Analysis of the Comprehensive Tensile Relationship in Electrospun Silk Fibroin/Polycaprolactone Nanofiber Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
3. Results and Discussion
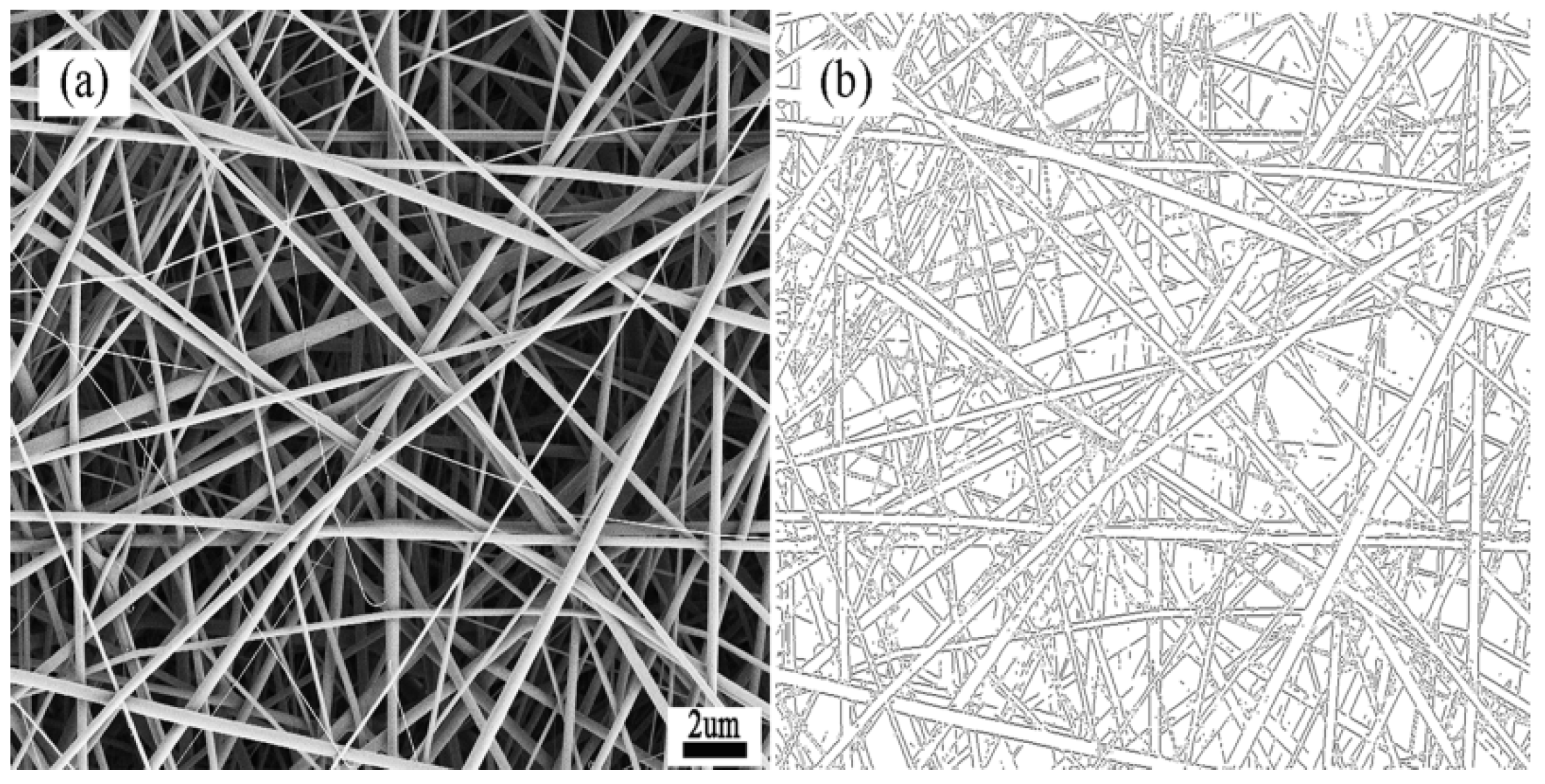
