Thermal Pre-Treatment of Sewage Sludge in a Lab-Scale Fluidized Bed for Enhancing Its Solid Fuel Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
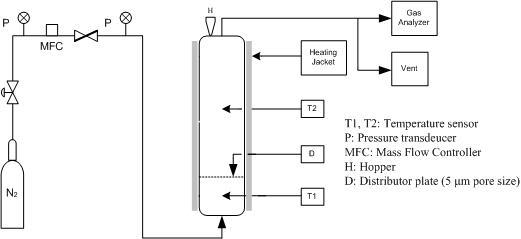
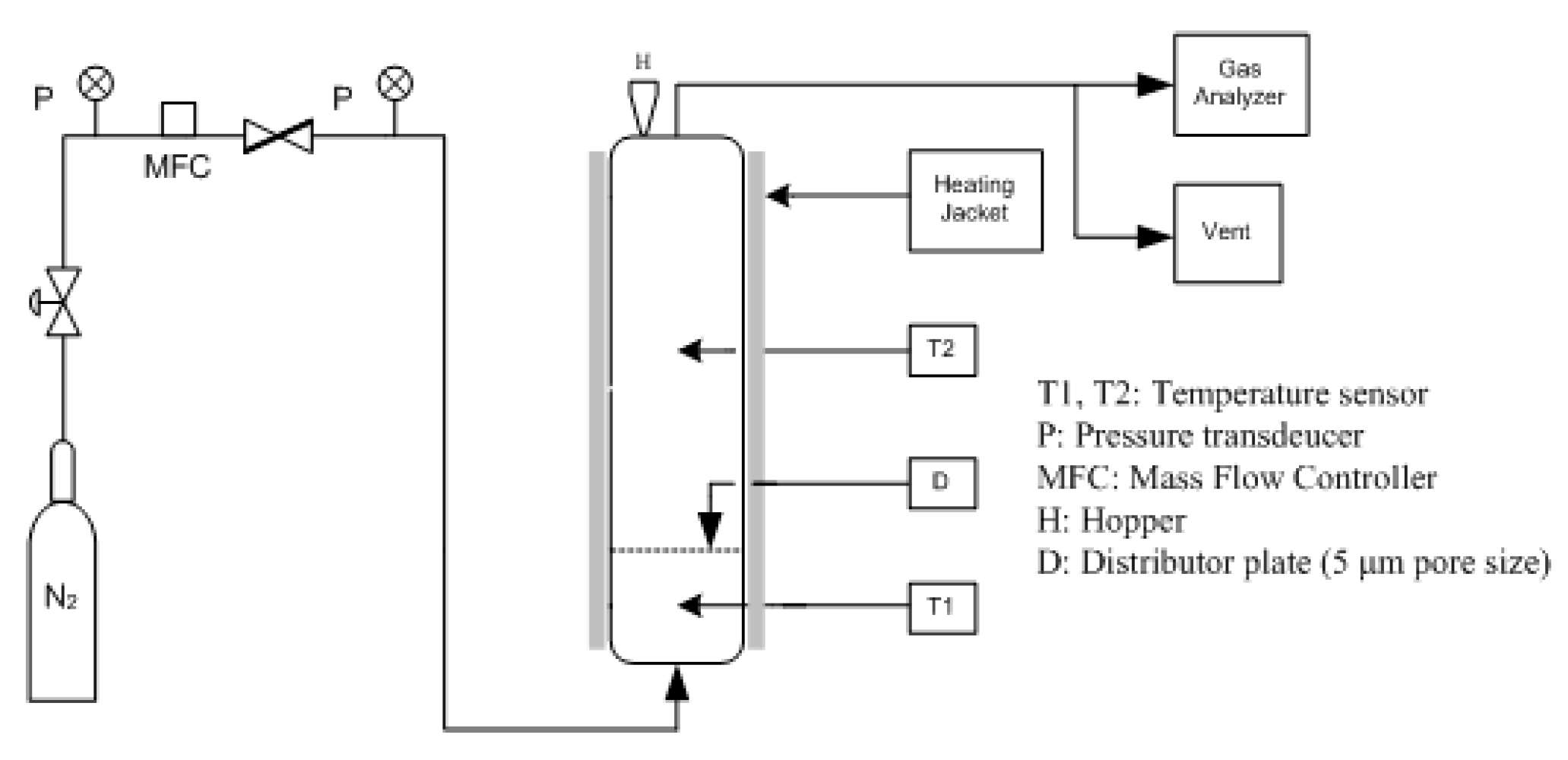
2.2. Experimental Apparatus and Method
