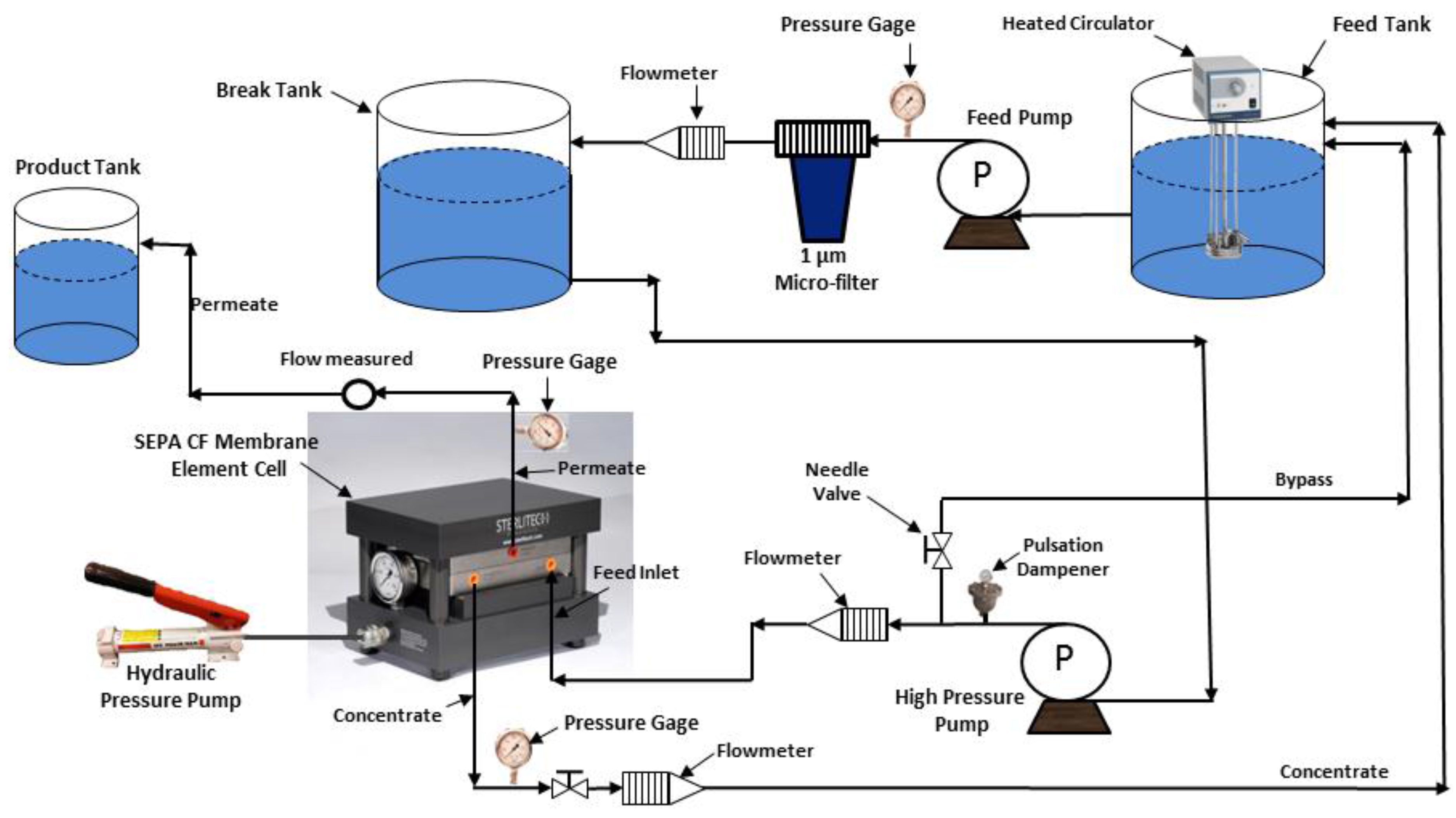
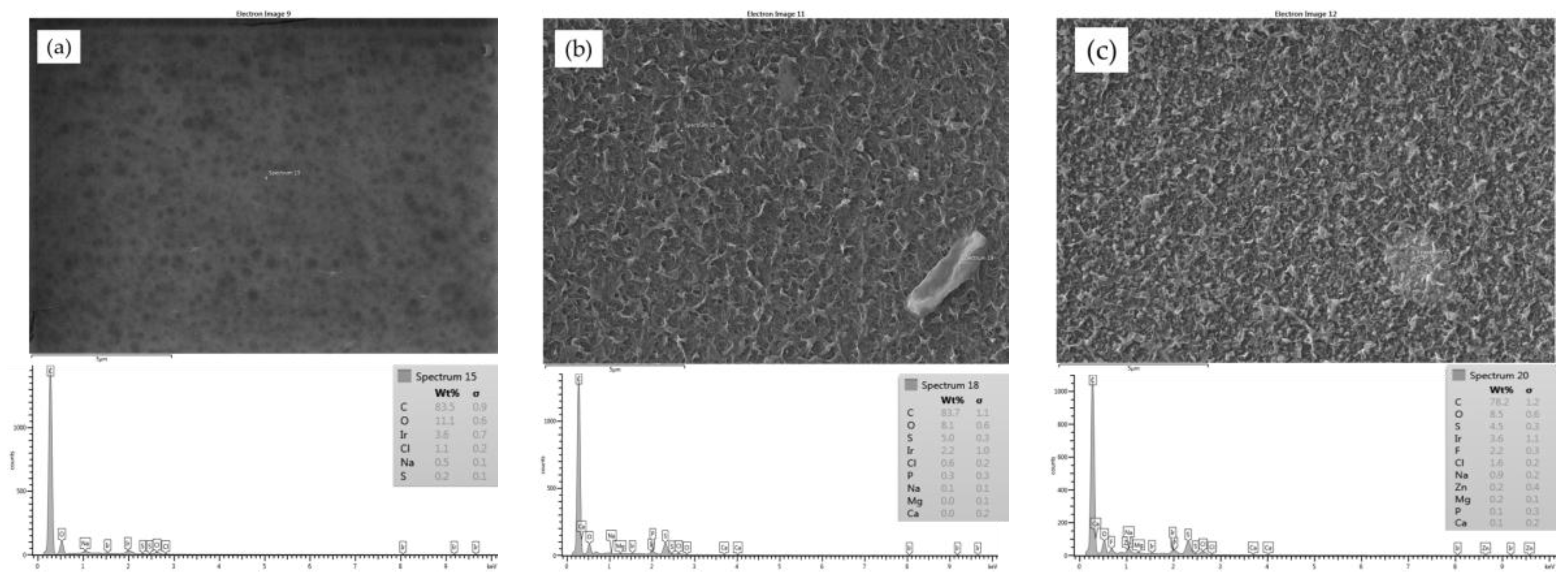
Initially, samples from the clean CE, SE and AD membranes were imaged by the SEM, and corresponding EDXS spectra were taken to reveal the morphology of the membrane surfaces and to also compare them to the fouled membranes surfaces. The SEM images of the unused membranes showed that the surface of the cellulose acetate (CE) membrane had a very smooth morphology, as shown in
Figure 2a, compared to a rough surface morphology of the SE and AD membranes, as shown in
Figure 2b,c, respectively. The EDXS spectra of the three membranes showed that all membranes had a high percentage of carbon which can be caused by the aliphatic functional groups in the cellulose acetate (CE) membrane, aromatic functional groups in the polyamide (AD) membrane, and aliphatic and aromatic functional groups in the thin film composite (SE) membrane [
2,
20]. A considerable percentage of sulfur is present in the spectra of both the SE and AD membranes as a result of the microporous substrate, which is typically polysulphone [
2,
21].
