Influence of Size Reduction of Fly Ash Particles by Grinding on the Chemical Properties of Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Sample Preparation
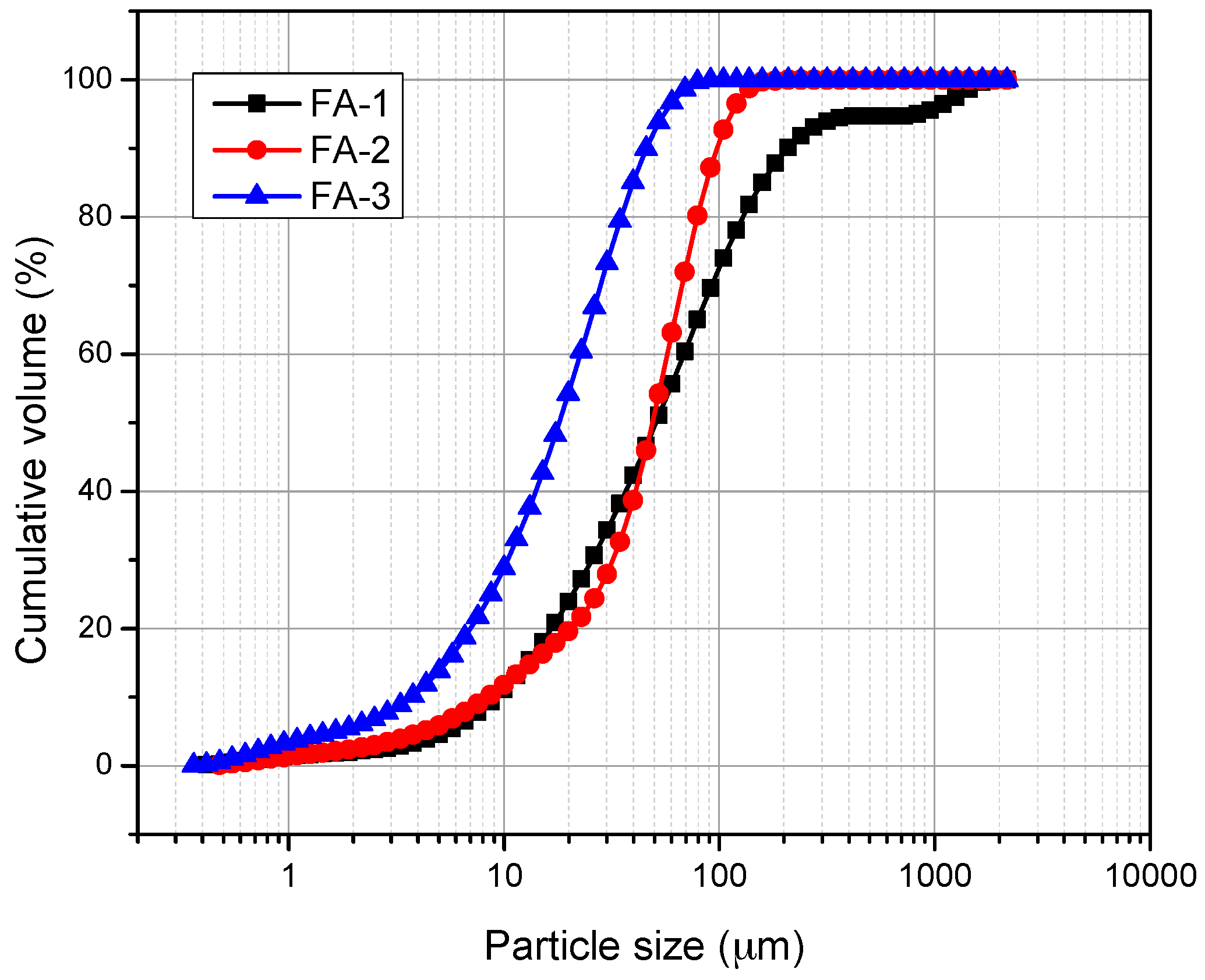
2.3. Characterization of FA
3. Results and Discussion
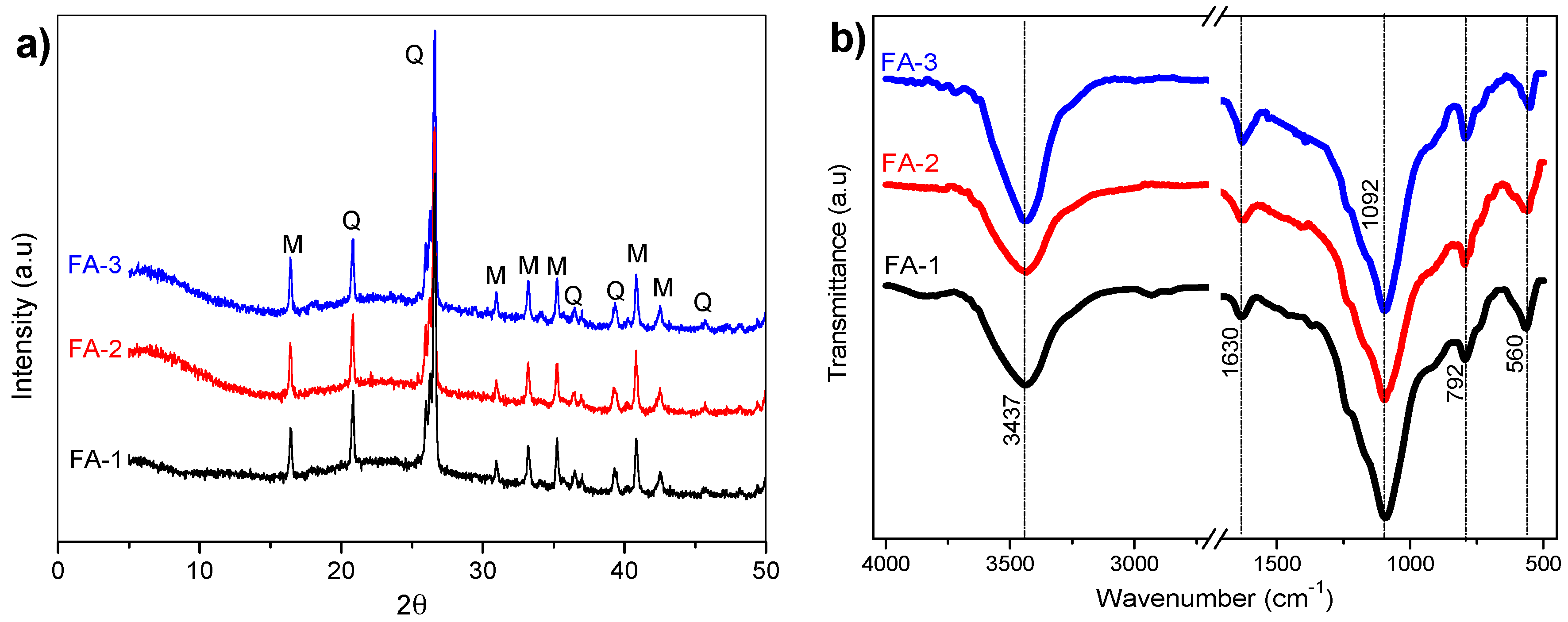
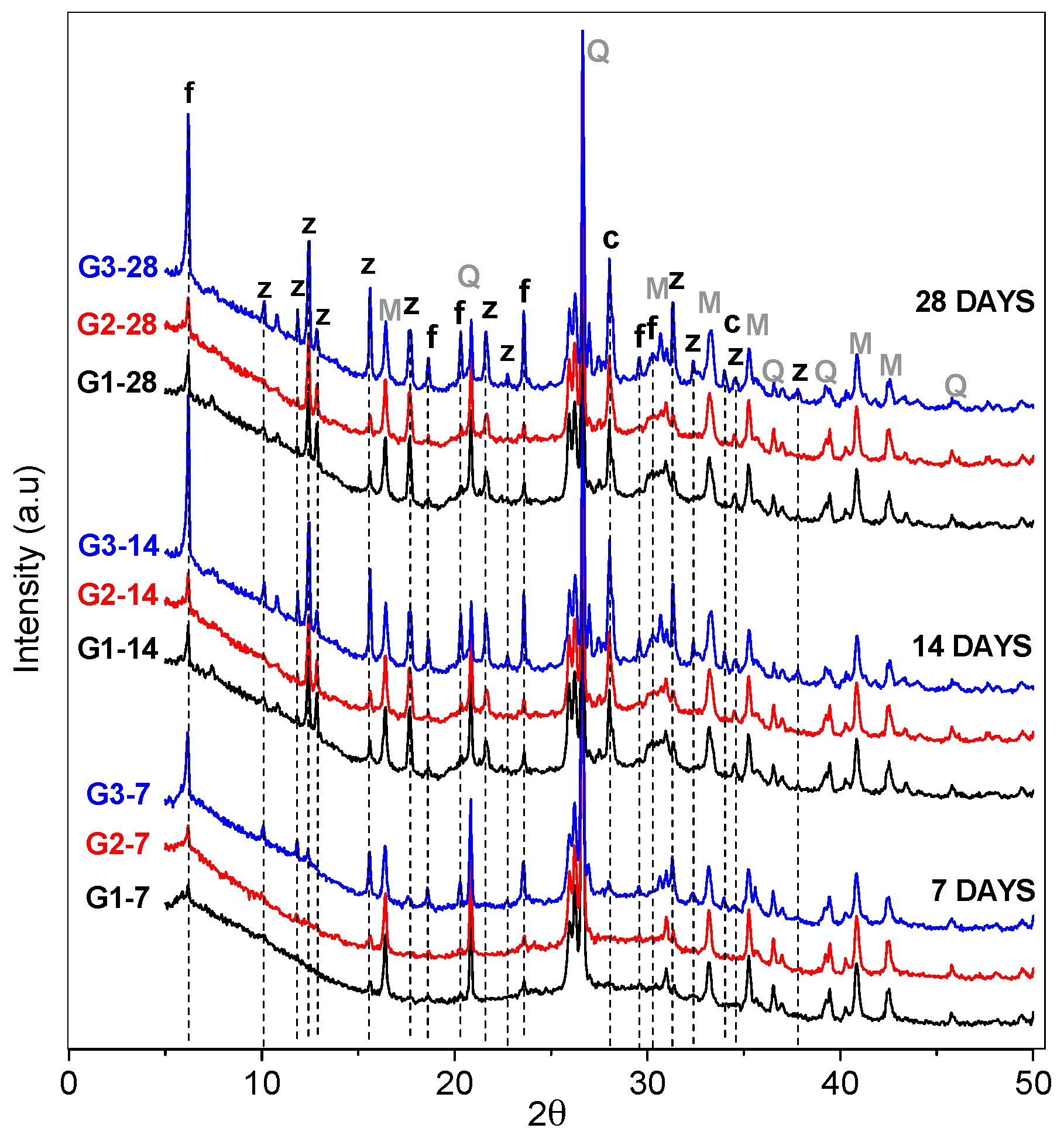
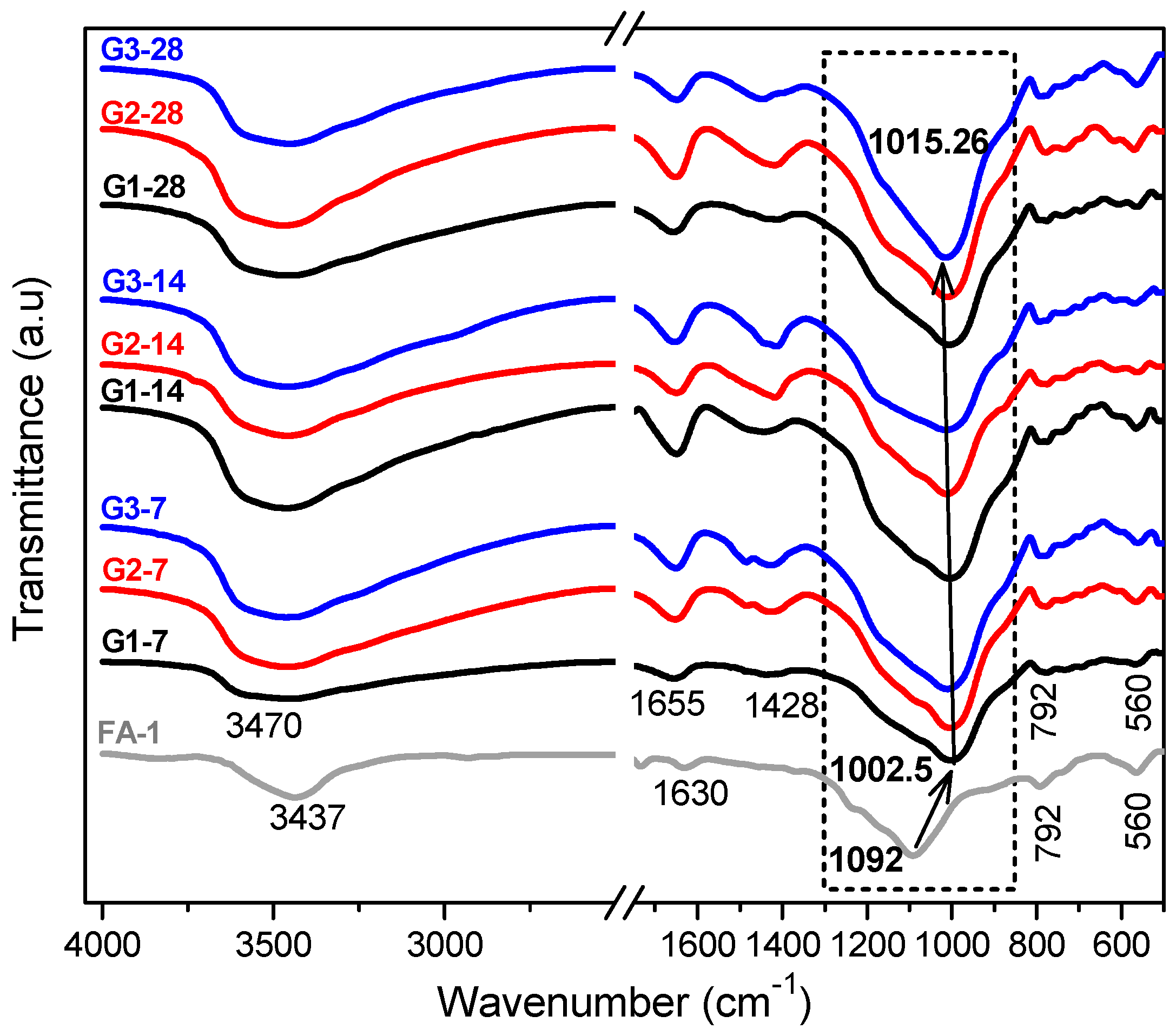
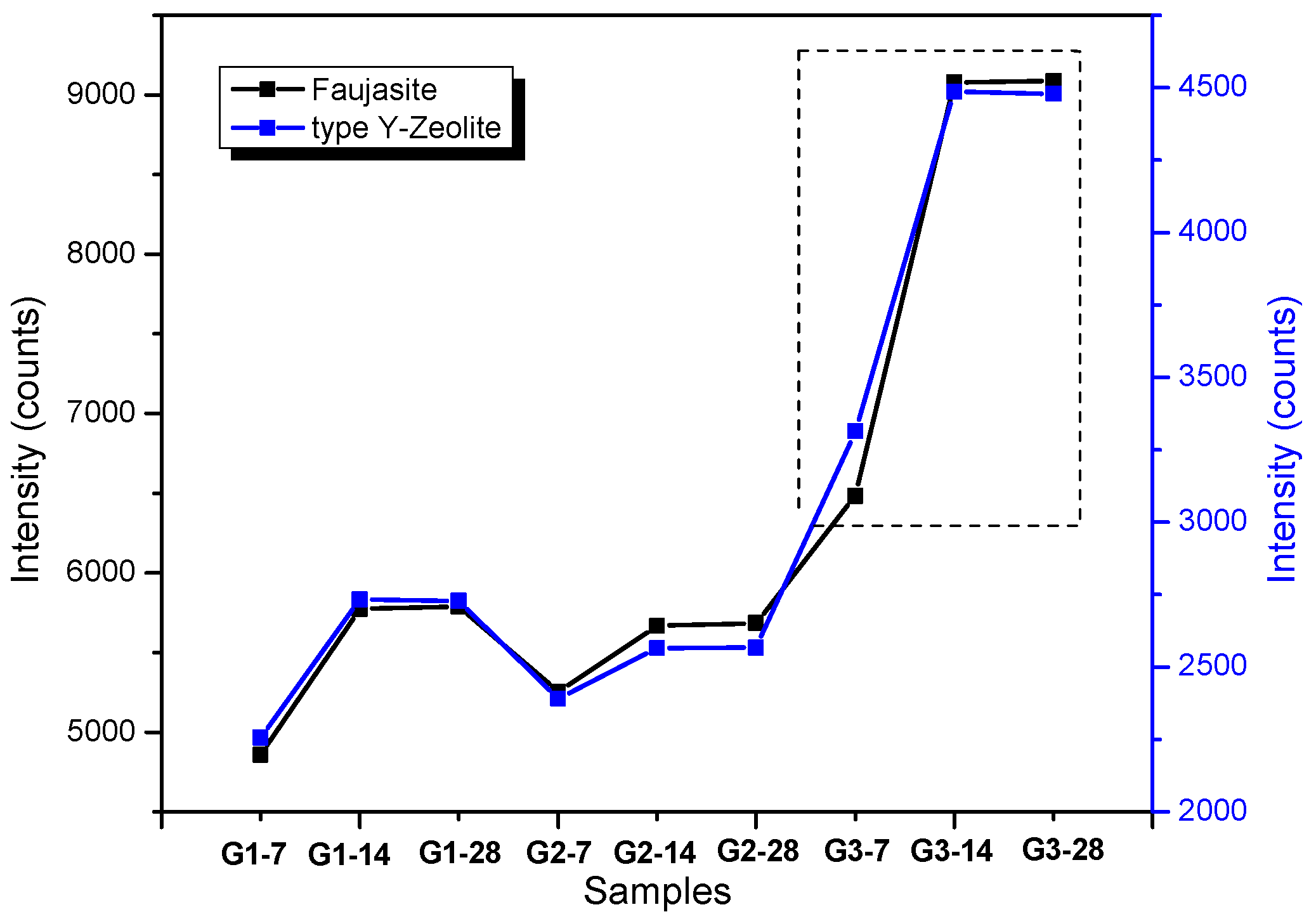
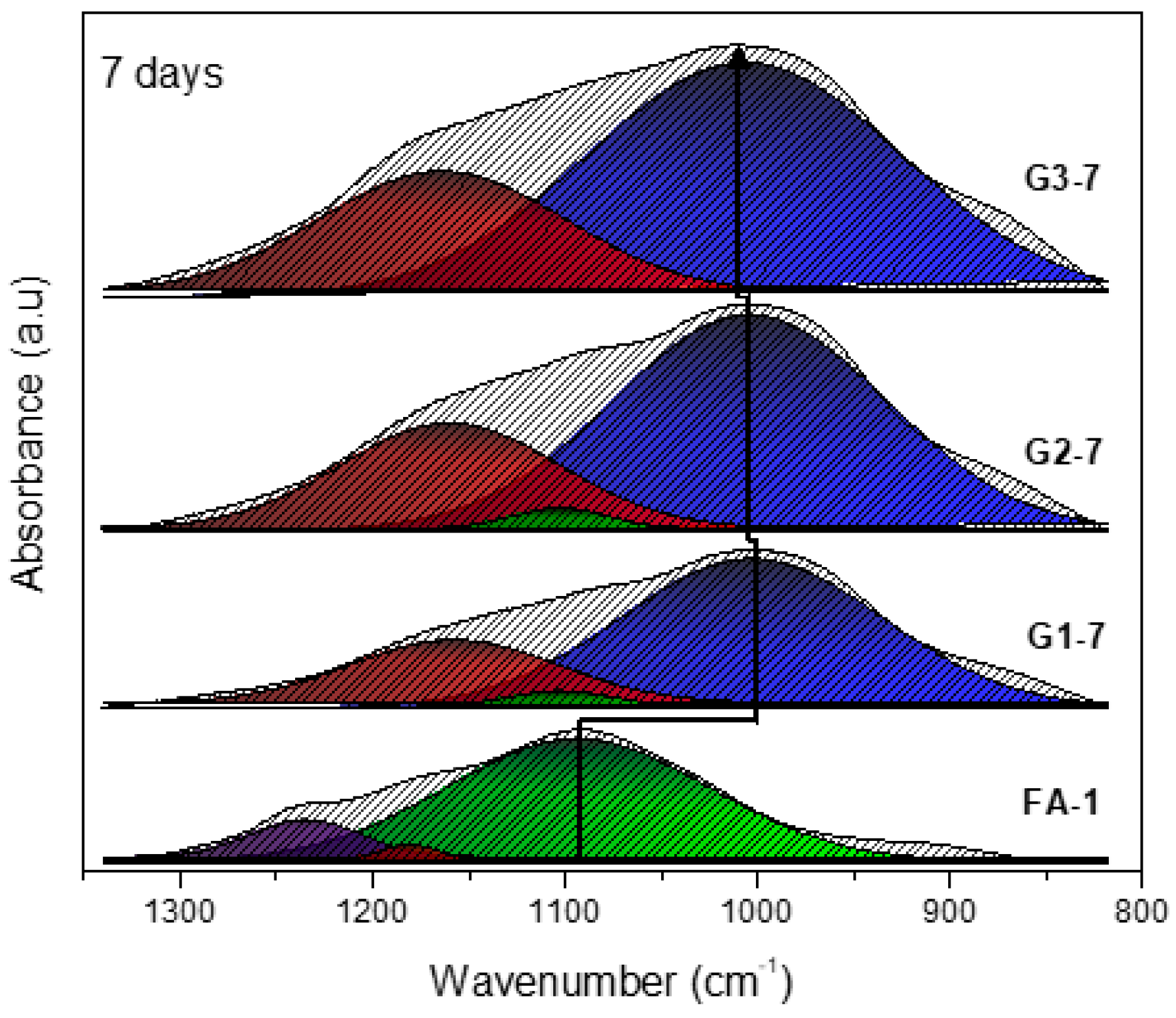
3.1. XRD and FT-IR Analysis
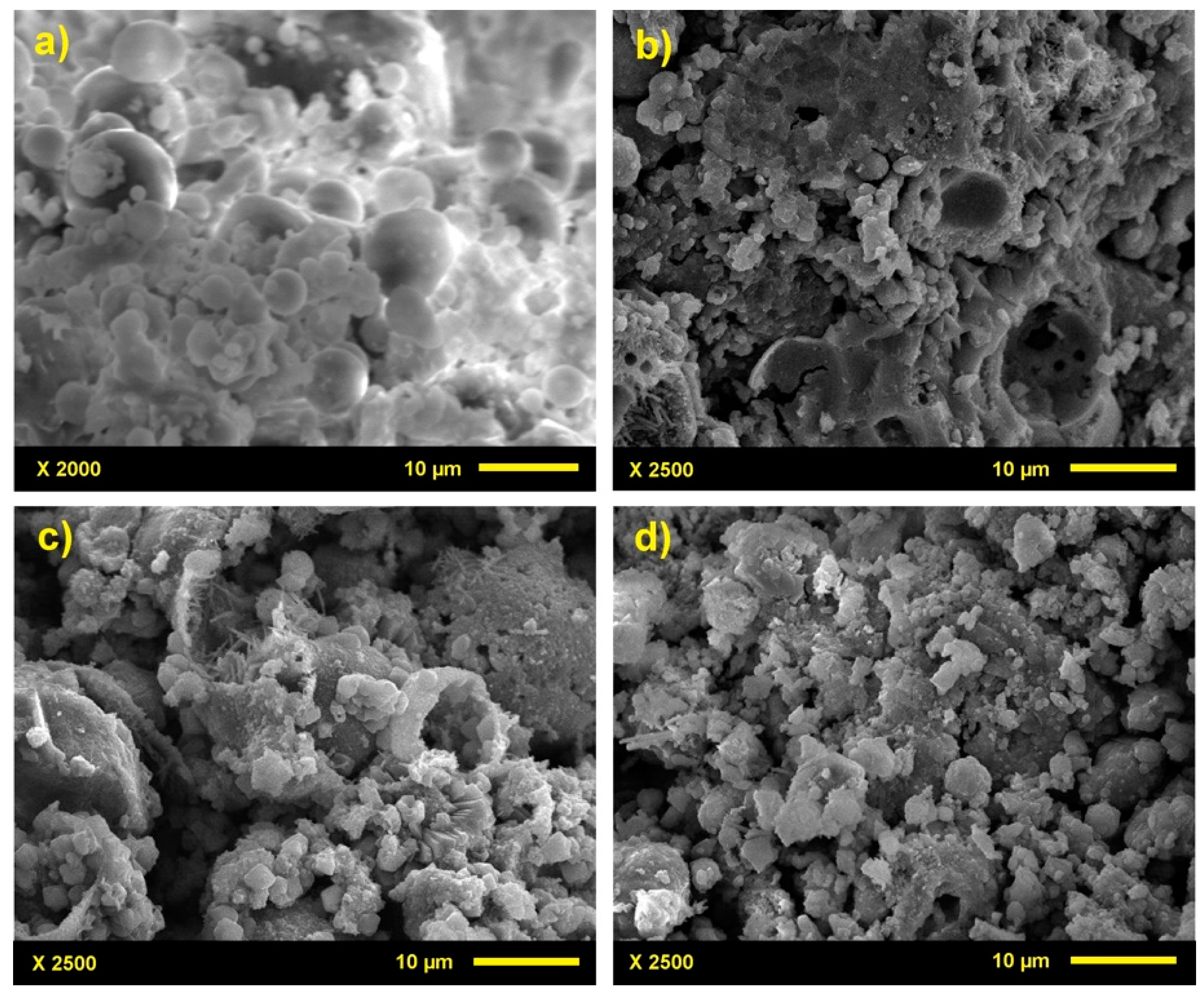
3.2. Microstructure
4. Conclusions
- The reduction of fly ash particle size by grinding allows a greater dissolution in the alkaline activation of the raw material.
- Deconvolutions show the displacement of the bands with greater precision, and in the same way establish whether the activation process was carried out and elucidate the evolution of how the bonds are restructured over time.
