Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
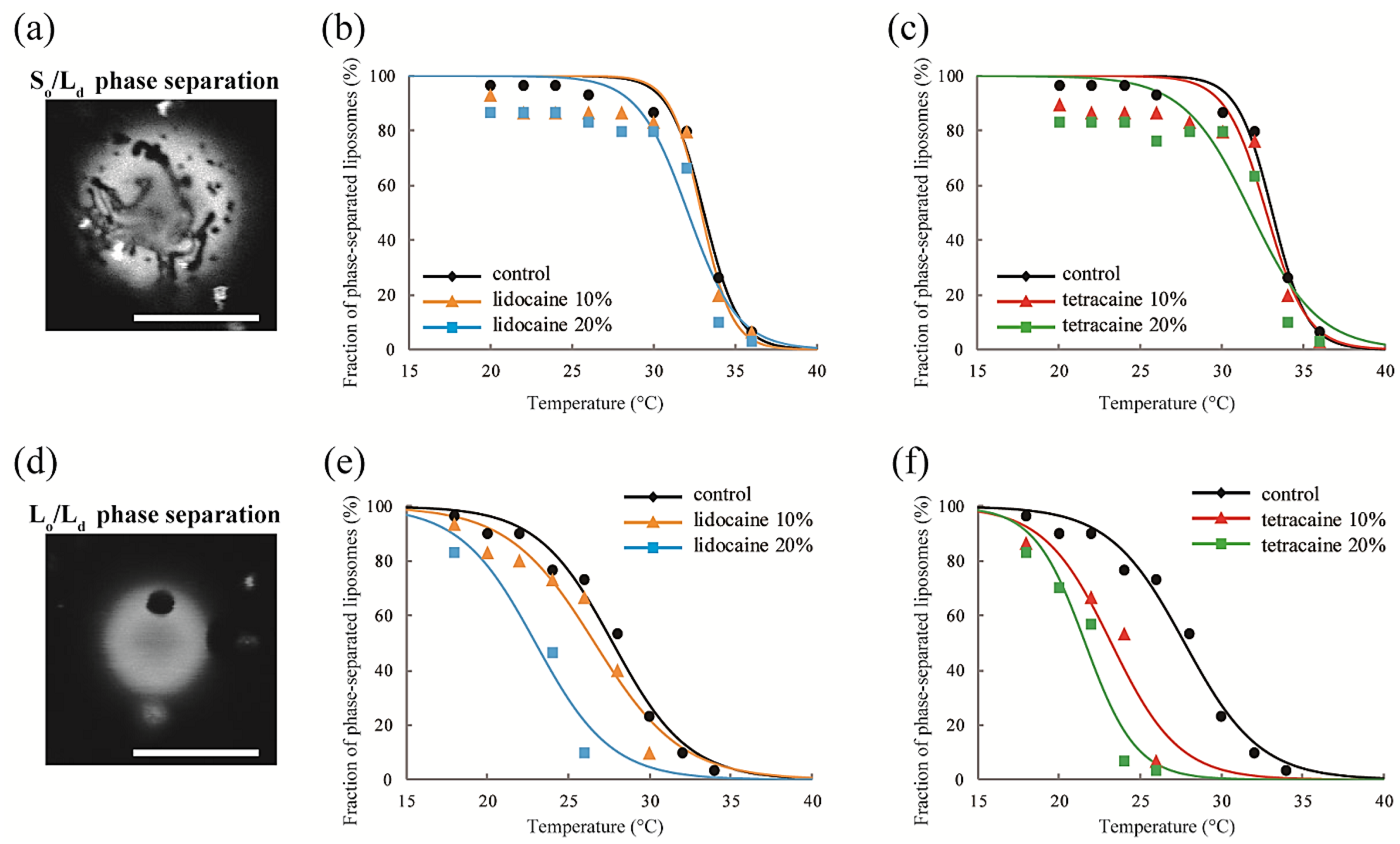
2.2.1. Microscopic Observation of Phase-Separated Structure on Liposomes
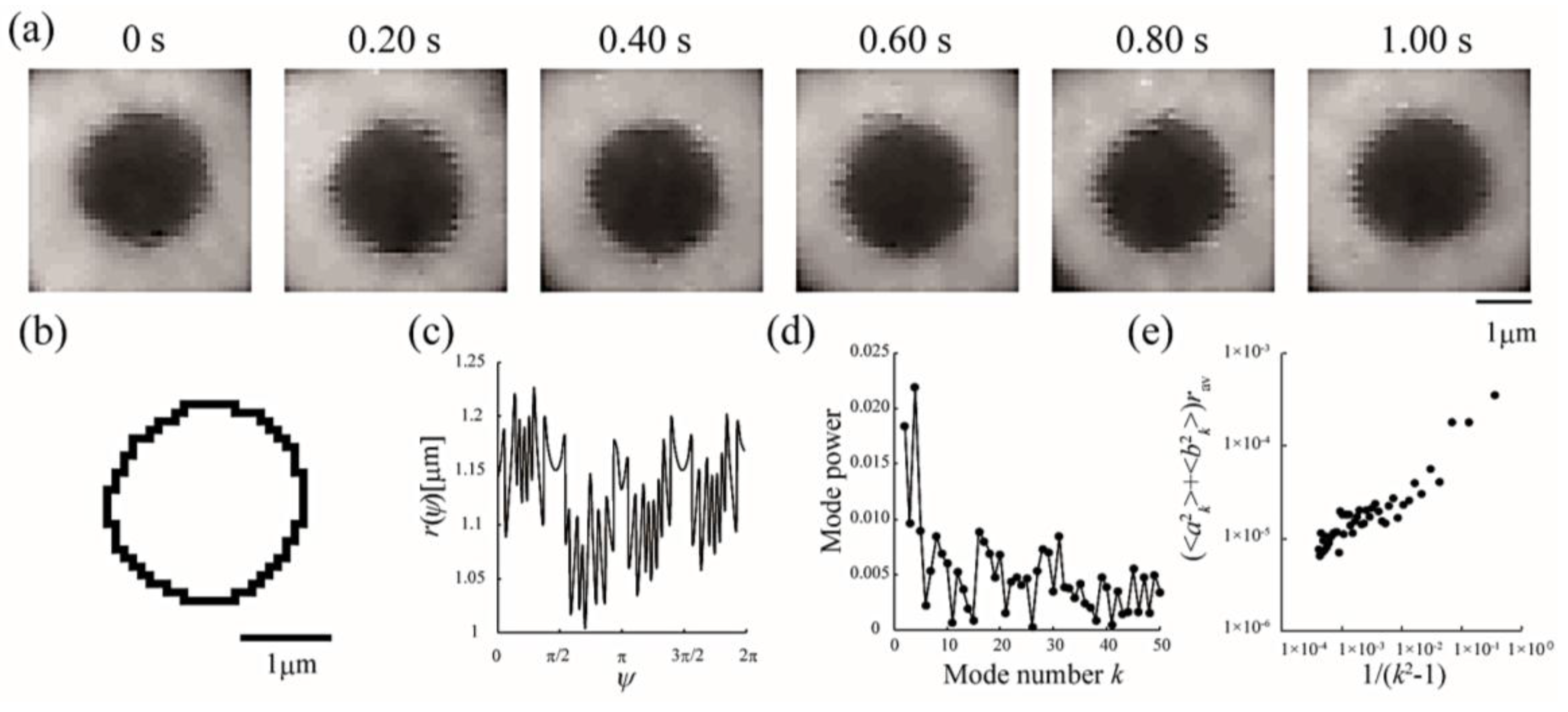
2.2.2. Line Tension Measurement by Flicker Spectroscopy of Domain Boundary Fluctuation
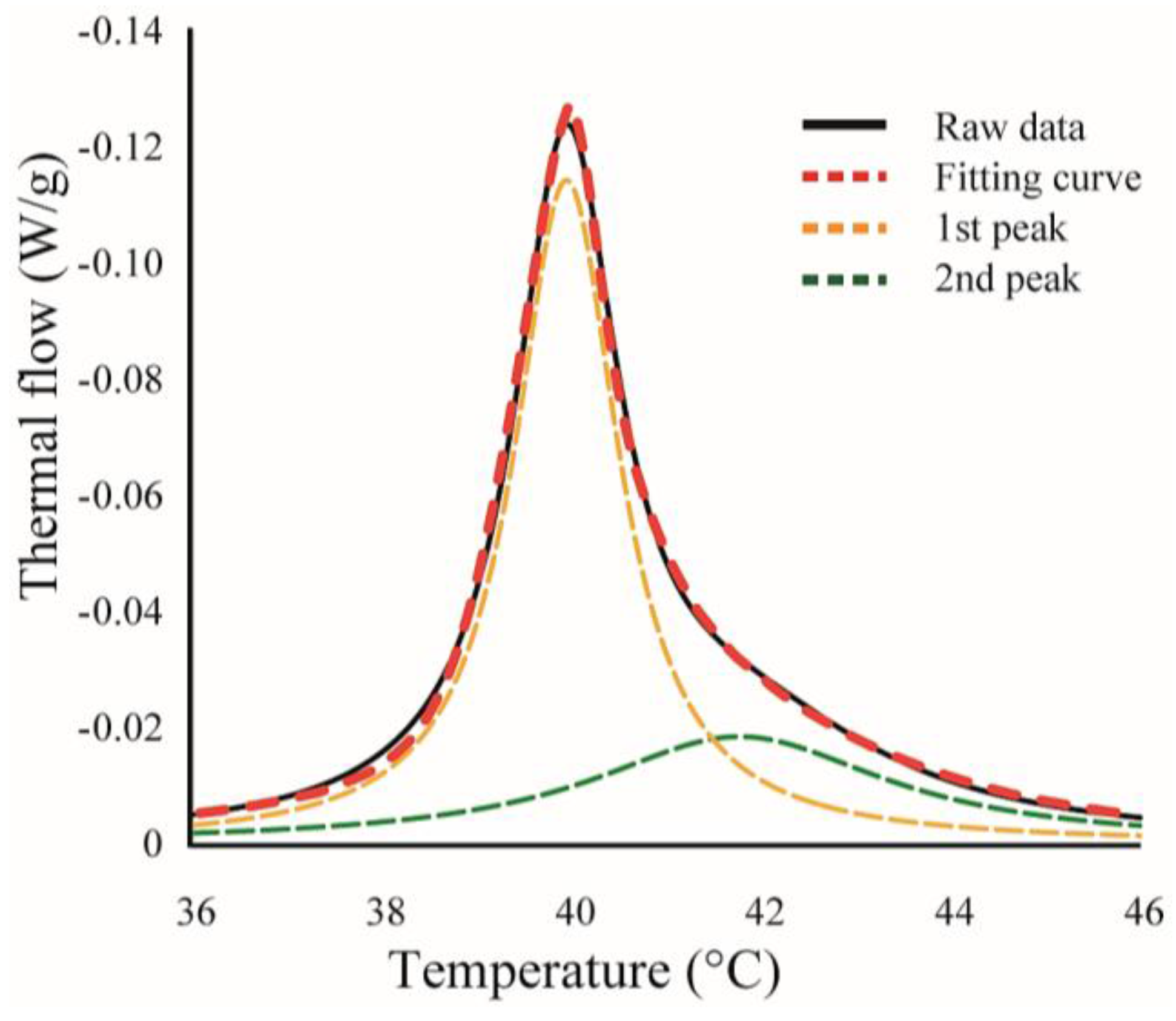
2.2.3. DSC Measurements
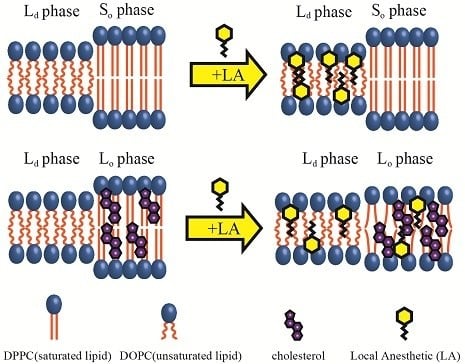
3. Results
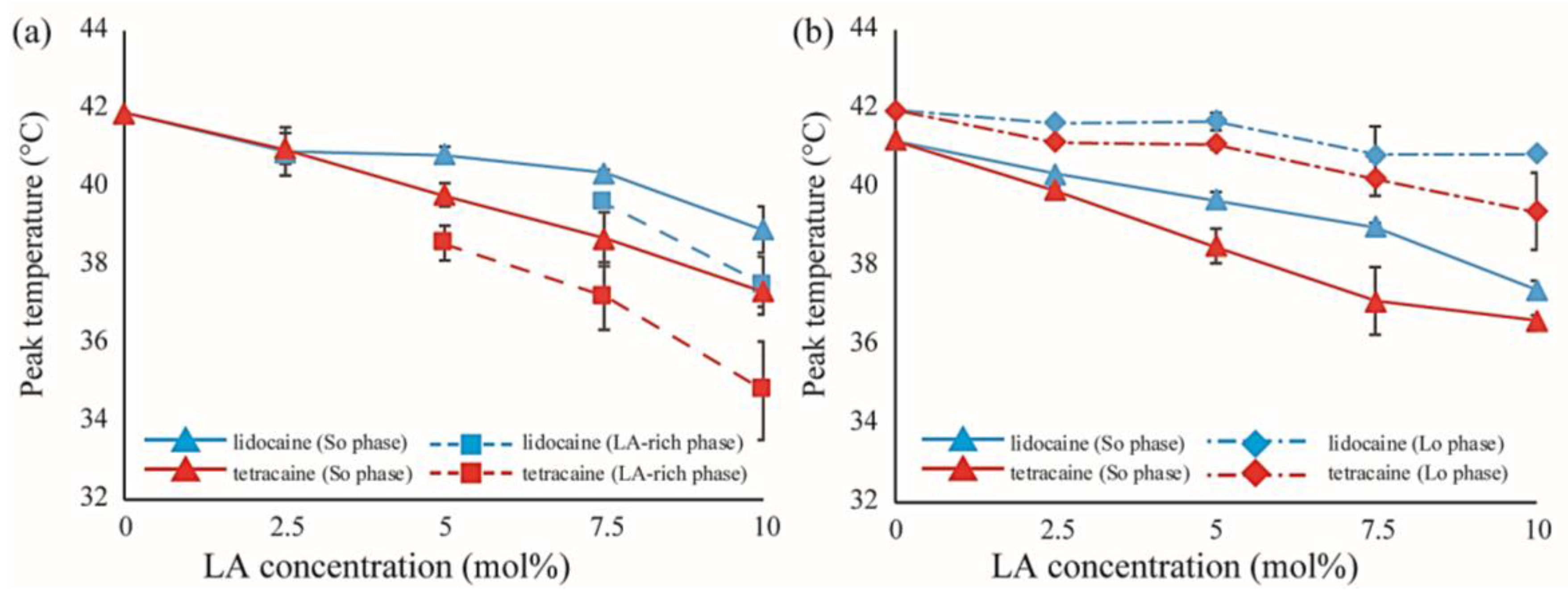
3.1. Miscibility Temperature Measurement in LA-Containing Lipid Membranes
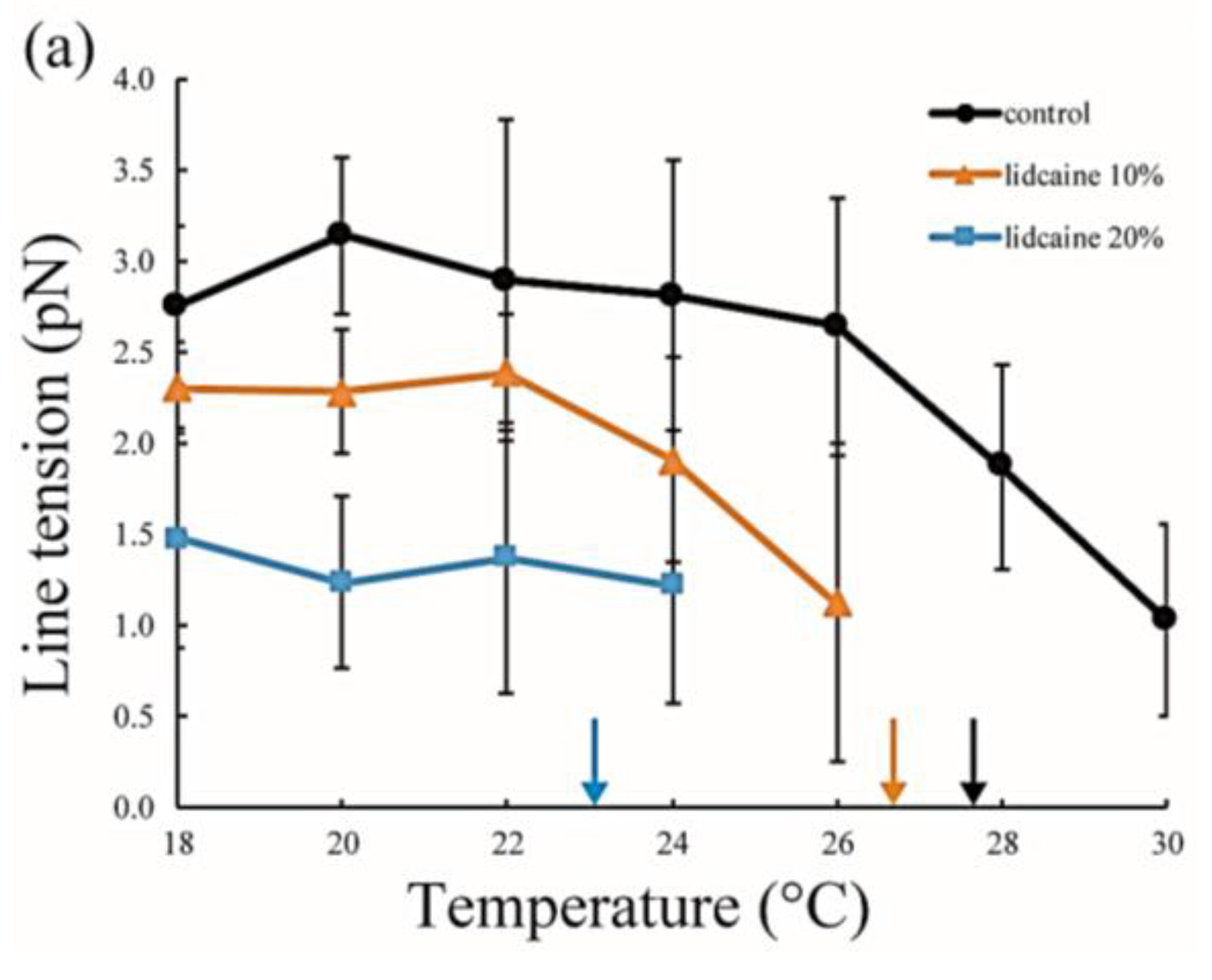
3.2. Line Tension Measurement at the Liquid Domain Boundary in LA-Containing Lipid Membranes
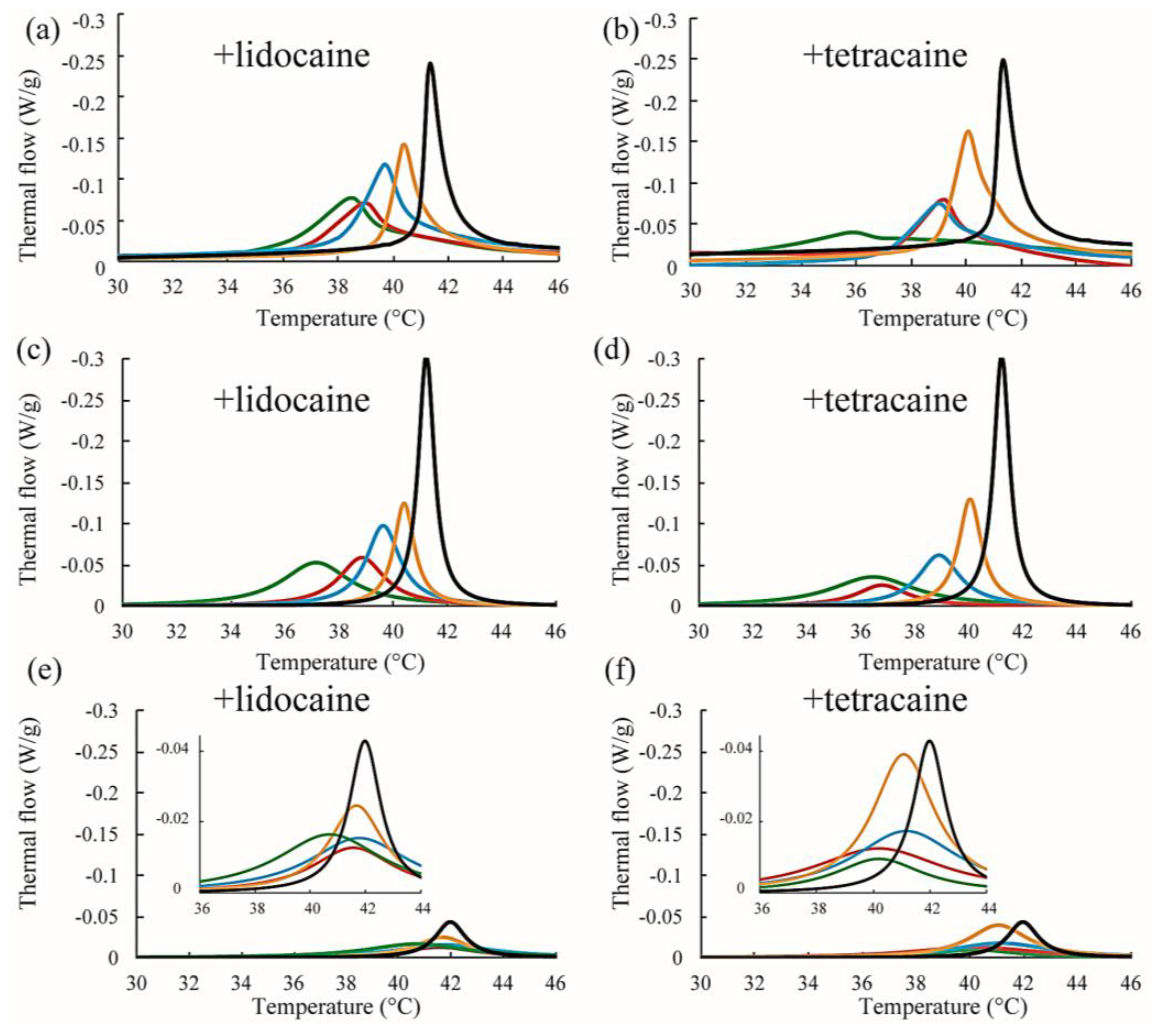
3.3. DSC Measurement in LA-Containing Lipid Membranes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Becker, D.E.; Reed, K.L. Local anesthetics: Review of pharmacological considerations. Anesth. Prog. 2012, 59, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, D.S.; McPhee, J.C.; Scheuer, T.; Catterall, W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA 1996, 93, 9270–9275. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, C.; Vorobyov, I.; French, R.J.; French, C.; Yarov-Yarovoy, V.; Allen, T.W. Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA 2014, 111, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. From Ionic Currents to Molecular Mechanisms: The Structure and Function of Voltage-Gated Sodium Channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: Mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesthesiol. Res. Pract. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Komai, H.; McDowell, T.S. Differential effects of bupivacaine and tetracaine on capsaicin-induced currents in dorsal root ganglion neurons. Neurosci. Lett. 2005, 380, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Tateuchi, R.; Sagawa, N.; Shimada, Y.; Goto, S. Enhancement of the 1-Octanol/Water Partition Coefficient of the Anti-Inflammatory Indomethacin in the Presence of Lidocaine and Other Local Anesthetics. J. Phys. Chem. B 2015, 119, 9868–9873. [Google Scholar] [CrossRef] [PubMed]
- De Paula, E.; Schreier, S.; Jarrell, H.C.; Fraceto, L.F. Preferential location of lidocaine and etidocaine in lecithin bilayers as determined by EPR, fluorescence and 2H NMR. Biophys. Chem. 2008, 132, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Weizenmann, N.; Huster, D.; Scheidt, H.A. Interaction of local anesthetics with lipid bilayers investigated by 1H MAS NMR spectroscopy. Biochim. Biophys. Acta Biomembr. 2012, 1818, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- Booth, P.J.; Riley, M.L.; Flitsch, S.L.; Templer, R.H.; Farooq, A.; Curran, A.R.; Chadborn, N.; Wright, P. Evidence that bilayer bending rigidity affects membrane protein folding. Biochemistry 1997, 36, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Brohawn, S.G.; Su, Z.; MacKinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; McMahon, H.T. The structural era of endocytosis. Science 1999, 285, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Pristerà, A.; Baker, M.D.; Okuse, K. Association between tetrodotoxin resistant channels and lipid rafts regulates sensory neuron excitability. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV preparation and imaging: Minimizing artifacts. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Brown, D.A.; London, E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [Google Scholar] [CrossRef] [PubMed]