3.1.2. Effect of Membrane Type on Scale Formation
To investigate the difference in the scale morphology among the three types of the membranes (SE, CE and AD), three SEM images of these membranes were taken after the membranes were operated under identical conditions (i.e., feed water quality of location 6, temperature of 37 °C, feed pressure of 2620–2757 kPa, and feed flow rate of 2.27 Lpm). First,
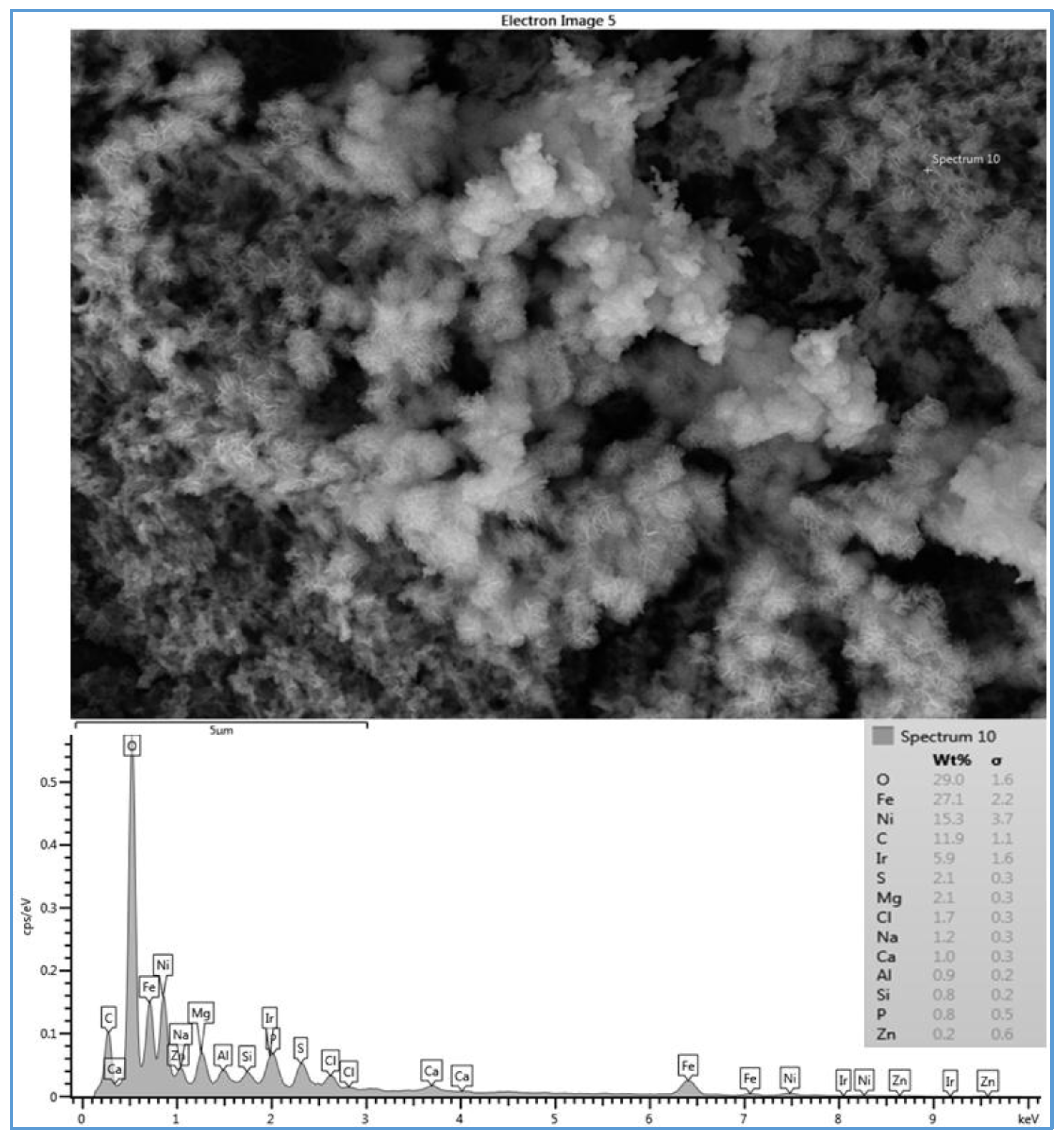
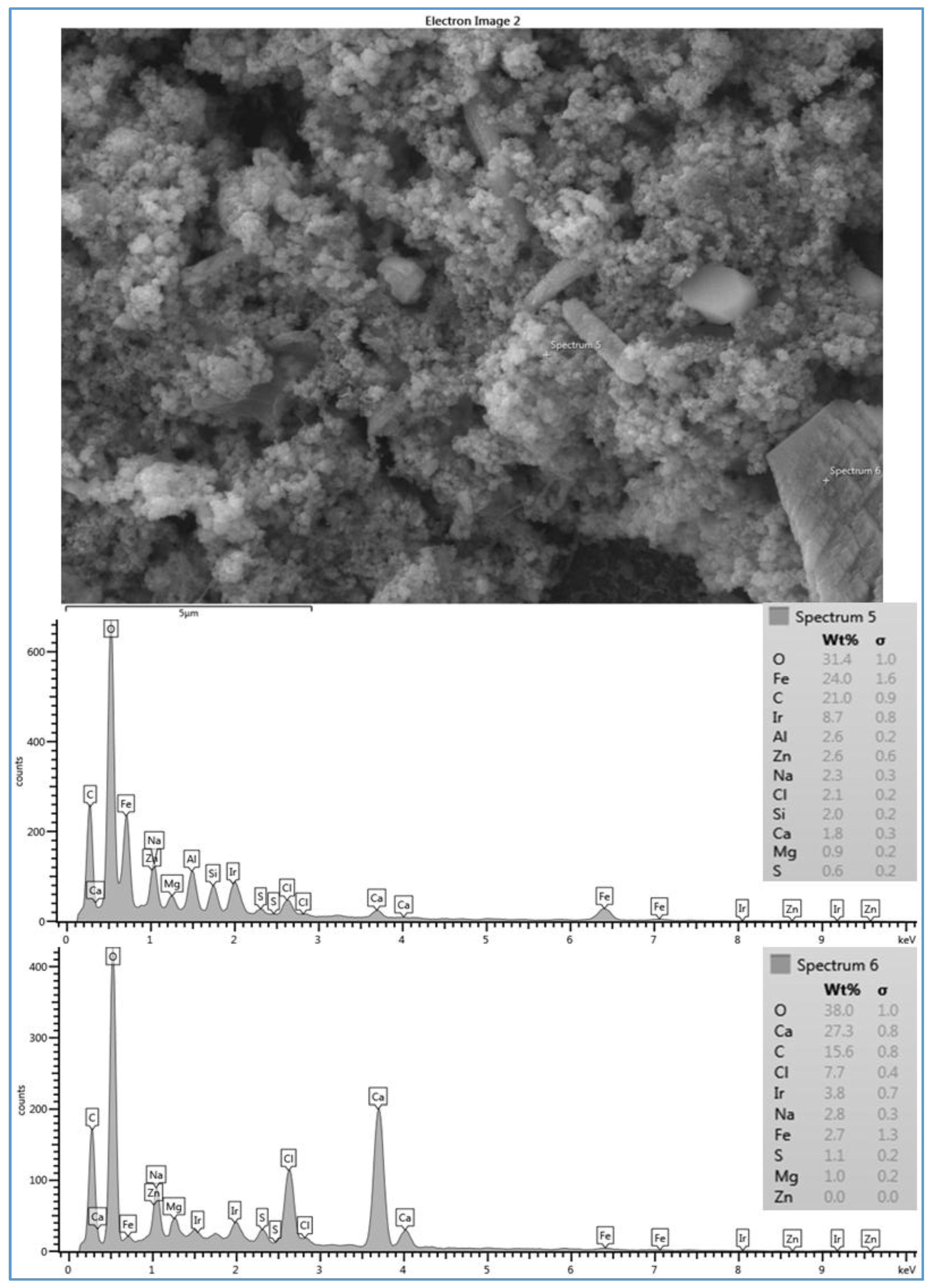
Figure 4 shows the SEM image and the EDSX spectrum of the fouled SE membrane (run 1). The EDSX analysis unexpectedly showed that the scale formation had high level of Ni and Fe which were not highly present in the feed water of location 6. As expected, O, C, Na
+, Ca
2+, Mg
2+, and Cl
− were present in the spectrum. The data suggest that the fouling was due to a combination of organic matter and inorganic material. Although Fe was observed in the feed water in low quantity, it was probably due to a leaching from the system parts. For example, some rust was seen on the submerged part of the heated immersion circulator indicating a leach of iron into the feed water. In addition, although it was not found in the feed water, Ni was also detected by the EDSX spectrum. It was apparently due to the rust as well. The part that was rusting was replaced with an aluminum part to minimize the corrosion during the rest of the experiment runs.
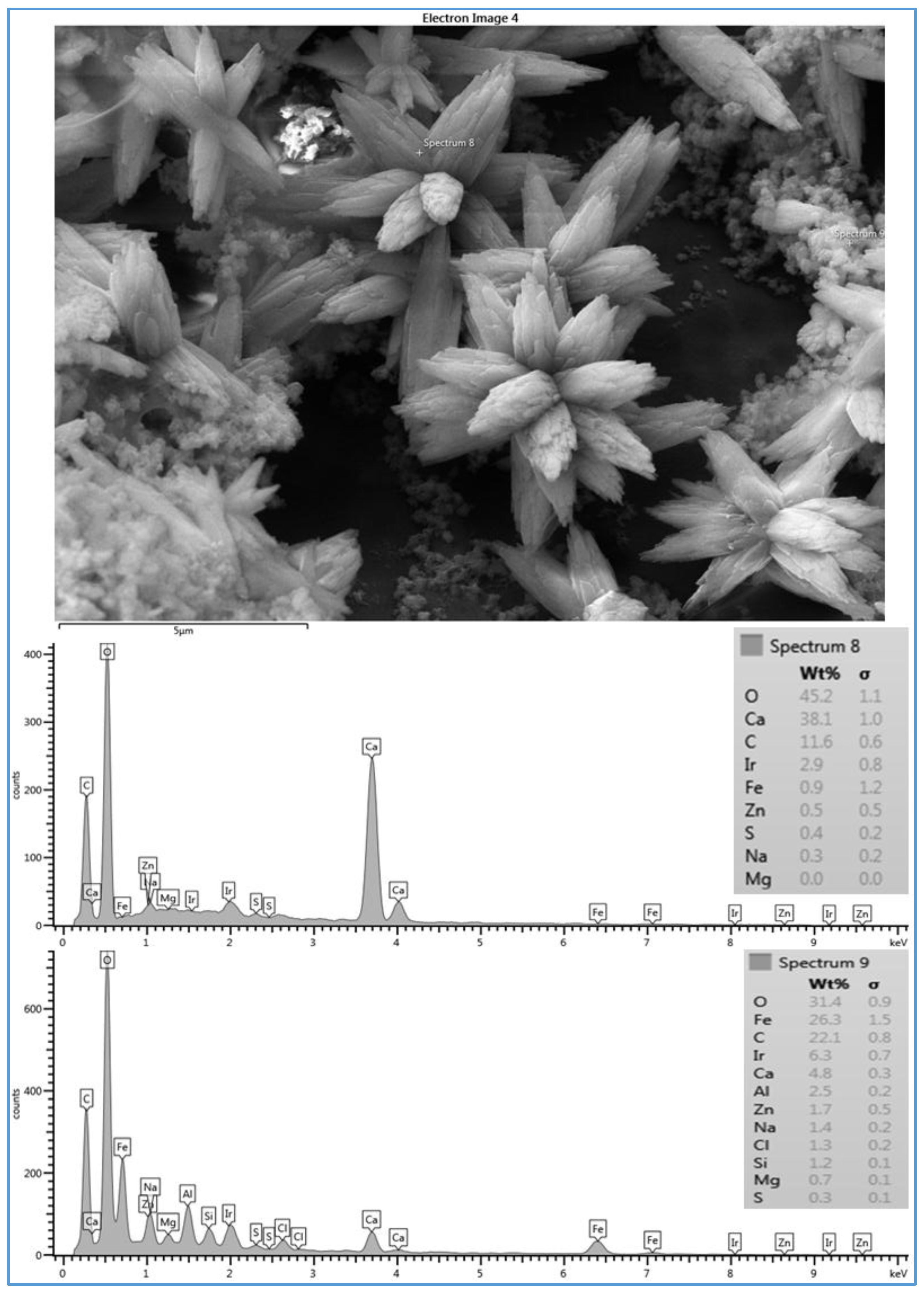
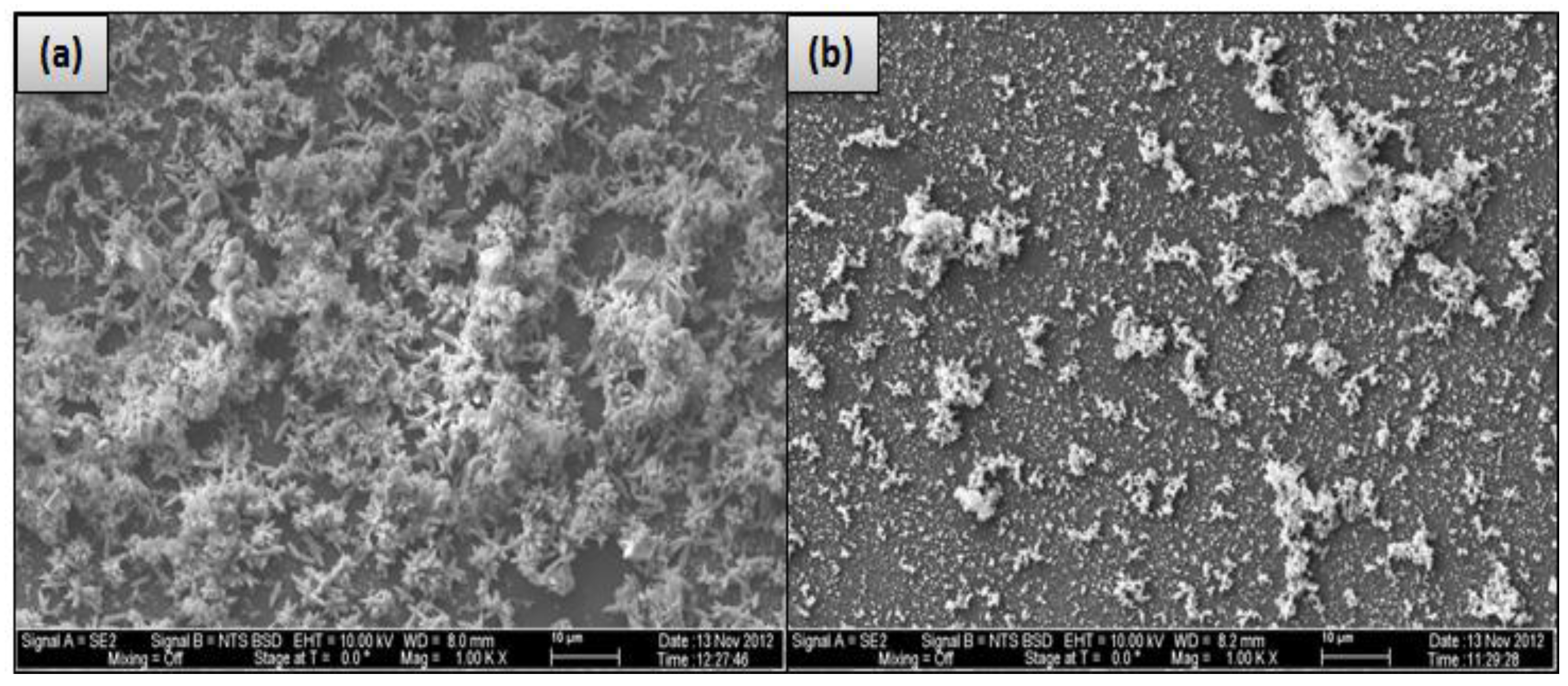
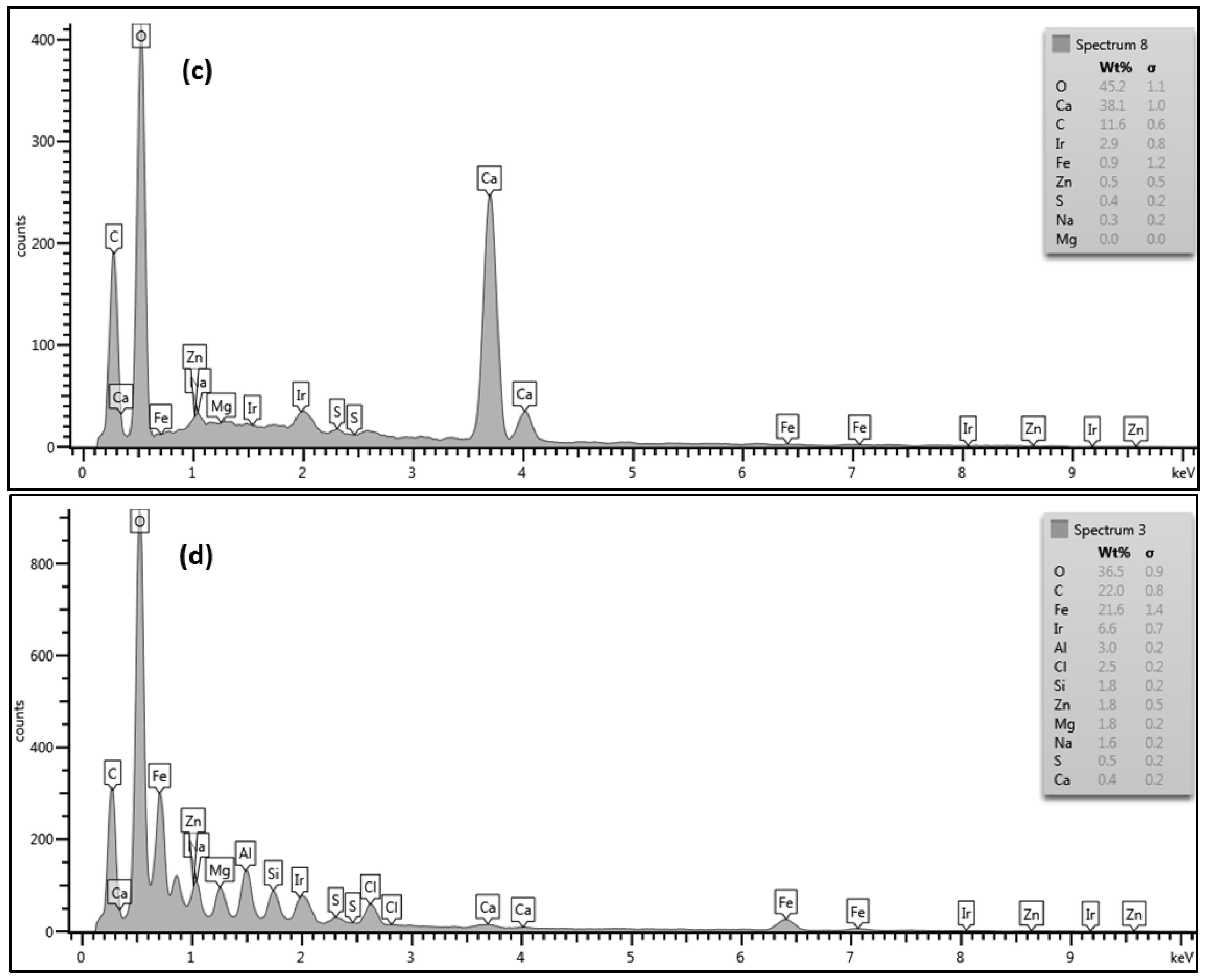
However, the deposited material on the CE membrane surface (run 3) mostly covered the membrane surface with a crystal structure (flowers) and lesser amounts of amorphous shapes, as illustrated in
Figure 5. To investigate both structures, two spectra were taken in both places. Spectrum 8, which was located on the flower structure, shows that a high level of Ca
2+ (38.1%) was present. Spectrum 9, which was located on the amorphous shape, shows that a lower level of Ca
2+ (4.8%) was present. Both spectra show high levels of C and O. Moreover, spectrum 9 also shows a high level of Fe
2+ (26.3%). The high peaks of O, C, and Ca
2+ that exist in spectrum 8 suggest that the structure of the deposited material was calcium carbonate (CaCO
3), which is consistent with that observed by Tzotzi [