- A smaller particle size requires less time to produce crystalline structures and gels that provide stability to the geopolymers, as well as more homogeneity in the matrix and more rigid bonds.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Energy Agency (IEA). Energy Technology Transitions for Industry. Strategies for the Next Industrial Revolution; OECD/IEA: Paris, France, 2009; p. 321. ISBN 978-92-64-06858-2. [Google Scholar]
- Baumert, K.A.; Herzog, T.; Pershing, J. Navigating the Numbers: Greenhouse Gas Data and International Climate Policy; World Resources Institute: Washington, DC, USA, 2005; p. 122. ISBN 1-56973-599-9. [Google Scholar]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Argiz, C.; Moragues, A.; Menéndez, E. Use of ground coal bottom ash as cement constituent in concretes exposed to chloride environments. J. Clean. Prod. 2018, 170, 25–33. [Google Scholar] [CrossRef]
- Manz, O.E. Coal fly ash: A retrospective and future look. Fuel 1999, 78, 133–136. [Google Scholar] [CrossRef]
- Giaccio, G.M.; Malhotra, V.M. Concrete incorporating high volumes of ASTM Class F fly ash. Cem. Concr. Aggreg. 1988, 10, 88–95. [Google Scholar]
- Argiz, C.; Sanjuán, M.A.; Menéndez, E. Coal Bottom Ash for Portland Cement Production. Adv. Mater. Sci. J. Eng. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Argiz, C.; Gálvez, J.C.; Moragues, A. Effect of silica fume fineness on the improvement of Portland cement strength performance. Constr. Build. Mater. 2015, 96, 55–64. [Google Scholar] [CrossRef]
- ASTM C618. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Kriven, W.M. Geopolymer-based composites. In Comprehensive Composite Materials II; Oxford Academic Press: Oxford, UK, 2018; Volume 5, pp. 269–280. [Google Scholar]
- Masi, G.; Rickard, W.D.; Bignozzi, M.C.; Van Riessen, A. The effect of organic and inorganic fibres on the mechanical and thermal properties of aluminate activated geopolymers. Compos. Part B Eng. 2015, 76, 218–228. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard. Mater. 2003, 96, 201–216. [Google Scholar] [CrossRef]
- Argiz, C.; Menéndez, E.; Moragues, A.; Sanjuán, M.A. Fly ash characteristics of Spanish coal-fired power plants. Afinidad 2015, 72, 269–277. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- İlkentapar, S.; Atiş, C.D.; Karahan, O.; Avşaroğlu, E.G. Influence of duration of heat curing and extra rest period after heat curing on the strength and transport characteristic of alkali activated class F fly ash geopolymer mortar. Constr. Build. Mater. 2017, 151, 363–369. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42.9, 2917–2933. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; De La Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Atiş, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; Ilkentapar, S.; Luga, E. Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Erdoğdu, K.; Türker, P. Effects of fly ash particle size on strength of Portland cement fly ash mortars. Cem. Concr. Res. 1998. 28, 1217–1222. [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterization of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; Van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Proc. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujiwara, H.; Noguchi, H.; Hoshino, T.; Ohdomari, I. Novel interatomic potential energy function for Si, O mixed systems. Jpn. J. Appl. Phys. 1999, 38, L366. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Gadsden, J.A. Infrared Spectra of Minerals and Related Inorganic Compounds; Butterworths: London, UK, 1975; p. 277. ISBN 0408706651. [Google Scholar]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S. Effect of source materials on geopolymerization. Ind. Eng. Chem. Res. 2003, 42, 1698–1706. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; p. 454. ISBN 978-1845694494. [Google Scholar]