3. Results and Discussions
3.1. Thermogravimetric Analysis
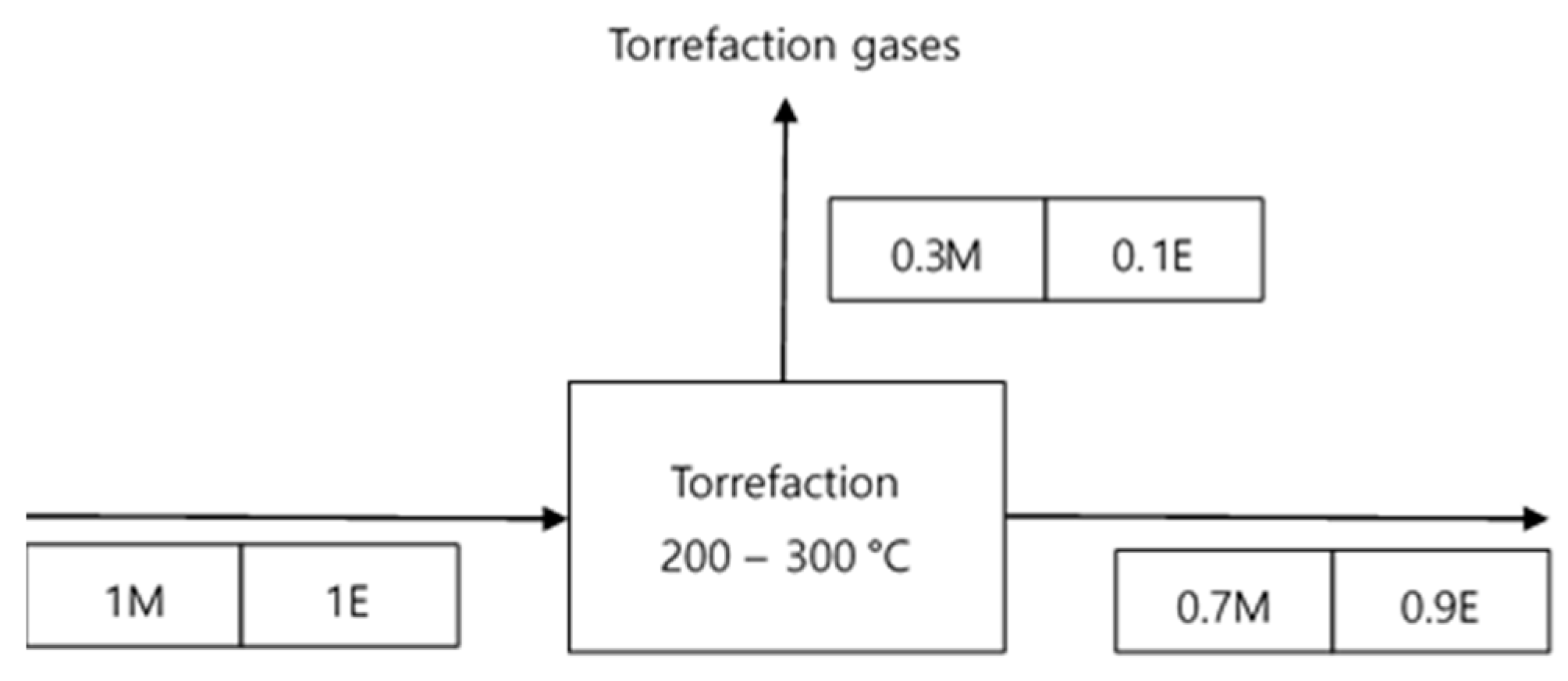
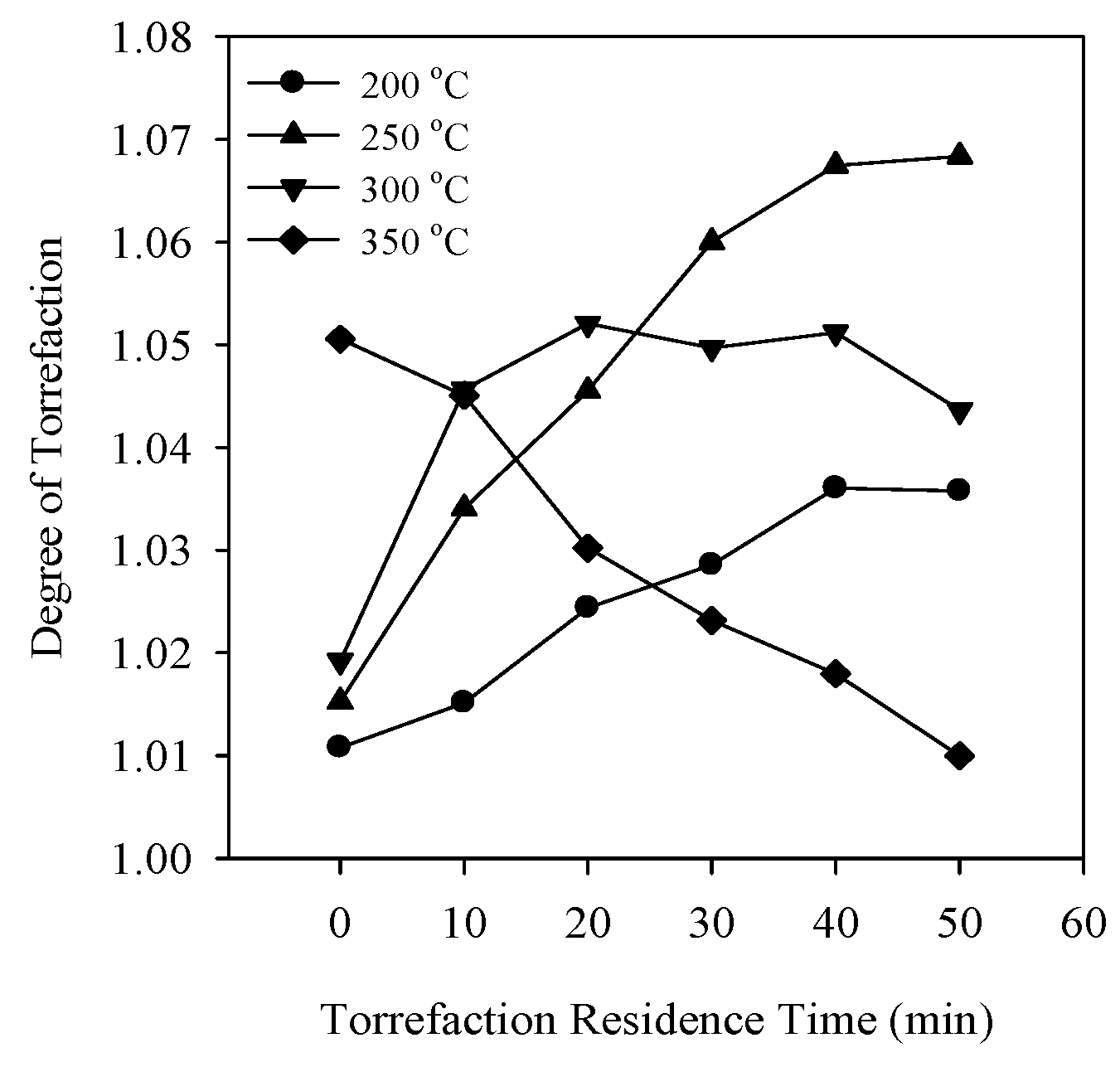
3.2. Degree of Torrefaction
3.3. Torrefaction Index
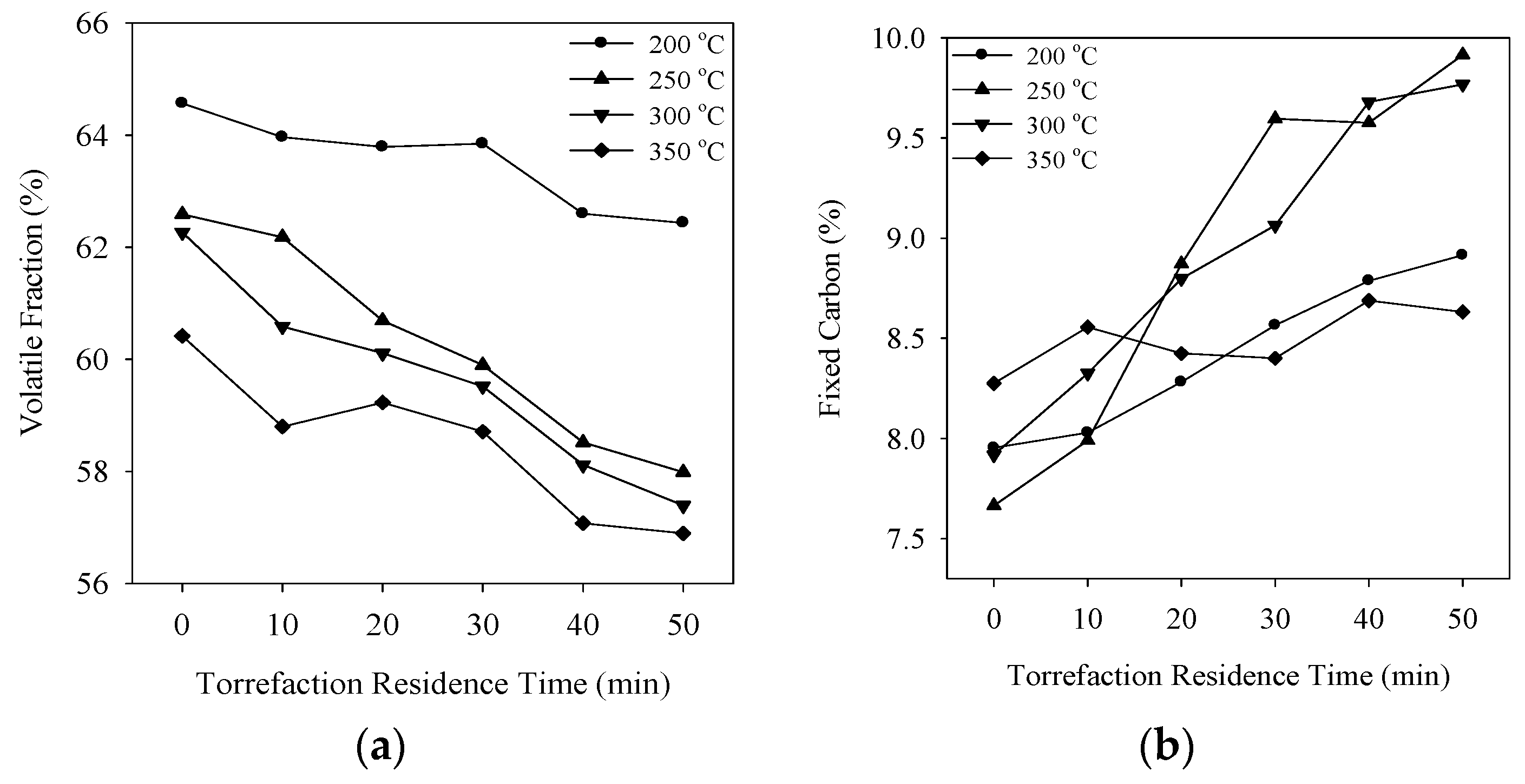