22] on a thin film composite polyamide membrane surface. The data presented in spectra 8 and 9 on the CE membrane surface suggest that the crystal forms were accompanied by a small amount of a mixture of organic matter and inorganic materials. In addition, the smooth black spots on the SEM image in
Figure 5 are parts of the CE membrane surface. Membrane fouling can be affected by the membrane surface characteristic. Smooth surface such as that of CE membrane is less susceptible to fouling. However, membrane with rough surface such as that of SE membrane is more susceptible to fouling by materials that can be accumulated in the valleys or channels of the surface [
17]. Therefore, the fouled material of run 3 partially covers the CE membrane surface compared to that of run 1, which apparently covers the entire surface of the SE membrane, as illustrated in
Figure 4. This provides one potential cause for the rapid decline in the permeate flux of run 1 compared to that of run 3. This observation agrees with the hypothesis that the permeate flux decline increases linearly with the increase of the scale formation on the membrane surface [
22,
23].
Figure 6 shows that the fouling layer deposited on the AD membrane surface (run 5) has different shapes. Some large crystal shapes exist as part of the scale formation on the membrane surface. Also, two spectra were taken to investigate the morphology of the two different shapes of the fouling material on the membrane surface. Like the observation in spectrum 8 of run 3, spectrum 6, which was taken on the crystal shape, shows a high level of Ca
2+ (27.3%) compared to a lower level of Ca
2+ (1.8%) on the amorphous shaped material, as shown by spectrum 5. Likewise, both spectra (5 and 9) show a high level of C and O as well as a high level of Fe
2+ on the amorphous shaped material. In addition, spectrum 5 shows a low level of silica present in the deposited material, and spectrum 6 shows a high peak of Cl
− on the crystal shape. The SEM image and the data of spectra 5 and 6 suggest that the fouling materials deposited on the AD membrane surface have different configurations and composition. The fouling materials included different crystal shapes and a mixture of organic and inorganic matter. As a result of high levels of O, C and Ca
2+, the large crystal form is also possibly due to the formation of CaCO
3, which was reported as a common scale with all feed types by Greenlee [
5], Kucera [
2], and Antony [
23].