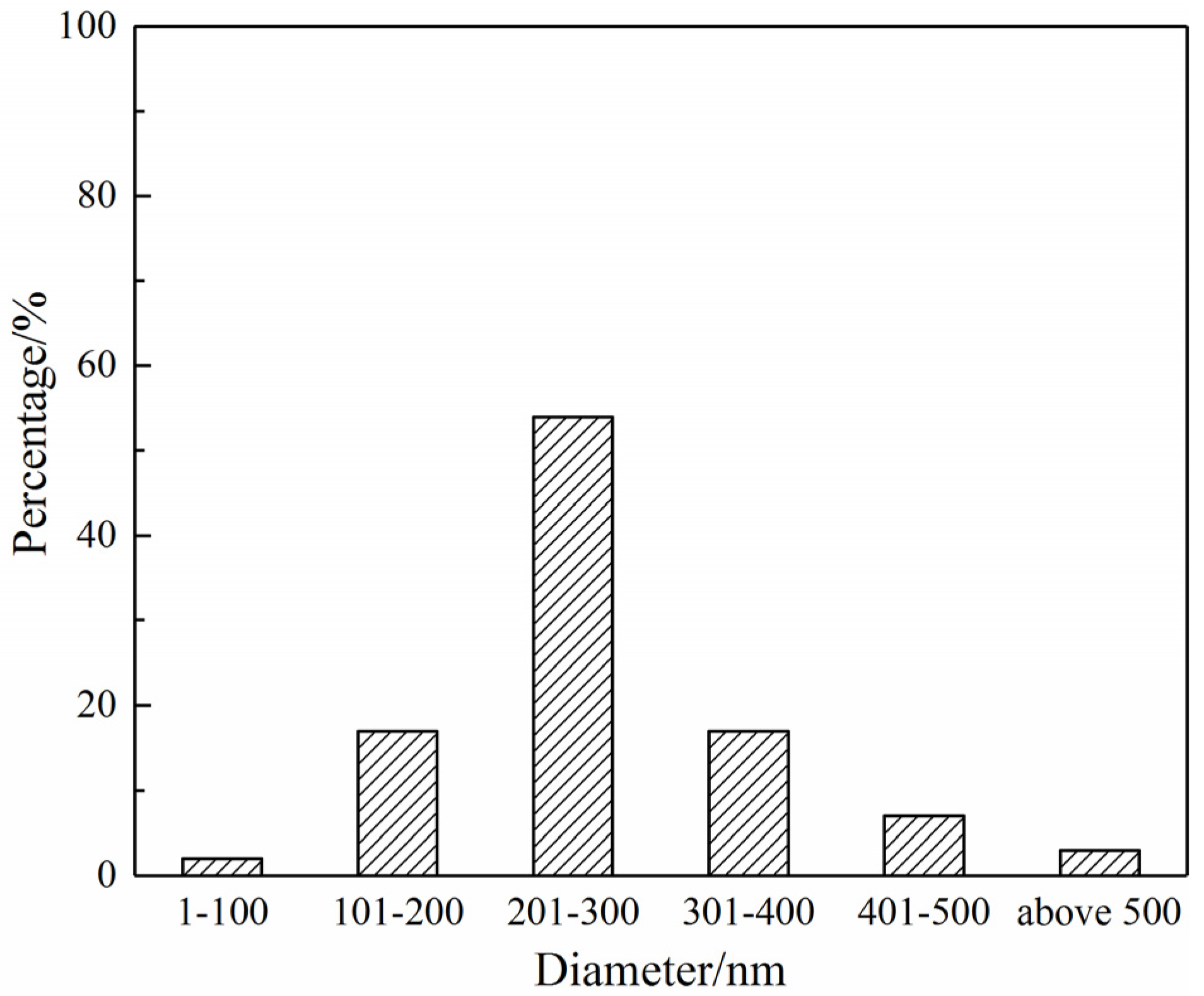
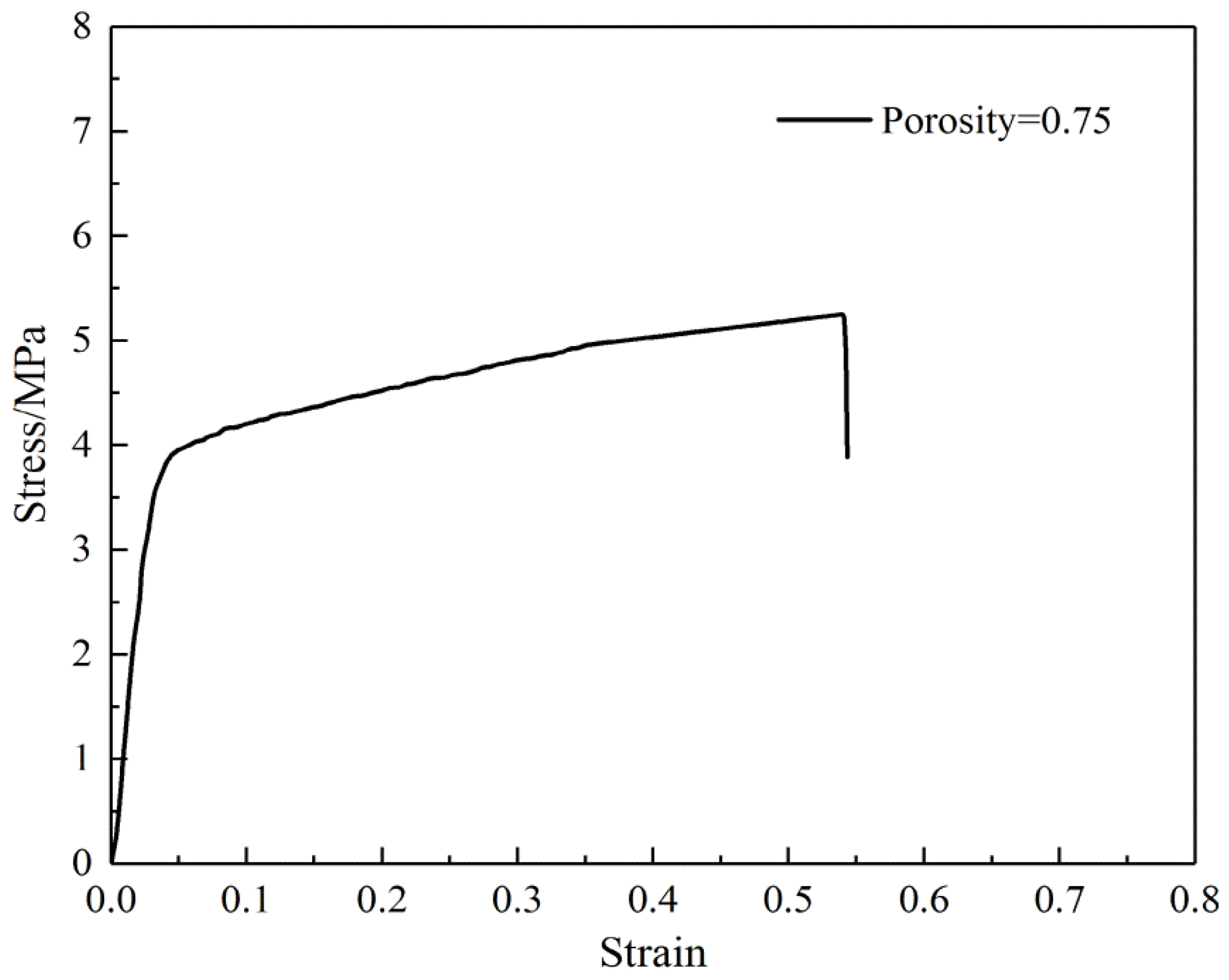
3.1. Morphology and Structural Features of the SF/PCL Nanofiber Membranes
3.2. Geometry and Tensile Strength Analysis
- 1
- All the fibers in the RVE had the same diameter and a circular cross section; they were present as straight fibers (the force required for the crimp and buckling of fibers was negligible with respect to the tension of fibers); the materials were incompressible.