- Heberle, F.A.; Feigenson, G.W. Phase Separation in Lipid Membranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004630. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim. Biophys. Acta Biomembr. 2009, 1788, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.G.; Paradiso, P.; Serro, A.P.; Fernandes, A.; Saramago, B. Interaction of local and general anaesthetics with liposomal membrane models: A QCM-D and DSC study. Coll. Surf. B Biointerfaces 2012, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Serro, A.P.; Galante, R.; Kozica, A.; Paradiso, P.; da Silva, A.M.P.S.G.; Luzyanin, K.V.; Fernandes, A.C.; Saramago, B. Effect of tetracaine on DMPC and DMPC + cholesterol biomembrane models: Liposomes and monolayers. Coll. Surf. B Biointerfaces 2014, 116, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Shimokawa, N.; Takagi, M. Destabilization of phase-separated structures in local anesthetic-containing model biomembranes. Chem. Lett. 2015, 9–11. [Google Scholar] [CrossRef]
- Arai, Y.C.P.; Ikeuchi, M.; Fukunaga, K.; Ueda, W.; Kimura, T.; Komatsu, T. Intra-articular injection of warmed lidocaine improves intraoperative anaesthetic and postoperative analgesic conditions. Br. J. Anaesth. 2006, 96, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Bandeiras, C.; Serro, A.P.; Luzyanin, K.; Fernandes, A.; Saramago, B. Anesthetics interacting with lipid rafts. Eur. J. Pharm. Sci. 2013, 48, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.; Karslake, J.; Machta, B.B.; Veatch, S.L. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys. J. 2013, 105, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Tian, A.; Melamed, S.; Johnson, C.; Tee, S.-Y.; Baumgart, T. Flicker spectroscopy of thermal lipid bilayer domain boundary fluctuations. Biophys. J. 2007, 93, 3169–3181. [Google Scholar] [CrossRef] [PubMed]
- Stottrup, B.L.; Heussler, A.M.; Bibelnieks, T.A. Determination of line tension in lipid monolayers by Fourier analysis of capillary waves. J. Phys. Chem. B 2007, 111, 11091–11094. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Johnson, C.; Wang, W.; Baumgart, T. Line tension at fluid membrane domain boundaries measured by micropipette aspiration. Phys. Rev. Lett. 2007, 98, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, N.; Mukai, R.; Nagata, M.; Takagi, M. Formation of modulated phase and domain rigidification in fatty acids-containing lipid membranes. Phys. Chem. Chem. Phys. 2017, 19, 13252–13263. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takashima, A.; Nishio, I. Effect of dibucaine hydrochloride on raft-like lipid domains in model membrane systems. Medchemcomm 2015, 6, 1444–1451. [Google Scholar] [CrossRef]
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006, 7, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Konyakhina, T.M.; Goh, S.L.; Amazon, J.; Heberle, F.A.; Wu, J.; Feigenson, G.W. Control of a nanoscopic-to-macroscopic transition: Modulated phases in four-component DSPC/DOPC/POPC/Chol giant unilamellar vesicles. Biophys. J. 2011, 101, L8–L10. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.L.; Amazon, J.J.; Feigenson, G.W. Toward a better raft model: Modulated phases in the four-component bilayer, DSPC/DOPC/POPC/CHOL. Biophys. J. 2013, 104, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Konyakhina, T.M.; Wu, J.; Mastroianni, J.D.; Heberle, F.A.; Feigenson, G.W. Phase diagram of a 4-component lipid mixture: DSPC/DOPC/POPC/chol. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, S.; Zhang, S.; Lee, T.R.; Schwartz, D.K. Linactants: Surfactant analogues in two dimensions. Phys. Rev. Lett. 2008, 100, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Sriram, I.; Singhana, B.; Lee, T.R.; Schwartz, D.K. Line tension and line activity in mixed monolayers composed of aliphatic and terphenyl-containing surfactants. Langmuir 2012, 28, 16294–16299. [Google Scholar] [CrossRef] [PubMed]