The flower crystals of CaCO
3 seen in
Figure 5 and the large and elongated crystals seen in
Figure 6 are probably due to the high TOC (4.6–4.8 mg/L) and carbonate (72–82.4 mg/L) concentrations of the feed water at location 6, and CaCO
3 is likely a calcite crystal form, as suggested by Koyuncu and Wiesner [
13]. Furthermore, they reported that the ratio of the organic matter/calcium influences the shape of the CaCO
3 crystal in the cake formation. Moreover, Koyuncu [
24] proposed that organic matter may play a role in reducing calcium diffusivity in the cake formation. In addition, Lee [
25] reported that natural organic matter (NOM) can reduce free calcium ions by producing NOM-calcium complexes on the membrane surface. Also, they claimed that NOM-calcium complexes form a compressed layer on the membrane surface, which severely decreases the permeate flux. Therefore, some spectra did not show high peaks of calcium, particularly when the scale formation on the membrane surface was a more sludge-like deposit than a crystal formation, such as those of runs 1 (see
Figure 4 and
Figure 7). The results of the various configurations of the scale formations deposited under the same conditions on the three types of the RO membranes indicate that the surface roughness and the material of the membrane itself affect the scale formation, which ultimately impacts the permeate flux [
22,
26]. Therefore, it is noted that the ranges of the permeate fluxes of the three membranes, SE, CE and AD, were 0.656–0.327 Lpm/m
2, 0.481–0.314 Lpm/m
2 and 0.467–0.325 Lpm/m
2, respectively. The corresponding total time of these permeate fluxes of SE, CE, and AD membranes to achieve 70% of water recovery was 75.4 h, 80.8 h, and 85.6 h, respectively. The permeate productivity of the SE membrane (run 1) was significantly higher than that of CE (run 3) and AD (run 5) membranes. However, the salt rejection of the SE membrane (98.7%) is lower than that of the AD membrane (99.3%), but higher than that of the CE membrane (97.5%). This indicates that the salt rejection of the membrane was also affected by the surface roughness and the material of the membrane itself.