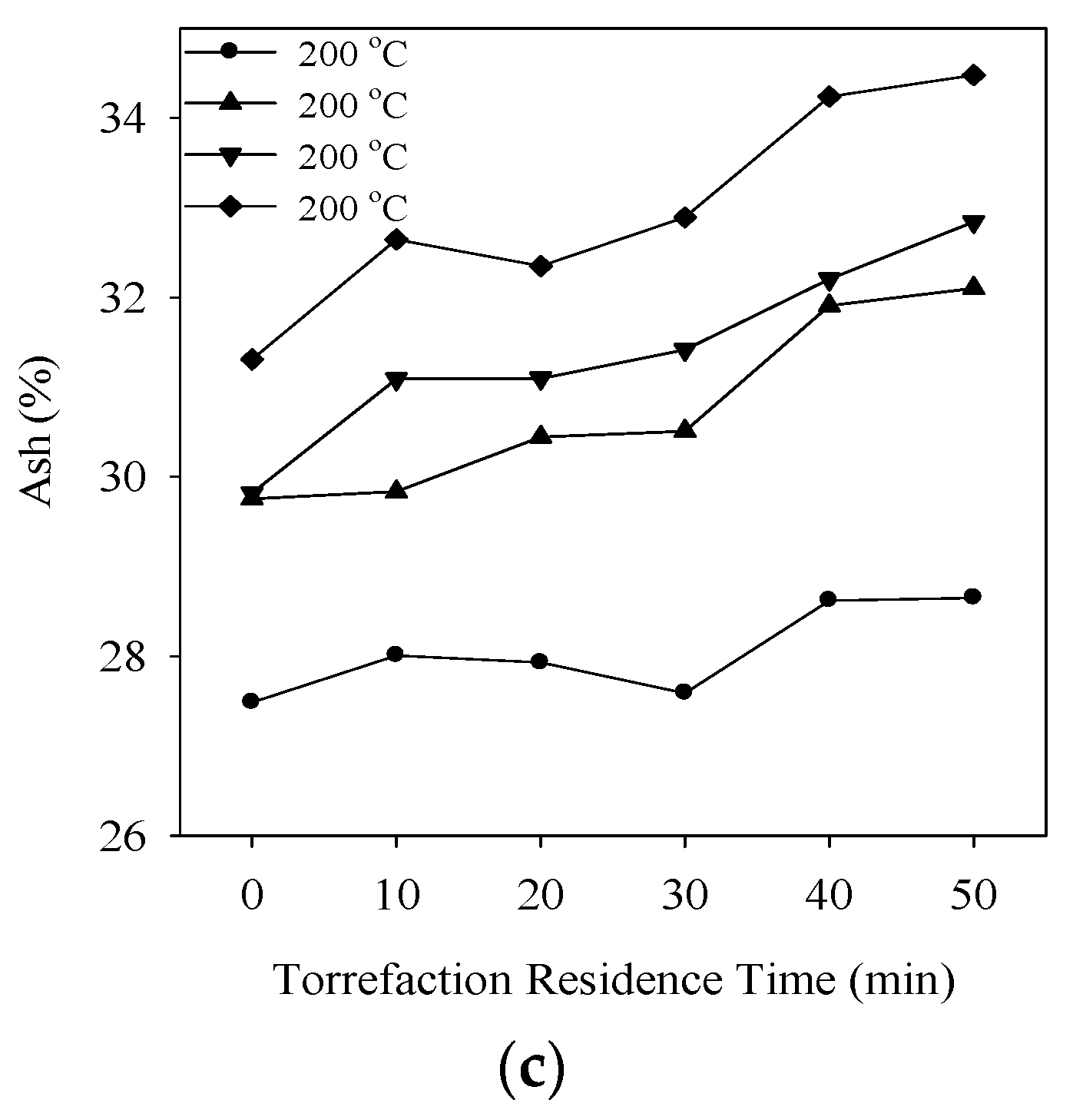
3.4. Proximate Analysis
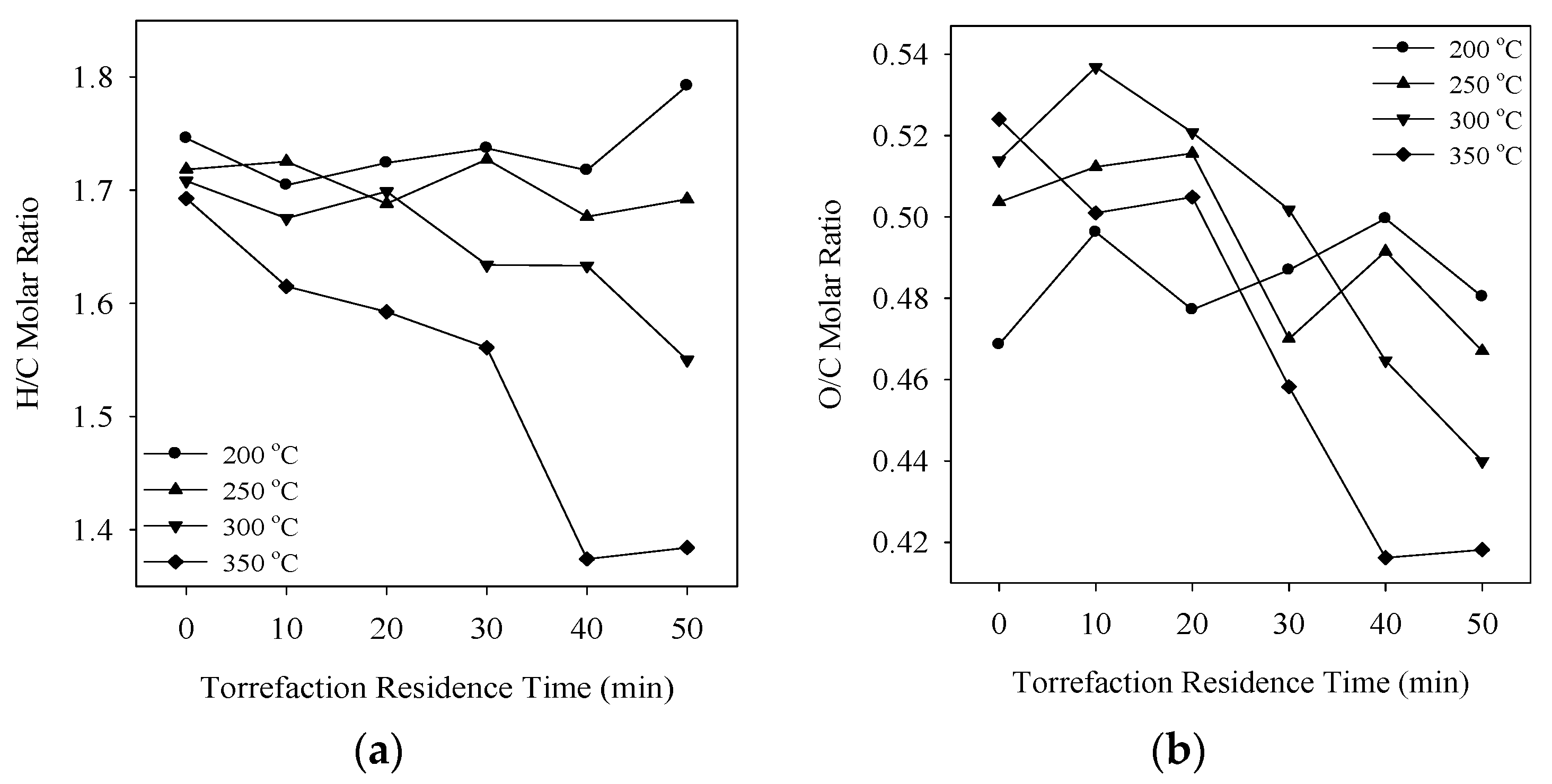
3.5. Ultimate Analysis
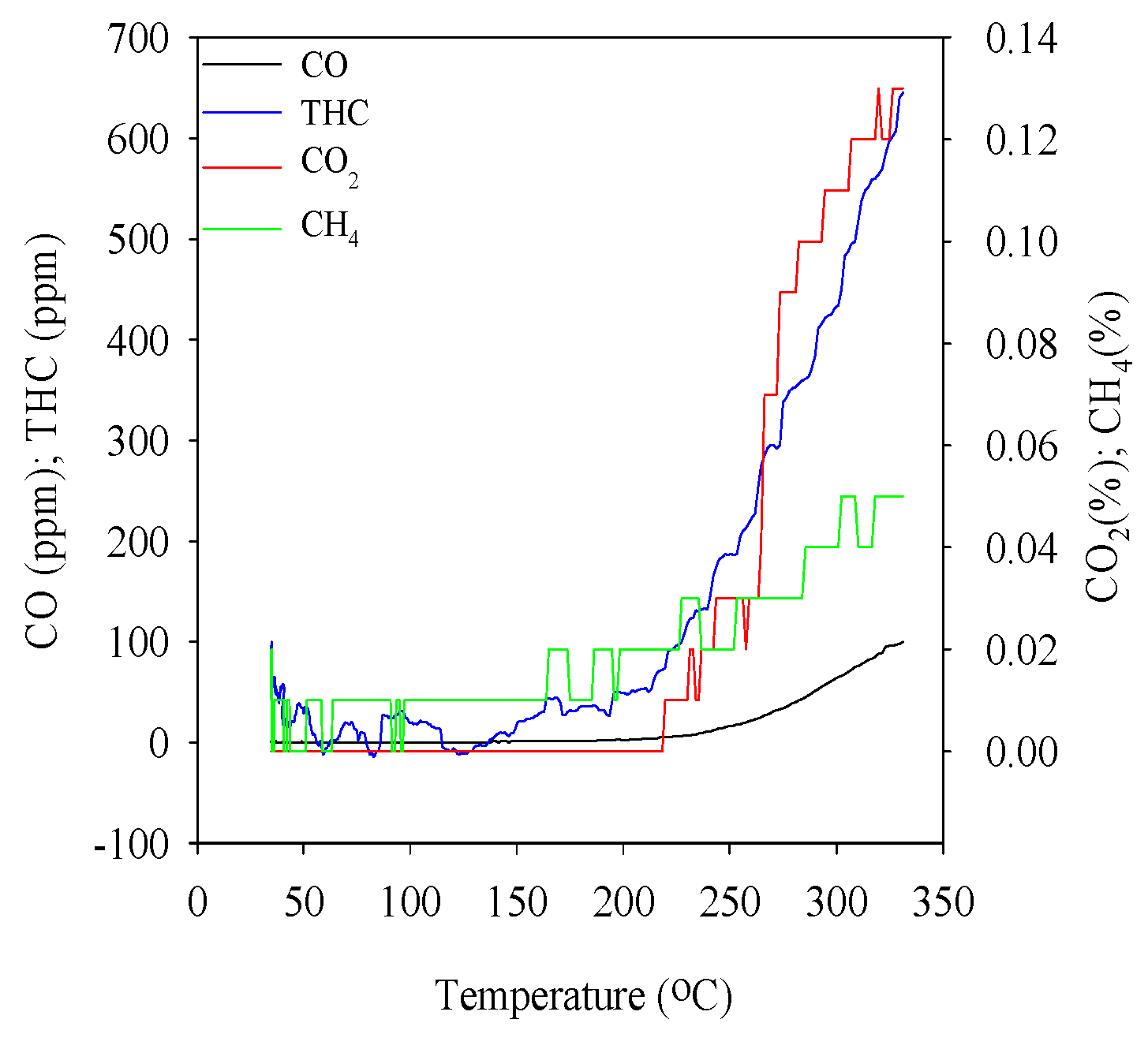
3.6. Gas Analysis
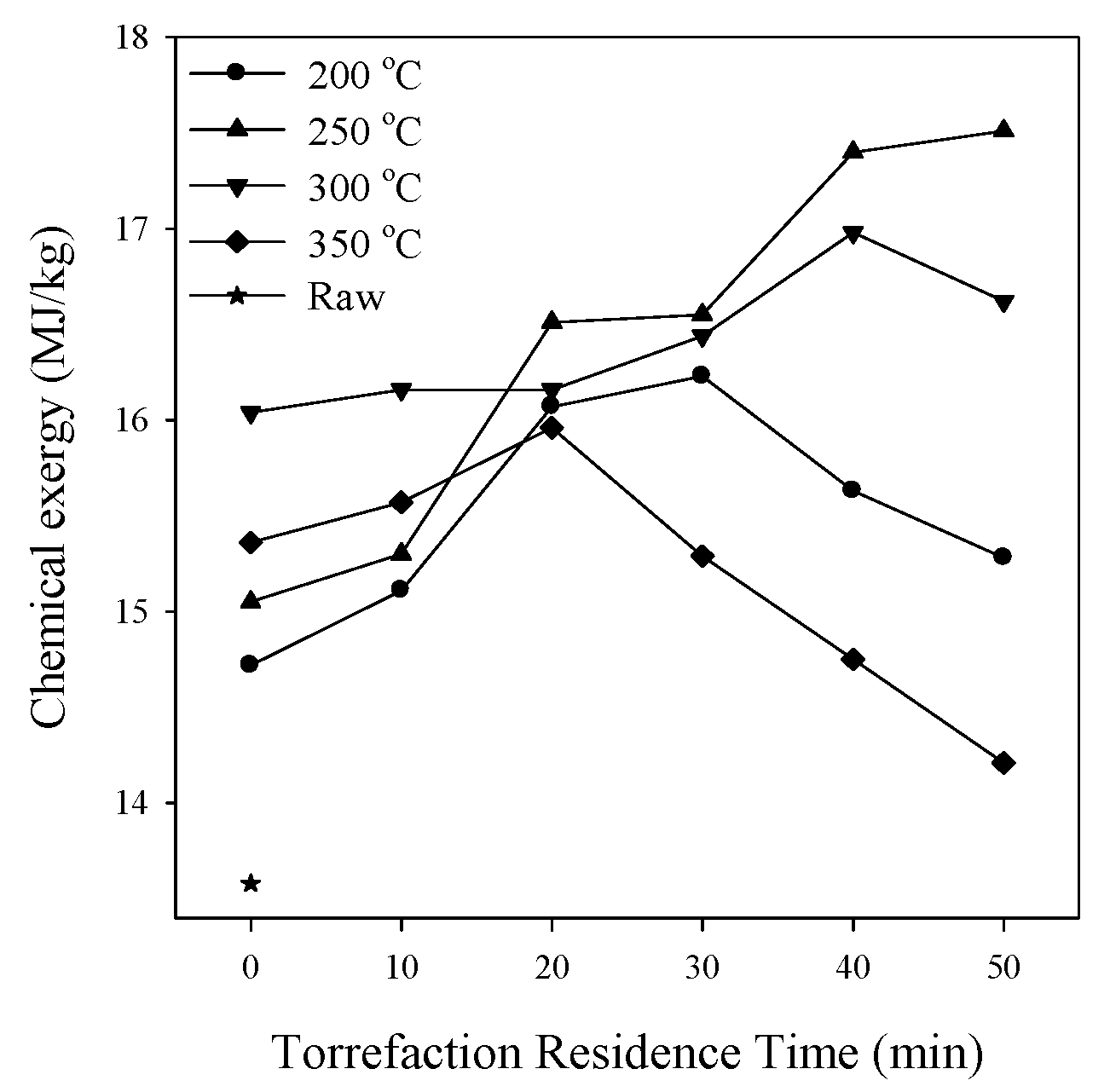
3.7. Chemical Exergy
3.8. Validating HHV Correlation for Torrefied Biomass Using Proximate and Ultimate Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| ASTM | American Society for Testing and Materials |
| C | Carbon |
| CH4 | Methane |
| CO | Carbon-monoxide |
| CO2 | Carbon-dioxide |
| DTG | Derivative Thermogravimetric Analysis |
| FC | Fixed Carbon |
| FTIR | Fourier-transform Infrared Spectroscopy |
| H | Hydrogen |
| HHV | Higher Heating Value |
| O | Oxygen |
| THC | Thermal Hydro-Carbon |
| TI | Torrefaction Index |
| VF | Volatile Fraction |
| TG-DTA | Thermogravimetric Analysis-Differential Thermal Analysis |
References
- Korean Government, Water and Wastewater Policy office of South Korea. 2012. Available online: http://eng.me.go.kr/eng/web/index.do?menuId=288 (accessed on 12 December 2017).