- 2
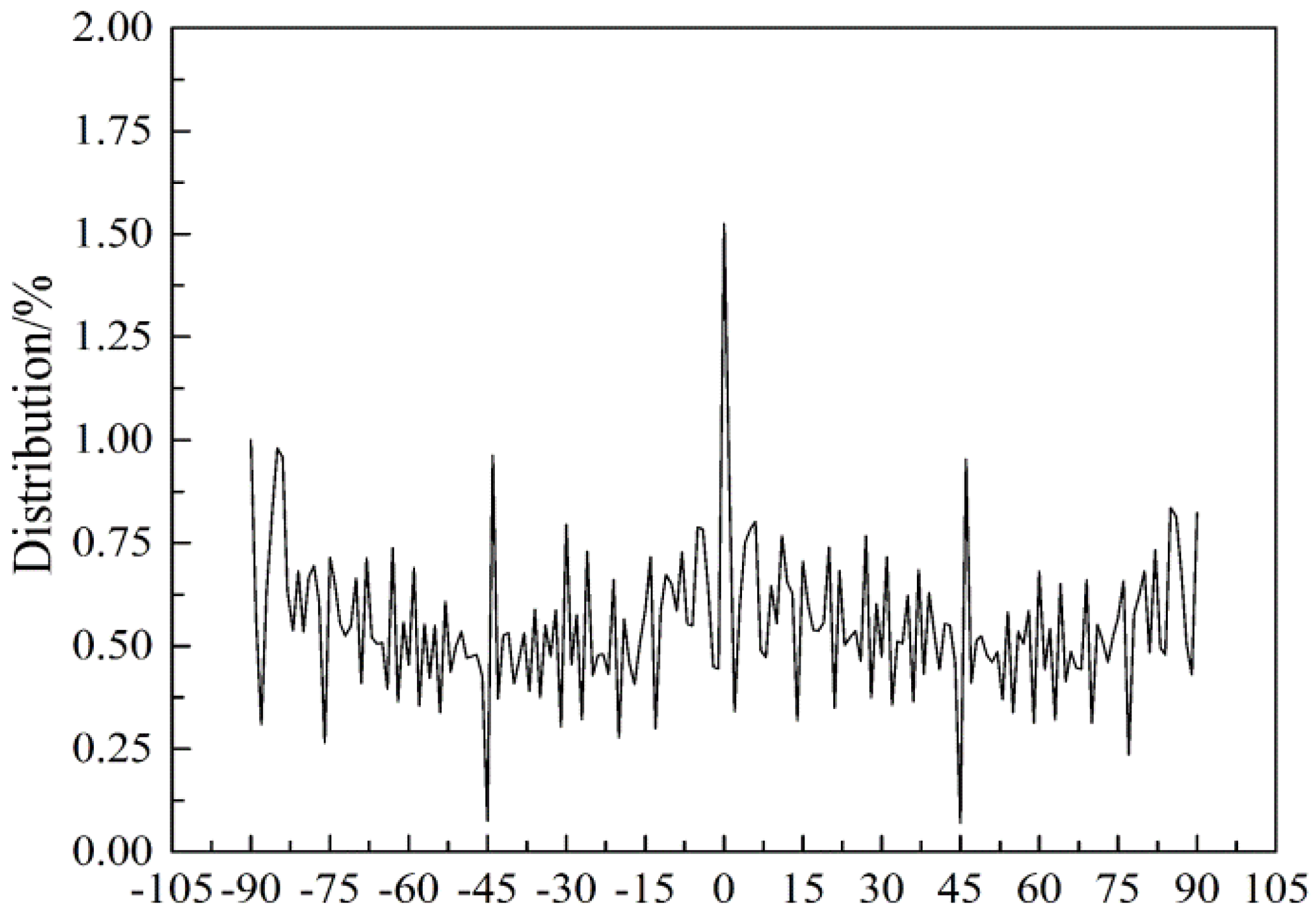
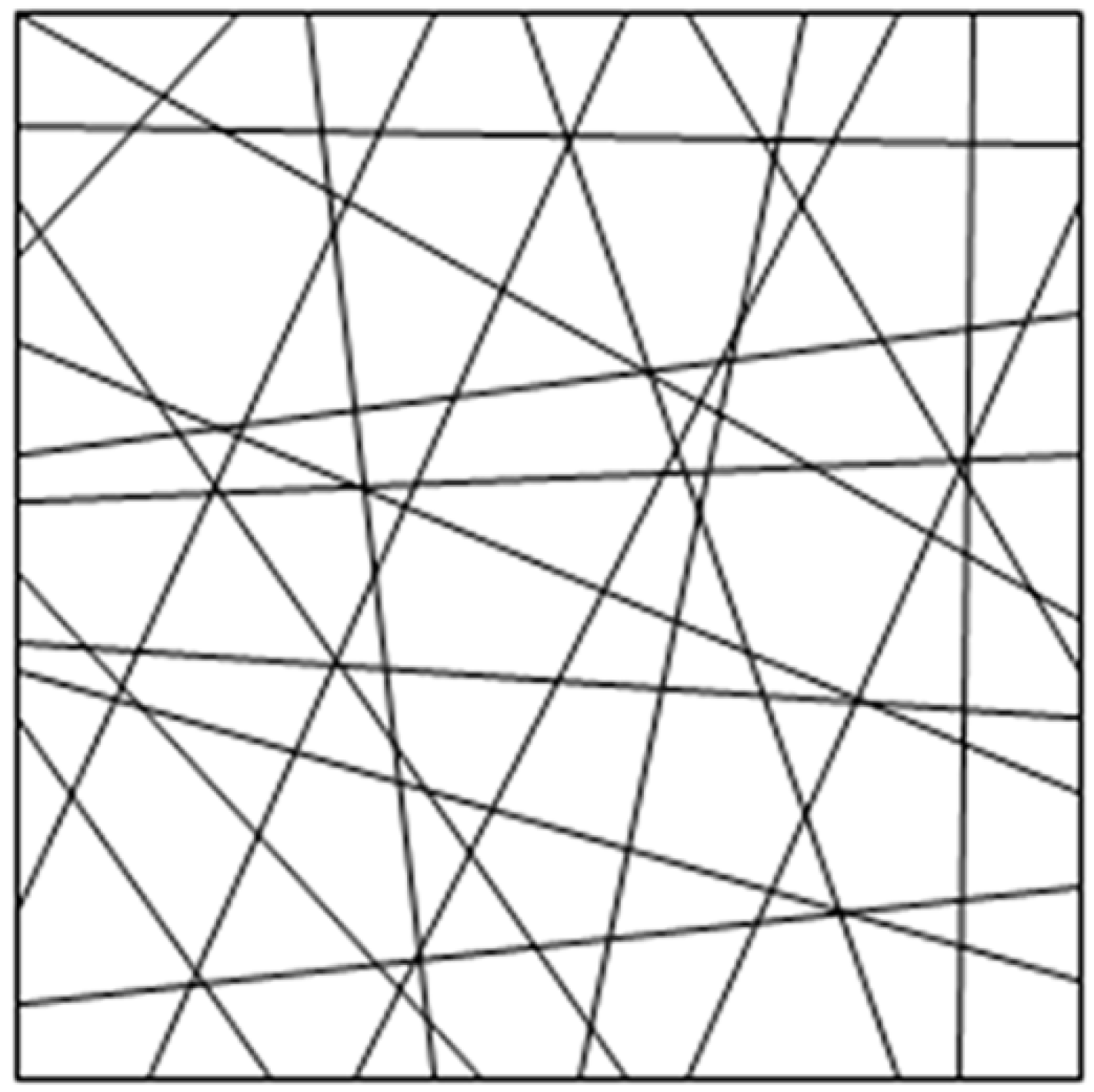
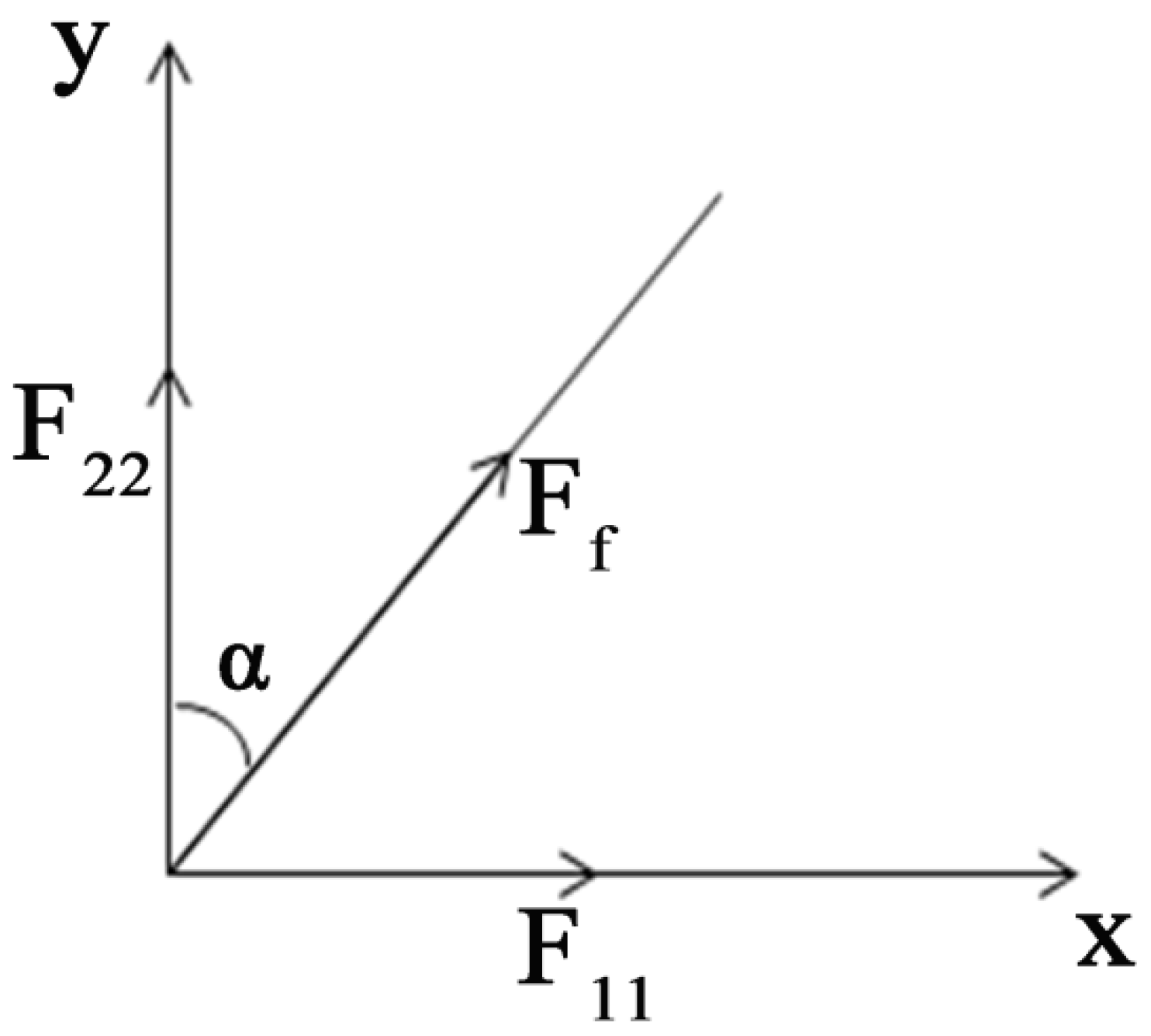
- There was no bonding among fiber intersections in the RVE and the force was negligible; the fiber web was a flat network structure and the fiber orientation was evenly distributed , where α was the angle of orientation.
- 3
- The thickness and pore distribution of membranes were uniform and the interaction between the layers could be neglected.
- 4
- The change of fiber length in the RVE was sufficiently regular to be considered to be an arithmetic progression.
3.2.1. Parameter Relationship in RVE