3.1.3. Effect of Feed Water Temperature on Scale Formation
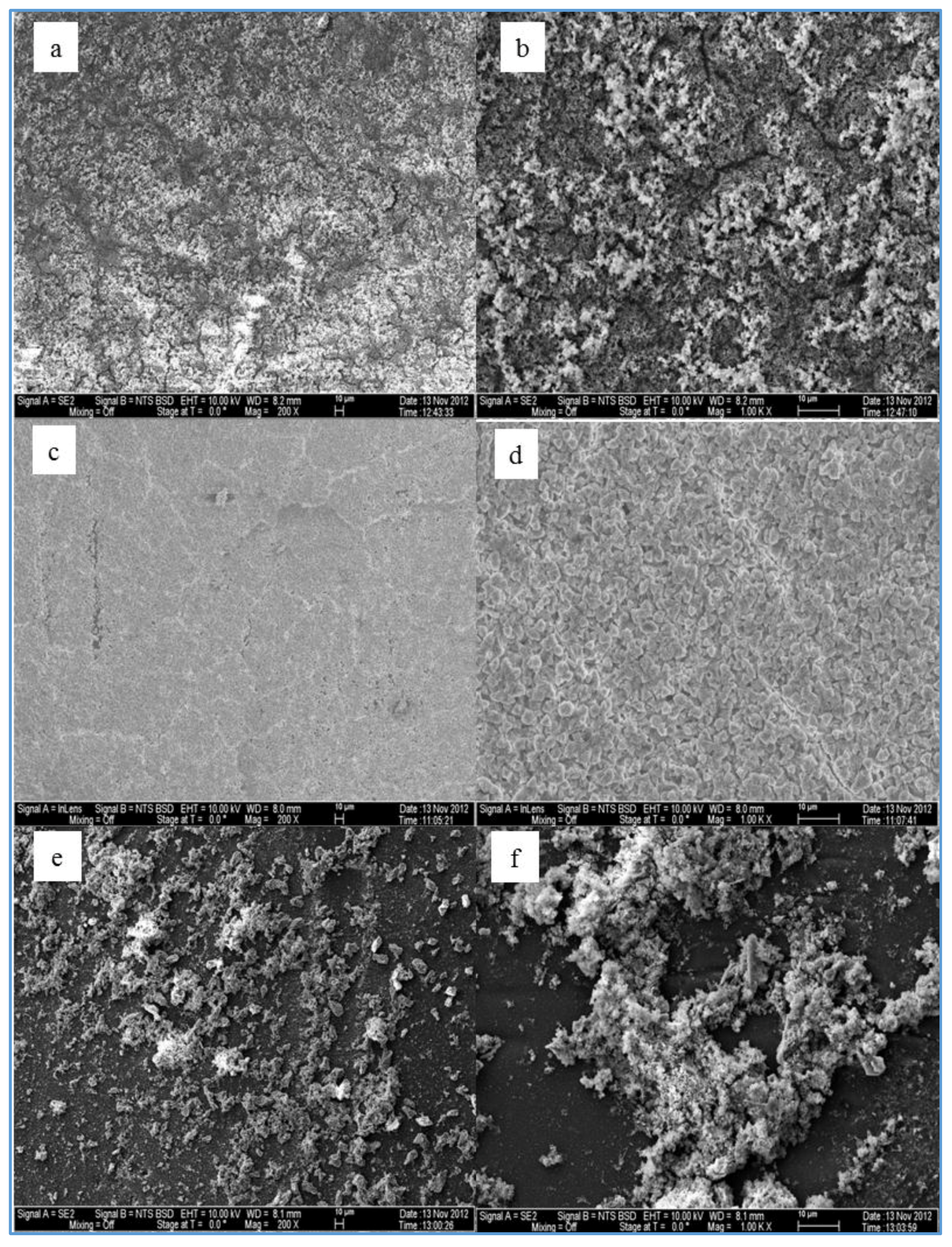
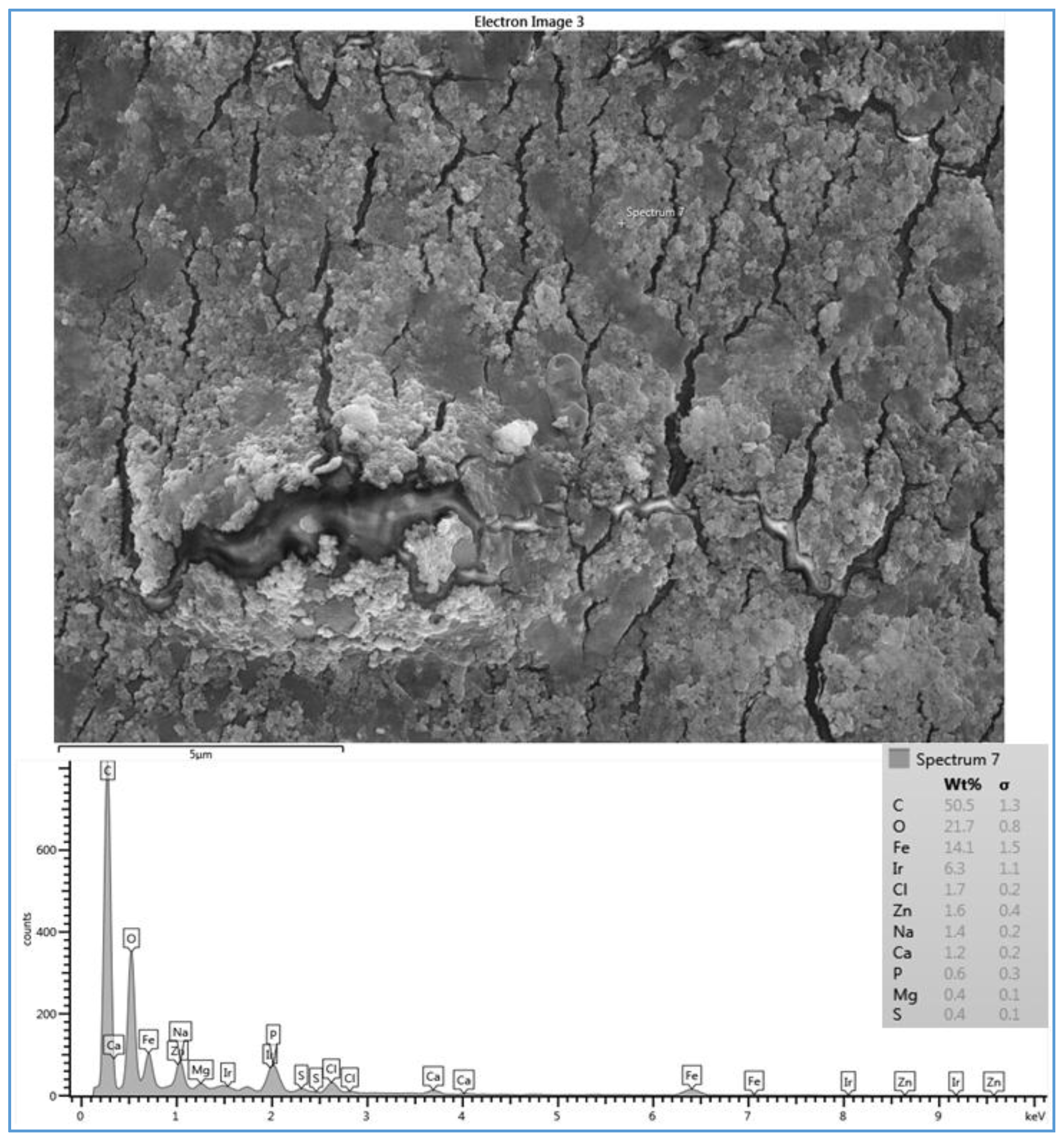
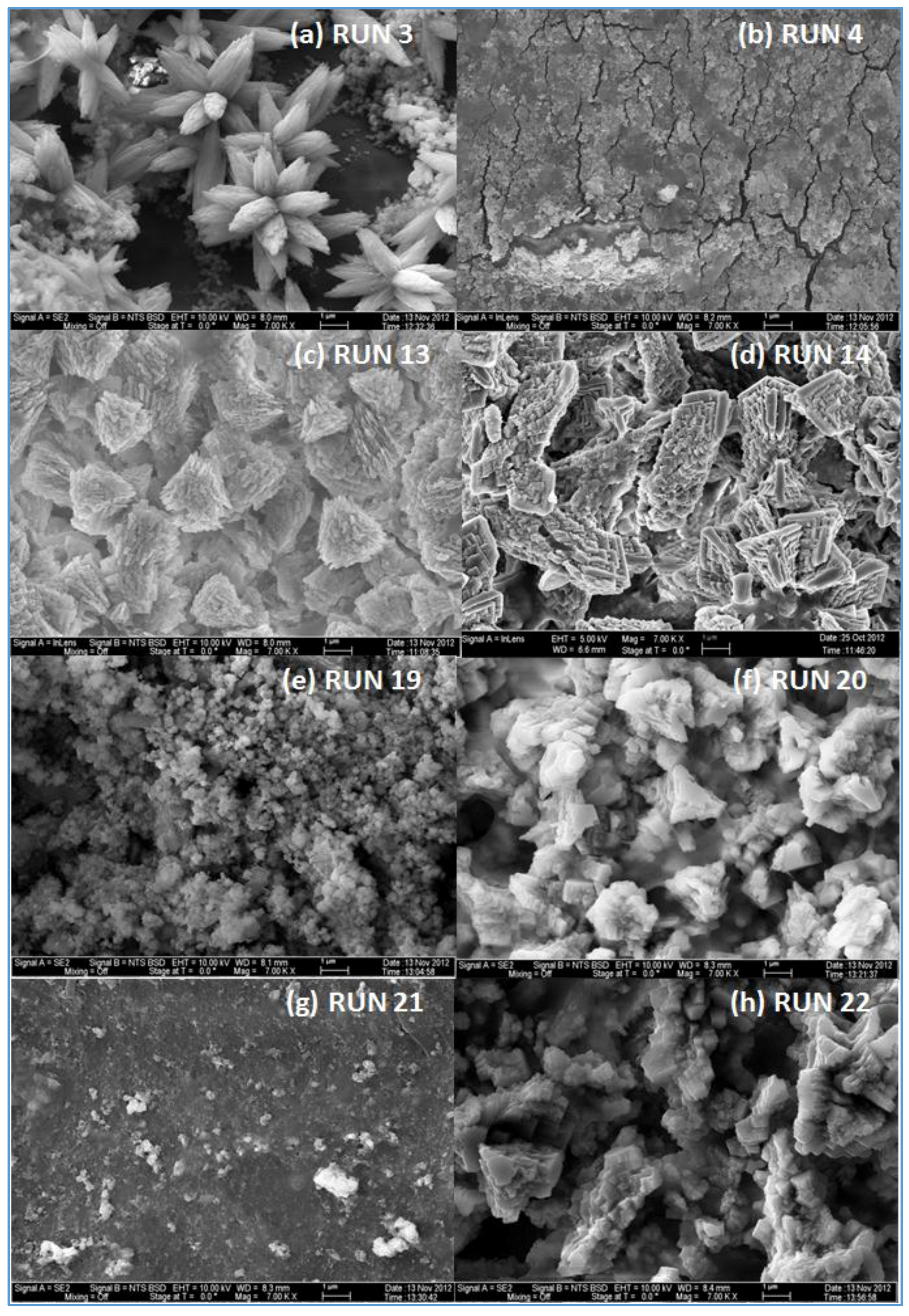
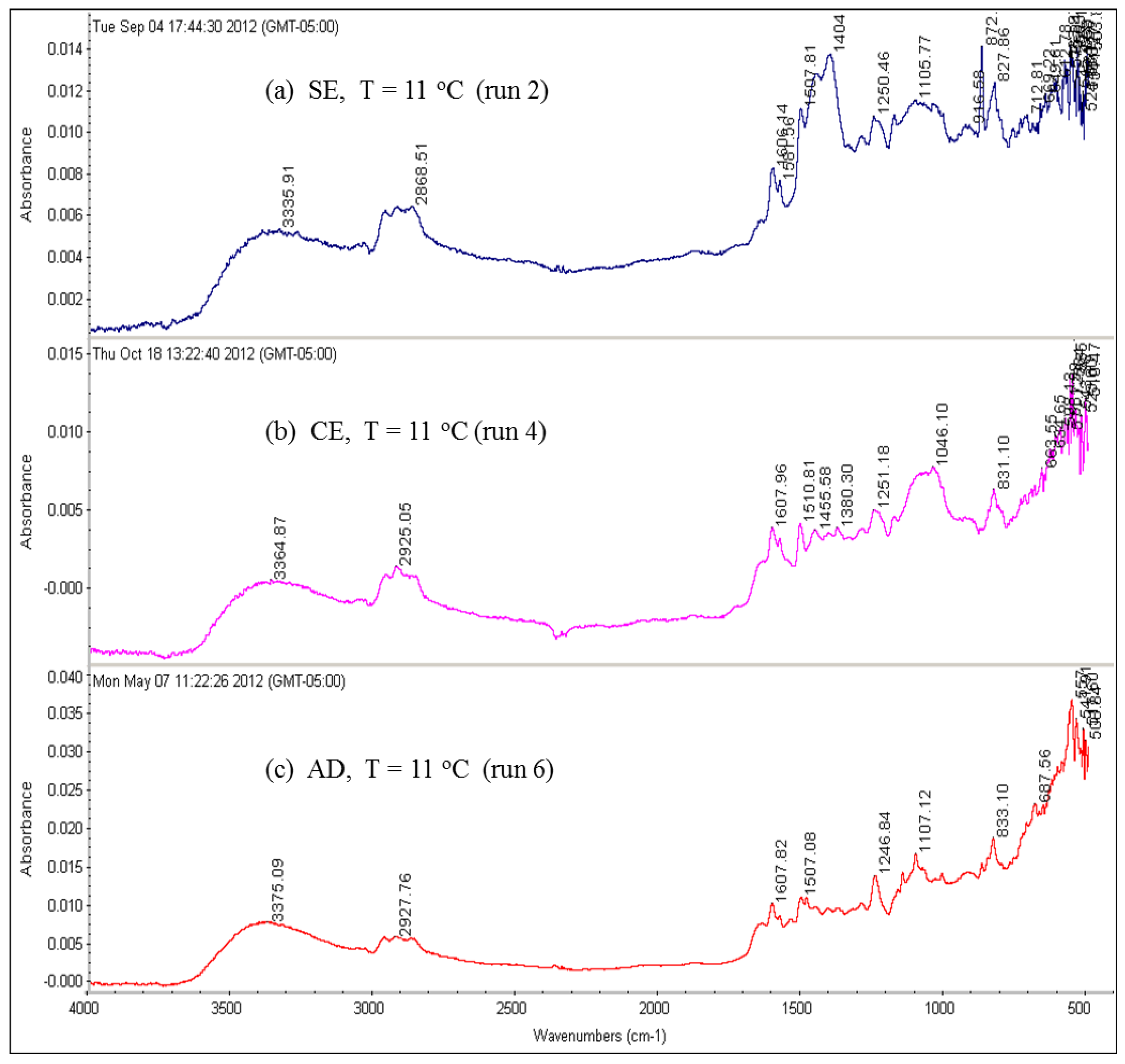
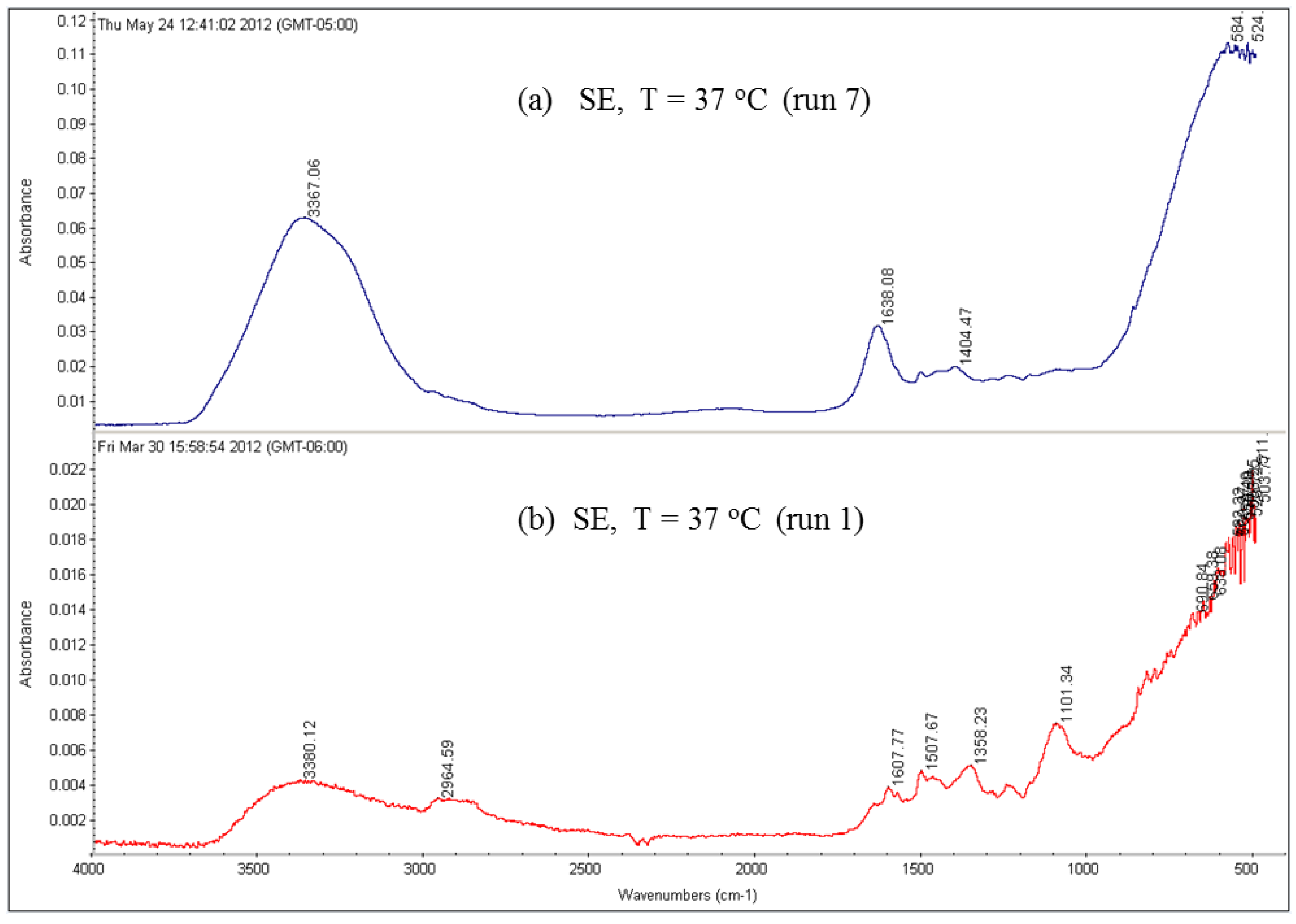
The effect of feed water temperature on the formation of the fouling material deposited on the membrane surface was also examined in this study. The SEM images in
Figure 8 shows a comparison between the scale formations on the surface of several RO membranes at high and low temperatures. The SEM images focus on the densest of accumulated materials on the selected RO membrane surfaces. The scale formation on the CE membrane at 11 °C (run 4) is quite different from that at 37 °C (run 3), as shown in
Figure 8a,b. The morphology of the fouled material on the CE membrane surface of run 3, as previously mentioned, is in more of a crystalline shape, which is mostly CaCO
3, while the morphology is a sludge-like deposit on the membrane surface of run 4. It is obvious that low temperature (11 °C) affected the deposited material on the CE membrane and resulted in the sludge layer. Jawor and Hoek [
27] reported that high temperature (25–35 °C) brackish feed water can enhance the crystal formation of the deposited material on the RO membrane surface, which agrees with what was observed in run 3. The EDSX spectrum of the fouled membrane of run 4, as shown in
Figure 7, shows that high levels of C and O, lesser amounts of Fe, Cl
−, Zn, Na
+ and Ca
2+, and a small amount of P, Mg
2+, and S were present in the scale formation. The results suggest that the scale formation on the CE membrane surface of run 4 is mostly organic or carbonaceous matter, which can be attributed to the high levels of TOC and carbonate of the feed water, and is accompanied by inorganic materials. It can be assumed that the higher total permeate flux drop of run 4 (42.8%), which was calculated after completion of the run, compared to that of run 3 (34.7%), is a consequence of the blockage of the CE membrane surface because of the sludge layer formation.