- Schowanek, D.; Carr, R.; David, H.; Douben, P.; Hall, J.; Kirchmann, H.; Patria, L.; Sequi, P.; Smith, S.; Webb, S. A risk-based methodology for deriving quality standards for organic contaminants in sewage sludge for use in agriculture—Conceptual framework. Regul. Toxicol. Pharmacol. 2004, 40, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Hospido, A.; Moreira, T.; Martín, M.; Rigola, M.; Feijoo, G. Environmental evaluation of different treatment processes for sludge from urban wastewater treatments: Anaerobic digestion versus thermal processes (10 pp). Int. J. Life Cycle Assess. 2005, 10, 336–345. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Fonts, I.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013, 222, 534–545. [Google Scholar] [CrossRef]
- Mills, N.; Pearce, P.; Farrow, J.; Thorpe, R.B.; Kirkby, N.F. Environmental & economic life cycle assessment of current & future sewage sludge to energy technologies. Waste Manag. 2014, 34, 185–195. [Google Scholar] [PubMed]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Torrefaction of tomato peel residues in a fluidized bed of inert particles and a fixed-bed reactor. Energy Fuels 2016, 30, 4858–4868. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A review of biomass densification systems to develop uniform feedstock commodities for bioenergy application. Biofuels Bioprod. Biorefin. 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Bergman, P.C.; Boersma, A.R.; Kiel, J.H.; Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction for Entrained-Flow Gasification of Biomass; Report ECN-RX-04-046; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cen, K.; Jing, X.; Gao, J.; Li, C.; Ma, Z. An approach for upgrading biomass and pyrolysis product quality using a combination of aqueous phase bio-oil washing and torrefaction pretreatment. Bioresour. Technol. 2017, 233, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rousset, P.; Aguiar, C.; Labbé, N.; Commandré, J. Enhancing the combustible properties of bamboo by torrefaction. Bioresour. Technol. 2011, 102, 8225–8231. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.C.; Boersma, A.; Zwart, R.; Kiel, J. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations “Biocoal”; Report No. ECN-C-05-013; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Kuang, J.; Zhang, Y.; Luo, Y. Pretreatment of agricultural residues for co-gasification via torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandre, J. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Dhungana, A.; Dutta, A.; Basu, P. Torrefaction of non-lignocellulose biomass waste. Can. J. Chem. Eng. 2012, 90, 186–195. [Google Scholar] [CrossRef]
- Ábrego, J.; Sánchez, J.L.; Arauzo, J.; Fonts, I.; Gil-Lalaguna, N.; Atienza-Martínez, M. Technical and energetic assessment of a three-stage thermochemical treatment for sewage sludge. Energy Fuels 2013, 27, 1026–1034. [Google Scholar] [CrossRef]
- Huang, Y.; Sung, H.; Chiueh, P.; Lo, S. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Gu, J.H.; Lim, Y.; Oh, S.C. Effect of Co-Torrefaction on the Properties of Sewage Sludge and Waste Wood to Enhance Solid Fuel Qualities. J. Residuals Sci. Technol. 2017, 14. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Mastral, J.F.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in an auger reactor. Energy Fuels 2014, 29, 160–170. [Google Scholar] [CrossRef]
- Cummins, E.J.; McDonnell, K.P.; Ward, S.M. Dispersion modelling and measurement of emissions from the co-combustion of meat and bone meal with peat in a fluidised bed. Bioresour. Technol. 2006, 97, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Henihan, A.M.; Leahy, M.J.; Leahy, J.; Cummins, E.; Kelleher, B. Emissions modeling of fluidised bed co-combustion of poultry litter and peat. Bioresour. Technol. 2003, 87, 289–294. [Google Scholar] [CrossRef]
- Hannula, I.; Kurkela, E. A semi-empirical model for pressurised air-blown fluidised-bed gasification of biomass. Bioresour. Technol. 2010, 101, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Pattiya, A. Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidised-bed reactor. Bioresour. Technol. 2011, 102, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Legros, R.; Bi, X.T.; Lim, C.J.; Sokhansanj, S. Torrefaction of sawdust in a fluidized bed reactor. Bioresour. Technol. 2012, 103, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Folkeson, B. Propensity of Bed Materials Used in Dual Fluidized Beds to Retain Ash-Forming Elements from Biomass Fuels; SLU, Department of Energy and Technology: Uppsala, Sweden, 2014. [Google Scholar]
- Alvarez, J.; Amutio, M.; Lopez, G.; Barbarias, I.; Bilbao, J.; Olazar, M. Sewage sludge valorization by flash pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2015, 273, 173–183. [Google Scholar] [CrossRef]
- Kongkeaw, N.; Patumsawad, S. Thermal upgrading of biomass as a fuel by torrefaction. In Proceedings of the 2nd International Conference on Environmental Engineering and Applications, Shanghai, China, 19–21 August 2011; Volume 17, pp. 38–42. [Google Scholar]
- Zanzi, R.; Ferro, D.T.; Torres, A.; Soler, P.B.; Bjornbom, E. Biomass torrefaction. In Proceedings of the 6th Asia-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002. [Google Scholar]
- Nimlos, M.N.; Brooking, E.; Looker, M.; Evans, R. Biomass torrefaction studies with a molecular beam mass spectrometer. Am. Chem. Soc. Div. Fuel Chem. 2003, 48, 590–591. [Google Scholar]
- Iroba, K.L.; Baik, O.; Tabil, L.G. Torrefaction of biomass from municipal solid waste fractions II: Grindability characteristics, higher heating value, pelletability and moisture adsorption. Biomass Bioenergy 2017, 106, 8–20. [Google Scholar] [CrossRef]