3.2.2. Tensile Loading Specimen Analysis
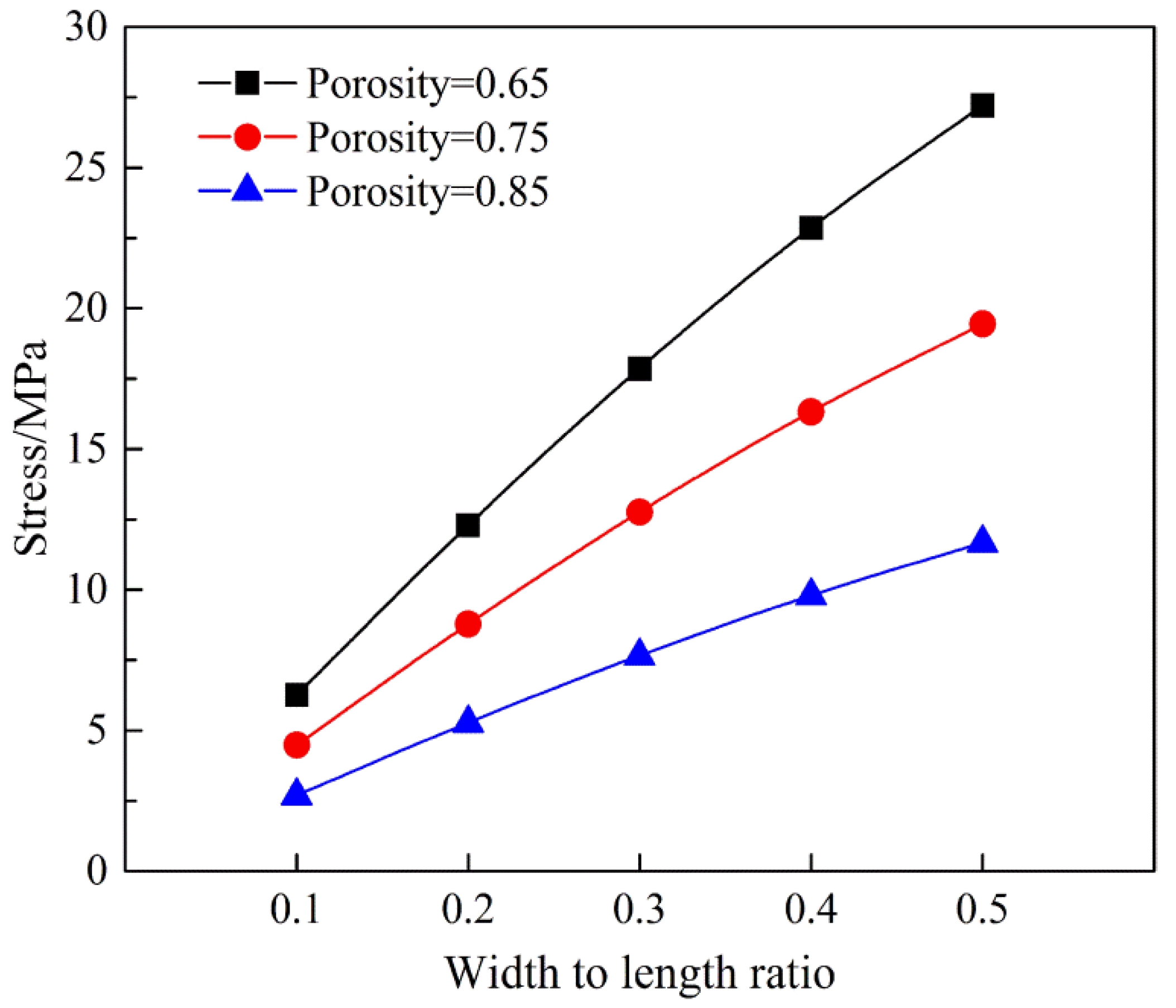
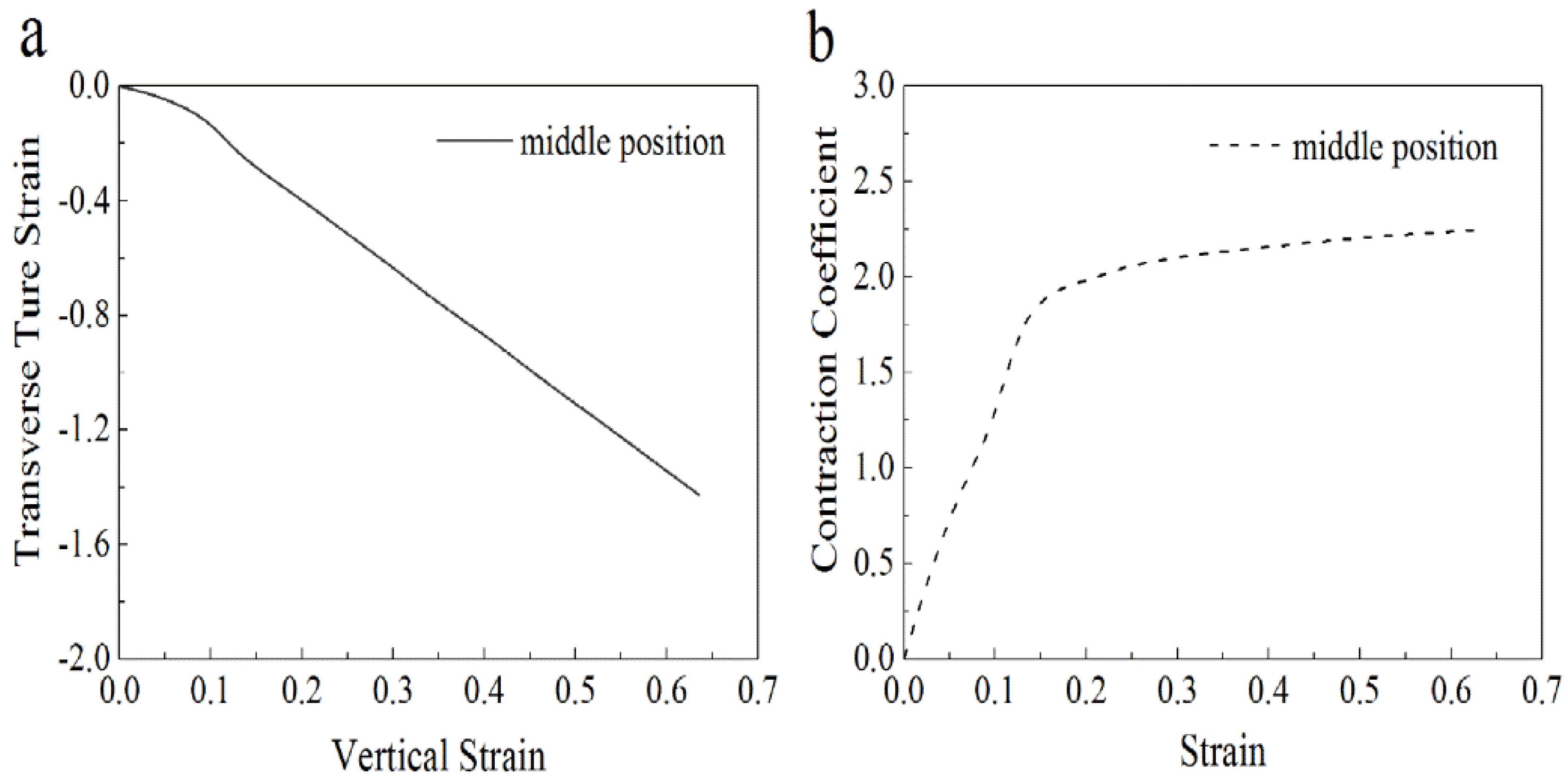
3.2.3. Analysis of Tensile Force of Nanofiber Membranes
3.3. Predicting the Mechanical Properties of Single Nanofiber
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Advan. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The effect of electrospun gelatin fibers alignment on schwann cell and axon behavior and organization in the perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of ccollagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Nakielski, P.; Kowalczyk, T.; Zembrzycki, K.; Kowalewski, T.A. Experimental