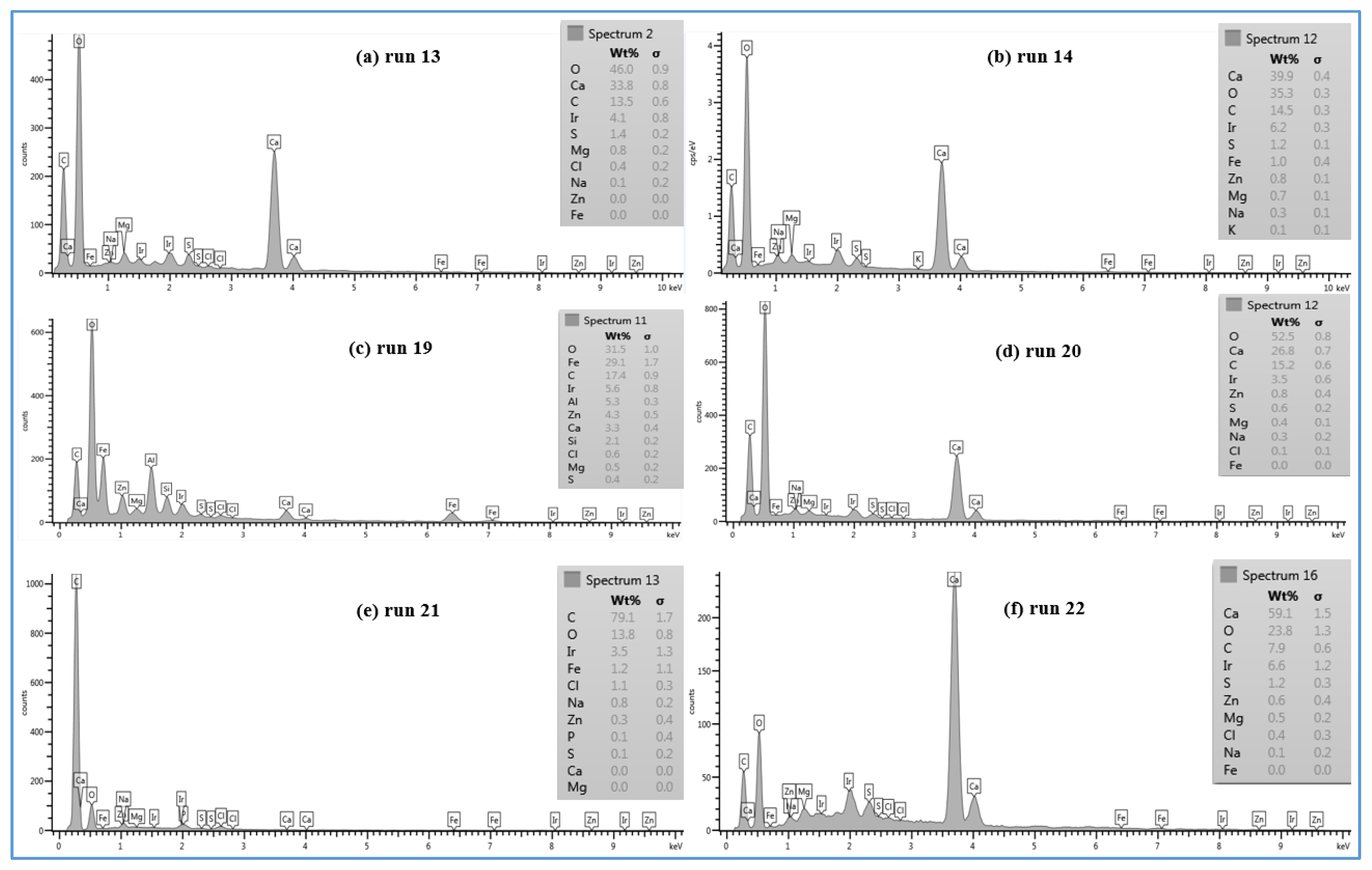
In lower TOC feed water (1.4–2.3 mg/L), the effect of the temperature on the morphology of the deposited material on the membrane surface is not the same as the one seen in the high TOC feed water (4.6–4.8 mg/L). The SEM images of the fouled SE membrane of run 13 and run 14 shown in
Figure 8c,d revealed that the formation of the fouled material from the feed water in location 1 deposited on the membranes surface is mostly in crystal shapes at both temperatures (37 and 11 °C). In addition, the results exhibited that the EDSX spectra of both fouled membranes, as displayed in
Figure 9a,b, is nearly identical. Both spectra have high peaks of C, O, and Ca
2+, indicating that the formed crystal on the membrane surface is likely CaCO
3. The relatively low TOC and high Ca
2+ (77.4–82 mg/L) and carbonate (73–94.2 mg/L) concentrations in feed water in location 1 apparently resulted in the uniform crystal shapes of CaCO
3. This observation is consistent with the findings noted by Koyuncu and Wiesner [
13].
Moreover, the SEM images of the deposited material from the feed water in location 5 at the temperature of 37 °C (run 19) and 11 °C (run 20), are shown in
Figure 8e,f, respectively. Both images show that the fouling materials extend across the entire surface of the SE membranes with a more crystalline morphology of the fouling developed on the membrane surface of run 20 at a low temperature (11 °C). The EDSX spectra shown in
Figure 9c and d revealed a high peak of Ca
2+ in addition to the high peaks of O and C on the fouled membrane surface of run 20, which indicates that most of the crystal morphology is CaCO
3. Also, the EDSX spectra shows that the fouling of run 19 and run 20 have a combination of organic matter and inorganic material. In addition, Inductively Coupled Plasma (ICP) spectrometer analysis showed that the Ca
2+ concentration of the permeate at low temperature (run 20) was higher than that at high temperature (run 19), as shown in
Figure 10. The Ca
2+ concentration is a consequence of more calcium deposits on the SE membrane surface, which resulted in forming more crystal morphology like CaCO
3.
In addition,
Figure 8g,h shows the SEM images of the scale formation of the fouled material from feed water in location 5 deposited on the CE membrane surface at the temperatures of 37 °C (run 21) and 11 °C (run 22), respectively. Like those of run 19 and run 20, the scale formation also extended across the entire surface of the CE membrane of runs 21 and 22. The fouling form of the high feed water temperature is apparently a sludge-like deposit on the membrane surface, while the fouling form of low feed water temperature is a combination of both sludge and crystalline shapes. The EDSX spectrum in
Figure 9e shows that the cake formation of run 21 has a carbon content of 79.1% and oxygen of 13.8%, suggesting that much of the fouling material is organic matter. However, the EDSX spectrum of
Figure 9f exhibits a high level of calcium (59.1%), besides the high level of the oxygen (23.8%) and lower level of carbon (7.9%) in the fouled material of run 22, suggesting that the large particles of the cake formation are like those of run 20, which are CaCO
3. Again, the low TOC and high carbonate concentrations of the feed water in location 5 were likely the main reason behind the formation of the CaCO
3 crystals. This observation agrees with that found by Koyuncu and Wiesner [
13]. Contradictory to the morphology observation of the high TOC and TDS feed water runs (for instance runs 3 and 4), the low temperature (11 °C) with the low TOC and TDS feed water (for instance runs 21 and 22) enhances the crystal formation of the deposited material on the RO membrane surface (see
Figure 8).
Overall, the results of the SEM images and EDSX spectra of the previously discussed runs revealed that the selected RO membranes have high rejection of organic matter, carbonate, and calcium, were also observed by Koyuncu [
24], Koyuncu & Wiesner [
13], Tran [
3] and Antony [
23].