- Recari, J.; Berrueco, C.; Puy, N.; Alier, S.; Bartrolí, J.; Farriol, X. Torrefaction of a solid recovered fuel (SRF) to improve the fuel properties for gasification processes. Appl. Energy 2017, 203, 177–188. [Google Scholar] [CrossRef]
- Basu, P.; Kulshreshtha, A.; Acharya, B. An index for quantifying the degree of torrefaction. BioResources 2017, 12, 1749–1766. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass torrefaction and CO2 capture using mining wastes—A new approach for reducing greenhouse gas emissions of co-firing plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Thomsen, T.; Stelte, W.; Henriksen, U.B. Changes of chemical and mechanical behavior of torrefied wheat straw. Biomass Bioenergy 2012, 40, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Poudel, J.; Ohm, T.; Lee, S.; Oh, S.C. A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag. 2015, 40, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Sadaka, S.; Negi, S. Improvements of biomass physical and thermochemical characteristics via torrefaction process. Environ. Prog. Sustain. Energy 2009, 28, 427–434. [Google Scholar] [CrossRef]
- Yang, Z.; Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Huhnke, R.L. Effects of torrefaction and densification on switchgrass pyrolysis products. Bioresour. Technol. 2014, 174, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Boateng, A.; Mullen, C. Fast pyrolysis of biomass thermally pretreated by torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 95–102. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Meng, J.; Lim, K.H.; Rojas, O.J.; Park, S. Transformation of lignocellulosic biomass during torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 199–206. [Google Scholar] [CrossRef]
- Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Patil, K.N.; Bellmer, D.D. Gasification performance of switchgrass pretreated with torrefaction and densification. Appl. Energy 2014, 127, 194–201. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Effect of Deep Drying and Torrefaction Temperature on Proximate, Ultimate Composition, and Heating Value of 2-mm Lodgepole Pine (Pinus contorta) Grind. Bioengineering 2016, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- White, R.H.; Dietenberger, M. Wood Products: Thermal Degradation and Fire; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Boardman, R.D.; Wright, C.T.; Hess, J.R. Some chemical compositional changes in miscanthus and white oak sawdust samples during torrefaction. Energies 2012, 5, 3928–3947. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Krieger Publishing Company: Boston, MA, USA, 1995. [Google Scholar]
- Nhuchhen, D.R. Studies on Advanced Means of Biomass Torrefaction. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 2016. [Google Scholar]
- Cordero, T.; Marquez, F.; Rodriguez-Mirasol, J.; Rodriguez, J.J. Predicting heating values of lignocellulosics and carbonaceous materials from proximate analysis. Fuel 2001, 80, 1567–1571. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]

| Elemental Analysis (wt %) * | C | 37.82 |
| H | 5.82 | |
| N | 4.14 | |
| O | 25.12 | |
| S | 1.44 | |
| Others | 25.66 | |
| Proximate Analysis (wt %) ** | Moisture (%) | 80.12 |
| Volatile Content (%) | 12.87 | |
| Ash (%) | 5.50 | |
| Fixed Carbon (%) | 1.51 | |
| HHV (MJ/kg) * | 15.81 | |
| Torrefaction Regimes | Temperature (°C) | TI [43] | TI (This Study) |
|---|---|---|---|
| Light | 200–235 | 0.93–0.95 | 0.96–0.99 |
| Medium | 235–275 | 0.95–0.97 | 0.97–1.02 |
| Severe | 275–300 | 0.97–1.0 | 0.97–1.0 |
| 350 | 1–0.96 |
| Torrefaction Residence Time (min) | 200 °C | 250 °C | 300 °C | 350 °C | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HHV1 | HHV2 | HHV3 | HHV4 | HHV * | HHV1 | HHV2 | HHV3 | HHV4 | HHV * | HHV1 | HHV2 | HHV3 | HHV4 | HHV * | HHV1 | HHV2 | HHV3 | HHV4 | HHV * | |
| 0 | 13.85 | 4.53 | 14.72 | 16.02 | 15.98 | 13.40 | 5.24 | 14.25 | 15.94 | 16.06 | 13.44 | 5.32 | 14.29 | 15.95 | 16.12 | 13.25 | 5.38 | 14.07 | 15.89 | 16.61 |
| 10 | 13.77 | 5.35 | 14.64 | 15.97 | 16.05 | 13.45 | 5.19 | 14.29 | 15.91 | 16.35 | 13.30 | 5.89 | 14.12 | 15.97 | 16.54 | 13.07 | 6.07 | 13.87 | 16.21 | 16.53 |
| 20 | 13.83 | 4.92 | 14.70 | 15.99 | 16.20 | 13.51 | 5.68 | 14.33 | 15.99 | 16.53 | 13.38 | 5.48 | 14.20 | 15.91 | 16.64 | 13.10 | 6.27 | 13.90 | 16.18 | 16.29 |
| 30 | 13.94 | 5.03 | 14.81 | 15.90 | 16.27 | 13.63 | 4.99 | 14.44 | 15.94 | 16.76 | 13.38 | 5.71 | 14.18 | 16.17 | 16.60 | 13.00 | 6.18 | 13.80 | 16.29 | 16.18 |
| 40 | 13.80 | 5.11 | 14.65 | 16.01 | 16.38 | 13.39 | 5.58 | 14.18 | 15.99 | 16.88 | 13.36 | 5.55 | 14.14 | 16.18 | 16.62 | 12.83 | 7.16 | 13.60 | 16.56 | 16.10 |
| 50 | 13.82 | 4.27 | 14.67 | 15.91 | 16.38 | 13.42 | 5.06 | 14.20 | 16.07 | 16.90 | 13.26 | 5.92 | 14.04 | 16.35 | 16.50 | 12.78 | 7.26 | 13.55 | 16.58 | 15.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karki, S.; Poudel, J.; Oh, S.C. Thermal Pre-Treatment of Sewage Sludge in a Lab-Scale Fluidized Bed for Enhancing Its Solid Fuel Properties. Appl. Sci. 2018, 8, 183. https://doi.org/10.3390/app8020183
Karki S, Poudel J, Oh SC. Thermal Pre-Treatment of Sewage Sludge in a Lab-Scale Fluidized Bed for Enhancing Its Solid Fuel Properties. Applied Sciences. 2018; 8(2):183. https://doi.org/10.3390/app8020183
Chicago/Turabian StyleKarki, Sujeeta, Jeeban Poudel, and Sea Cheon Oh. 2018. "Thermal Pre-Treatment of Sewage Sludge in a Lab-Scale Fluidized Bed for Enhancing Its Solid Fuel Properties" Applied Sciences 8, no. 2: 183. https://doi.org/10.3390/app8020183