- Brewster, R.; Pincus, P.A.; Safran, S.A. Hybrid lipids as a biological surface-active component. Biophys. J. 2009, 97, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.; Chachaty, C.; Hazarosova, R.; Tessier, C.; Nuss, P.; Momchilova, A.; Staneva, G. Docosahexaenoic acid promotes micron scale liquid-ordered domains. A comparison study of docosahexaenoic versus oleic acid containing phosphatidylcholine in raft-like mixtures. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, N.; Nagata, M.; Takagi, M. Physical properties of the hybrid lipid POPC on micrometer-sized domains in mixed lipid membranes. Phys. Chem. Chem. Phys. 2015, 17, 20882–20888. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, S.; Yonaha, T.; Tsuneyoshi, I. Local anesthetics with high lipophilicity are toxic, while local anesthetics with low pka induce more apoptosis in human leukemia cells. J. Anesth. Clin. Res. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Strichartz, G.R.; Sanchez, V.; Arthur, G.R.; Chafetz, R.; Martin, D. Fundamental properties of local anesthetics. II. Measured octanol:buffer partition coefficients and pKa values of clinically used drugs. Anesth. Analg. 1990, 71, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Himeno, H.; Shimokawa, N.; Komura, S.; Andelman, D.; Hamada, T.; Takagi, M. Charge-induced phase separation in lipid membranes. Soft Matter 2014, 10, 7959–7967. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and Function of Voltage-Gated Sodium Channels at Atomic Resolution. Exp. Physiol. 2014, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, N.L.C.; Brooks, N.J. Using High Pressure to Modulate Lateral Structuring in Model Lipid Membranes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 24. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugahara, K.; Shimokawa, N.; Takagi, M. Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics. Membranes 2017, 7, 33. https://doi.org/10.3390/membranes7030033
Sugahara K, Shimokawa N, Takagi M. Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics. Membranes. 2017; 7(3):33. https://doi.org/10.3390/membranes7030033
Chicago/Turabian StyleSugahara, Ko, Naofumi Shimokawa, and Masahiro Takagi. 2017. "Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics" Membranes 7, no. 3: 33. https://doi.org/10.3390/membranes7030033