Another observation is that although the feed waters in locations 1 and 6 had high concentrations of sulfate (312–320 mg/L and 681–687.4 mg/L, respectively), sulfur peaks were at very low levels, as shown in some of the spectra of the runs, indicating that some potentially low level of CaSO
4 precipitated on the membrane surface. The low levels of precipitated sulfate are due to the high rejection of the RO membranes in favor of the carbonate that outcompetes the sulfate ion, as interpreted by Koyuncu and Wiesner [
13]. It is also noted that the concentration of the sulfate in the concentrated stream of all conducted runs was very high when the desired water recovery was achieved. For example, the final sulfate concentrations in the concentrate stream of runs 1, 3 and 5 in location 6 were 2280 mg/L, 1813.3 mg/L and 1917.1 mg/L, respectively. The final sulfate concentrations in the concentrate stream of runs 19, 21 and 23 in location 5 were 331.4 mg/L, 548.8 mg/L and 466.94 mg/L, respectively. This observation provides additional evidence that only small amounts of sulfur precipitated on the surface of the fouled RO membranes.
3.1.4. Effect Pretreatment of Feed Water on Scale Formation
The effect of using a 0.1-micron MF membrane, which is considered the most appropriate method to remove larger particulates as a pretreatment unit for the feed water in advance of the RO membrane [
5], on the precipitated material on the membrane surface was also investigated.
Figure 11 shows an SEM image comparison of the fouled material of the un-pretreated (run 3) and pretreated (run 9) feed water in location 6 on the CE membrane surface. The SEM images in
Figure 11 show that the extent of the fouling across the surface of the CE membrane conducted without the MF membrane covers more than 70% of the membrane surface, compared to about 25% of that of the CE membrane conducted with the MF membrane. Supporting this idea was the fact that the initial permeate flux of the run conducted with the MF membrane was higher than the initial permeate flux of the run conducted without using the MF membrane. For example, the initial permeate flux of run 3, which was conducted without an MF membrane, is 0.481 Lpm/m
2, compared to 0.716 Lpm/m
2 for run 9 conducted with the MF membrane. Additionally, the formation of the fouled materials from the pretreated feed water by the MF membrane on the CE membrane surface (run 9) is apparently in clusters, and the EDSX spectrum of run 9, as shown in
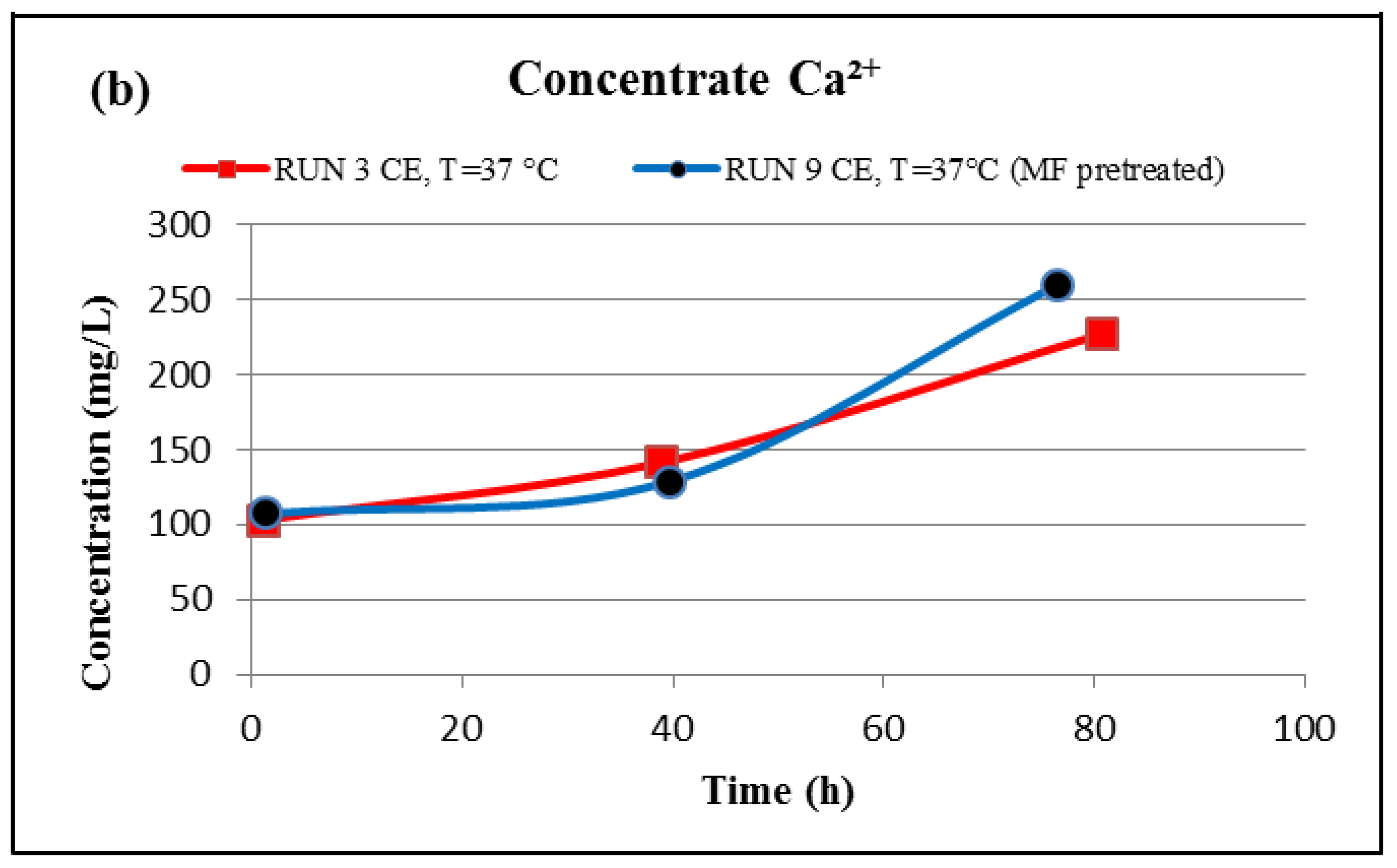
Figure 11d, displays high peaks of C and O, indicating that most of the fouling is organic matter. Furthermore, the EDSX spectra of run 3 and run 9 show that the precipitated calcium level on the membrane surface decreased from 38.1% to 0.4%. In addition, the permeate calcium concentration of run 9 conducted with the MF membrane is lower than that of run 3 conducted without an MF membrane, as presented in
Figure 12a, while the final concentration of the calcium in the concentrate stream of run 9 is higher than that of run 3, as displayed in
Figure 12b. The results suggest that more organic matter, which plays an important role in reducing calcium diffusivity in the fouled layer [
13,
24,
25], deposited on the RO membrane surface of run 9, and also more inorganic material was captured by the MF membrane. Therefore, the precipitated calcium on the surface of the fouled CE membrane was considerably reduced, and consequently the concentration of the calcium in the permeate stream of run 9 was reduced as well.
3.1.5. Investigation of the Cross Section of the Fouling Layer
An SEM/EDSX investigation of the cross section of the fouling layer on the SE membranes from the three water qualities was also done in this study. In general, an RO membrane sheet is comprised of either cellulose acetate membrane, which is made from a combination of cellulose diacetate and triacetate, or a thin-film composite polyamide membrane, which is manufactured by combining three structurally different layers. The first layer is typically made of a thin-film of cross-linked aromatic polyamide materials with a thickness of approximately 0.2 µm. The function of this layer is to reject dissolved and suspended solids in the feed water on one side and allow water to permeate. The second and the third layers are supporting layers which are polysulfone layer with a thickness of 50 µm and a non-woven polyester fabric layer with a thickness of 150 µm [
2,
21]. The SEM image in
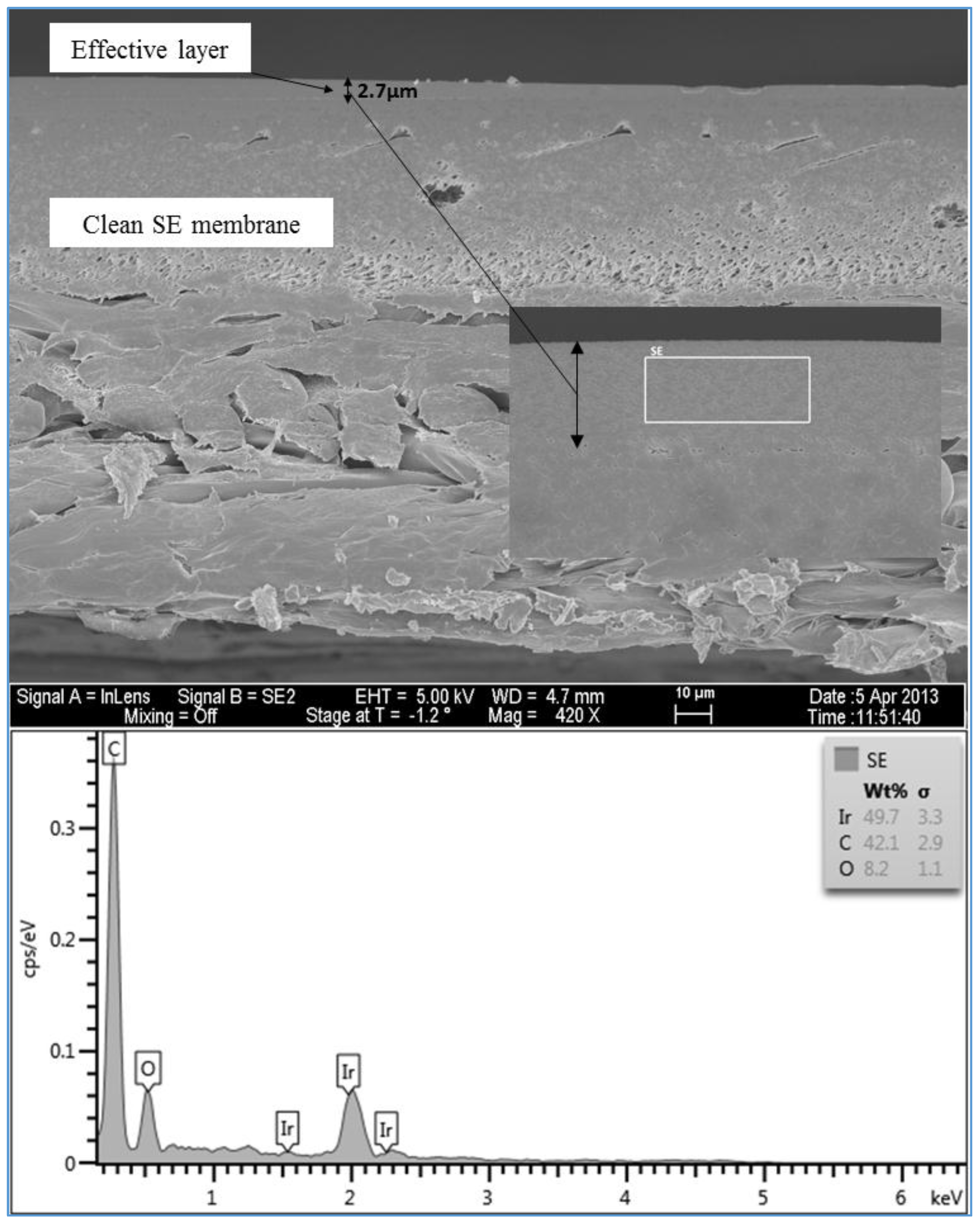
Figure 13 shows that the clean SE membrane has also three distinct layers. However, the top layer which is the effective layer has a thickness of about 2.7 µm. The spectrum of the clean membrane showed some peaks of Ir, which was used to coat the membrane samples to prevent the charge-up of the material surface.
