 2011, 19, 17244–17249. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Stewart, G.; Whitenett, S. Design of a tunable L-band multi-wavelength laser system for application to gas spectroscopy. Meas. Sci. Technol. 2006, 17, 1023. [Google Scholar] [CrossRef]
- Wei, W.; Chang, J.; Huang, Q.; Wang, Q.; Liu, Y.; Qin, Z. Water vapor concentration measurements using TDALS with wavelength modulation spectroscopy at varying pressures. Sens. Rev. 2017, 37, 172–179. [Google Scholar] [CrossRef]
- Homobono-Soares, V.; Renan Leal de Morais, C.; Ataide de Lima, R.; Fontana, E.; Ferreira Martins-Filho, J. Contributions to the optimization of an optical sensor for acetylene and carbon monoxide. In Proceedings of the 2011 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC 2011), Natal, Brazil, 29 October–1 November 2011; pp. 632–636. [Google Scholar]
- Austin, E.; van Brakel, A.; Petrovich, M.N.; Richardson, D.J. Fibre optical sensor for C2H2 gas using gas-filled photonic bandgap fibre reference cell. Sens. Actuators B 2009, 139, 30–34. [Google Scholar] [CrossRef]
- Ritari, T.; Tuominen, J.; Ludvigsen, H.; Petersen, J.C.; Sørensen, T.; Hansen, T.P.; Simonsen, H.R. Gas sensing using air-guiding photonic bandgap fibers. Opt. Express 2004, 12, 4080–4087. [Google Scholar] [CrossRef] [PubMed]
- Wynne, R.M.; Barabadi, B.; Creedon, K.J.; Ortega, A. Sub-Minute Response Time of a Hollow-Core Photonic Bandgap Fiber Gas Sensor. J. Lightwave Technol. 2009, 27, 1590–1596. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, A.P.; Ma, G.; Wang, B.; Kim, B.; Im, J.; He, S.; Chung, Y. Fiber-Optic Acetylene Gas Sensor Based on Microstructured Optical Fiber Bragg Gratings. IEEE Photonics Technol. Lett. 2011, 23, 1588–1590. [Google Scholar] [CrossRef]
- Dakin, J.P.; Gunning, M.J.; Chambers, P.; Xin, Z.J. Detection of gases by correlation spectroscopy. Sens. Actuators B 2003, 90, 124–131. [Google Scholar] [CrossRef]
- Rothman, L.S.; Jacquemart, D.; Barbe, A.; Chris Benner, D.; Birk, M.; Brown, L.R.; Carleer, M.R.; Chackerian, J.C.; Chance, K.; Coudert, L.H.; et al. The HITRAN 2004 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 139–204. [Google Scholar] [CrossRef]
- Gallegos-Arellano, E.; Vargas-Rodriguez, E.; Guzman-Chavez, A.D.; Cano-Contreras, M.; Cruz, J.L.; Raja-Ibrahim, R.K. Finely tunable laser based on a bulk silicon wafer for gas sensing applications. Laser Phys. Lett. 2016, 13, 065102. [Google Scholar] [CrossRef]
- Hoo, Y.L.; Liu, S.; Ho, H.L.; Jin, W. Fast Response Microstructured Optical Fiber Methane Sensor with Multiple Side-Openings. IEEE Photonics Technol. Lett. 2010, 22, 296–298. [Google Scholar] [CrossRef]






| Concentration (%) | m | n | (%) | Sensitivity (ppm) | |||
|---|---|---|---|---|---|---|---|
| 0–20 | 0.05174 | 0.02508 | 0.9971 | 9 | 0.522 | 2.9 × 10 | 521 |
| 20–32 | 0.02387 | 0.56405 | 0.9999 | 22 | 1.090 | 3.2 × 10 | 1340 |
| 32–51 | 0.01436 | 0.87236 | 0.9999 | 51 | 1.610 | 3.1 × 10 | 2158 |
| 51–100 | 0.00500 | 1.36072 | 0.9945 | 64 | 1.685 | 3.4 × 10 | 6800 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Rodriguez, E.; Guzman-Chavez, A.D.; Raja-Ibrahim, R.K.; Cardoso-Lozano, L.E. Gas Sensor Design Based on a Line Locked Tunable Fiber Laser and the Dual Path Correlation Spectroscopy Method. Appl. Sci. 2017, 7, 958. https://doi.org/10.3390/app7090958
Vargas-Rodriguez E, Guzman-Chavez AD, Raja-Ibrahim RK, Cardoso-Lozano LE. Gas Sensor Design Based on a Line Locked Tunable Fiber Laser and the Dual Path Correlation Spectroscopy Method. Applied Sciences. 2017; 7(9):958. https://doi.org/10.3390/app7090958
Chicago/Turabian StyleVargas-Rodriguez, Everardo, Ana Dinora Guzman-Chavez, Raja Kamarulzaman Raja-Ibrahim, and Luis Eusebio Cardoso-Lozano. 2017. "Gas Sensor Design Based on a Line Locked Tunable Fiber Laser and the Dual Path Correlation Spectroscopy Method" Applied Sciences 7, no. 9: 958. https://doi.org/10.3390/app7090958