- Kupwade-Patil, K.; Soto, F.; Kunjumon, A.; Allouche, E.N.; Mainardi, D.S. Multi-scale modeling and experimental investigations of geopolymeric gels at elevated temperatures. Comput. Struct. 2013, 122, 164–177. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Horpibulsuk, S.; Chanprasert, P.; Sukmak, P.; Arulrajah, A. Compressive strength development in fly ash geopolymer masonry units manufactured from water treatment sludge. Constr. Build. Mater. 2015, 82, 20–30. [Google Scholar] [CrossRef]
- Rodríguez, E.; Mejía, R.; Bernal, S.; Gordillo, M. Effect of the SiO2/Al2O3 and Na2O/SiO2 ratios on the properties of geopolymers based on MK. Rev. Fac. Ing. Univ. Antioq. 2009, 49, 30–41. [Google Scholar]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. Attenuated total reflectance Fourier transform infrared analysis of fly ash geopolymer gel aging. J. Langmuir. 2007, 23, 8170–8179. [Google Scholar] [CrossRef] [PubMed]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Du, H.; Hou, F.; Ren, S.; Geng, H. Structure formation process, heat-insulation property and cyclic compression–resilience performance of mullite fibres/whiskers frameworks. RSC Adv. 2014, 4, 9451–9456. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]



| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|
| % by weight | 56.9 | 26.24 | 5.56 | 4.25 | 0.72 | 1.21 | 0.346 | 0.71 | 1.21 | 2.47 |
| Sample | Specific Surface Area (m2/kg) |
|---|---|
| FA-1 | 975 |
| FA-2 | 1107 |
| FA-3 | 1371 |
| Band (cm−1) | Color | Assignment |
|---|---|---|
| 1230–1250 | Asymmetric stretching (Si-O-Si and Al-O-Si) | |
| 1150–1200 | Asymmetric stretching (Si-O-Si) | |
| 1050–1150 | Asymmetric stretching of amorphous phase (Si-O-Si and Al-O-Si) | |
| 980–1030 | Asymmetric stretching of N-A-S-H gel (Si-O-Si and Al-O-Si) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Cruz-Enríquez, A.; Corral-Higuera, R.; Pellegrini-Cervantes, M.D.J.; Gómez-Soberón, J.M.; Medina-Serna, T.D.J. Influence of Size Reduction of Fly Ash Particles by Grinding on the Chemical Properties of Geopolymers. Appl. Sci. 2018, 8, 365. https://doi.org/10.3390/app8030365
Rosas-Casarez CA, Arredondo-Rea SP, Cruz-Enríquez A, Corral-Higuera R, Pellegrini-Cervantes MDJ, Gómez-Soberón JM, Medina-Serna TDJ. Influence of Size Reduction of Fly Ash Particles by Grinding on the Chemical Properties of Geopolymers. Applied Sciences. 2018; 8(3):365. https://doi.org/10.3390/app8030365
Chicago/Turabian StyleRosas-Casarez, Carlos Antonio, Susana Paola Arredondo-Rea, Adriana Cruz-Enríquez, Ramón Corral-Higuera, Manuel De Jesús Pellegrini-Cervantes, José Manuel Gómez-Soberón, and Teresita De Jesús Medina-Serna. 2018. "Influence of Size Reduction of Fly Ash Particles by Grinding on the Chemical Properties of Geopolymers" Applied Sciences 8, no. 3: 365. https://doi.org/10.3390/app8030365