Figure 14 illustrates the thickness and morphology of the scale formation of the fouling on the SE membrane from the feed water of location 6 (high TOC and high TDS). The EDSX spectrum of run 1, shown in
Figure 14, showed that, as previously observed in the plan view SEM image (
Figure 4), the scale formation was due to a combination of organic matter and inorganic material. However, a higher level of Ca
2+ was noticed in the cross section SEM image than in the plan view SEM image. Also, a high level of Ni and Fe was noted in the fouling layer.
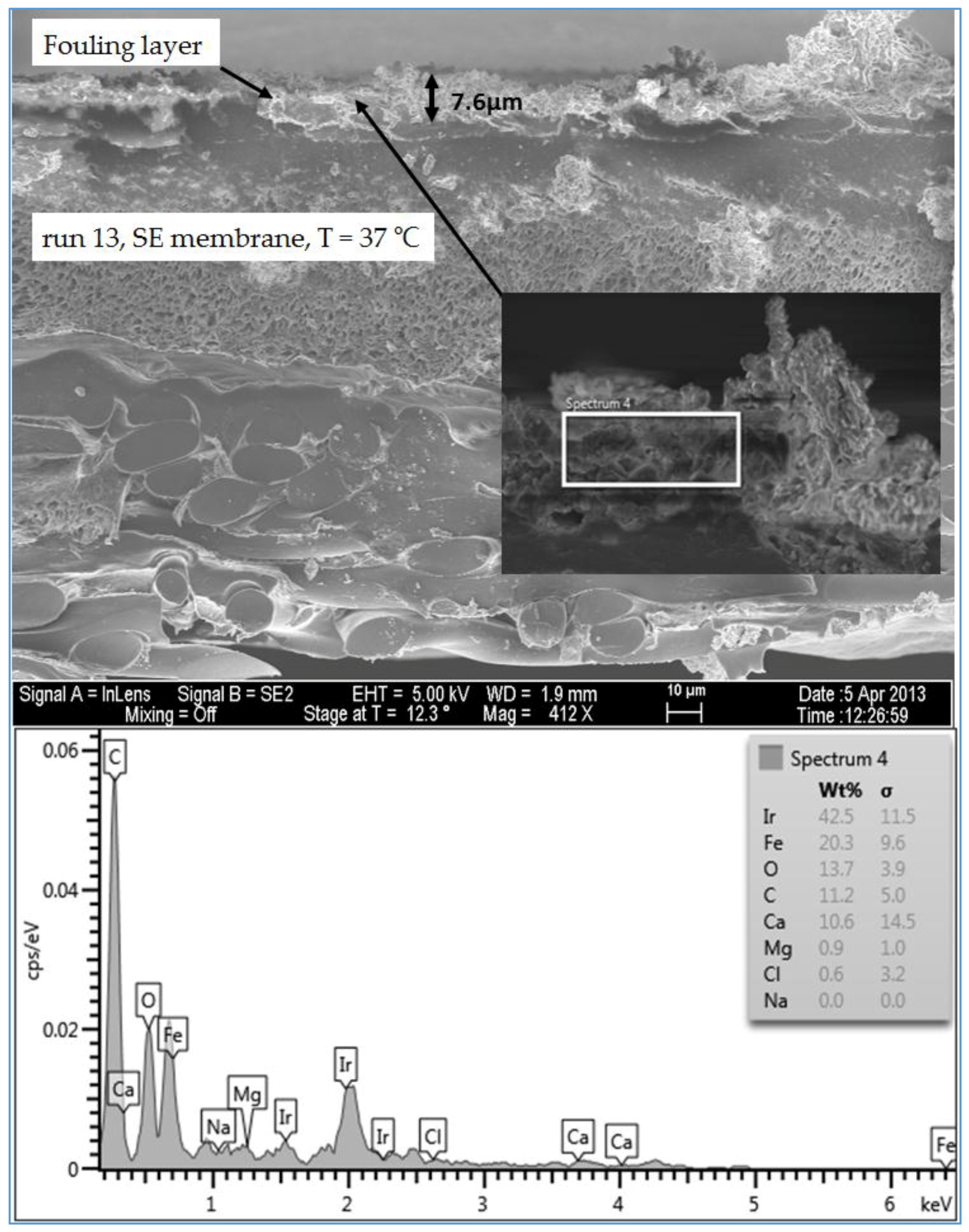
Figure 15 displays the SEM images of the cross section of the fouling on the SE membrane from location 1 (moderate TOC and moderate TDS). Like the observation seen in run 1, the spectrum of run 13, shown in
Figure 15, indicated that the fouling layer contained both organic and inorganic materials. However, the level of Ca
2+ of the cross section SEM image was lower than that of the plan view SEM image (
Figure 9). In addition,
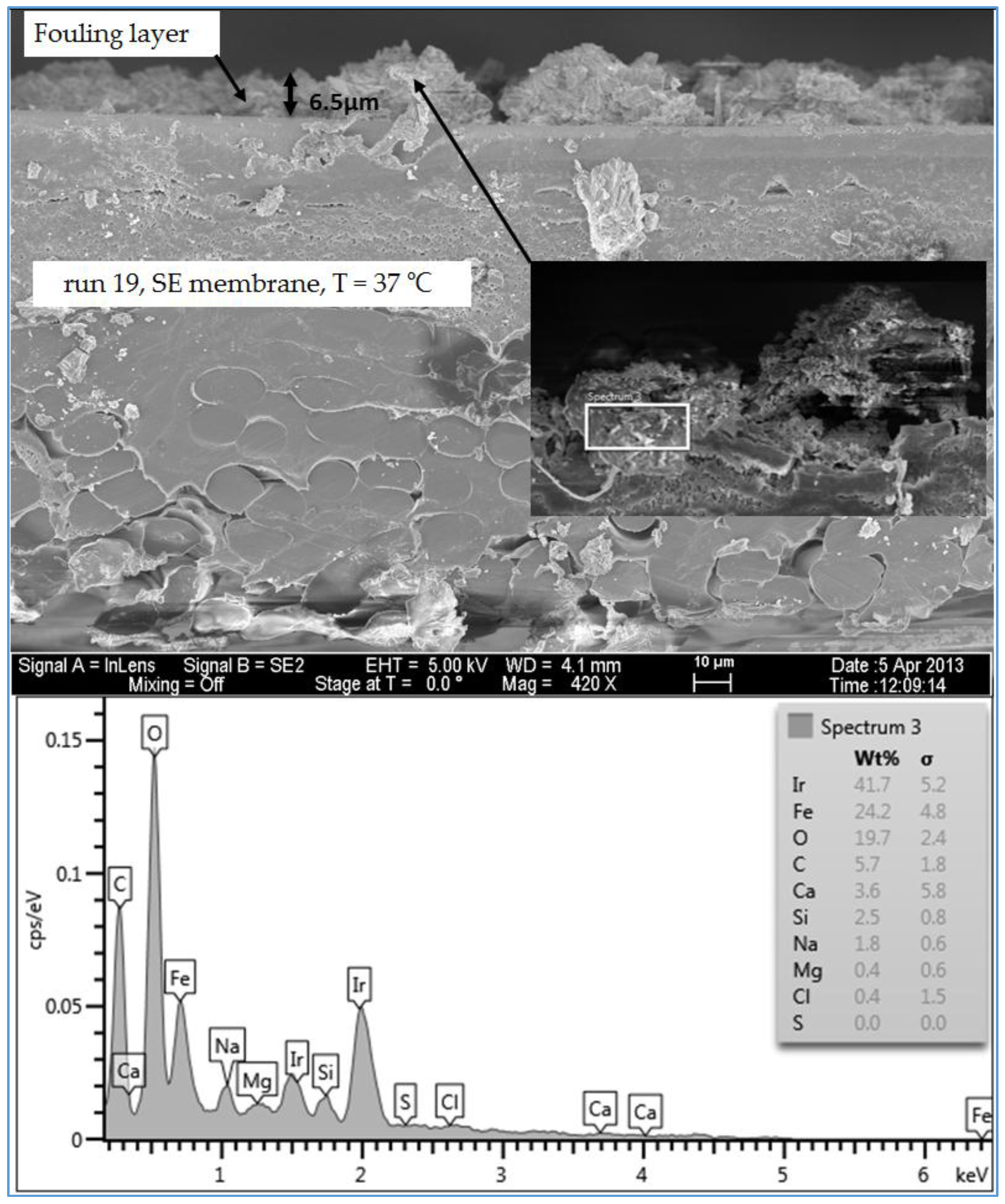
Figure 16 illustrates the thickness and the formation of the fouled materials from location 5 (low TOC and low TDS) on the SE membrane. Moreover, like the observation seen in the plan view SEM image (
Figure 9), the spectrum of the cross section SEM image of the fouling of run 19, illustrated in
Figure 16, exhibited high organic matter and lower inorganic material. Generally, the SEM images of the cross section of the scale formation showed that the composition of the fouled material was about the same through the entire thickness. In addition, the results revealed that there were no distinct layers present through the formation of the fouling, such as those observed by Tran [
3]. Therefore, the associated EDSX spectra were represented by area instead of using spots.