and numerical evaluation of drug release from nanofiber mats to brain tissue. J. Biomed. Mater. Res. 2014, 103, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Design 2015, 21, 1944–1959. [Google Scholar] [CrossRef]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Mechanical properties of single-brin silkworm silk. J. Appl. Polym. Sci. 2000, 75, 1270–1277. [Google Scholar] [CrossRef]
- Mkhabela, V.J.; Ray, S.S. Poly (epsilon-caprolactone) nanocomposite scaffolds for tissue engineering: A brief overview. J. Nanosci. Nanotechnol. 2014, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, C.H.; Kim, G.H. A polycaprolactone/silk-fibroin nanofibrous composite combined with human umbilical cord serum for subacute tympanic membrane perforation; an in vitro and in vivo study. J. Mater. Chem. B 2014, 2, 2703–2713. [Google Scholar] [CrossRef]
- Kharaziha, M.; Fathi, M.H.; Edris, H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. J. Mech. Behav. Biomed. 2013, 24, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Amit, R.; Kameswara, R.P.V.; Stephen, R.; Arjun, J. Effect of fiber orientation on pore size characteristics of nonwoven structures. J. Appl. Polym. Sci. 2010, 118, 2668–2673. [Google Scholar]
- Pourdeyhimi, B.; Dent, R.; Davis, H. Measuring fiber orientation in nonwovens, Part III: Fourier transform. Text. Res. J. 1997, 67, 143–151. [Google Scholar] [CrossRef]
- Pourdeyhimi, B.; Kim, H.S. Measuring fiber orientation in nonwovens: The hough transform. Text. Res. J. 2002, 72, 803–809. [Google Scholar] [CrossRef]
- Demirci, E.; Acar, M.; Pourdeyhimi, B.; Silberschmidt, V.V. Computation of mechanical anisotropy in thermally bonded bicomponent fibre nonwovens. Comp. Mater. Sci. 2012, 52, 157–163. [Google Scholar] [CrossRef]
- Xu, B.; Yu, L. Determining fiber orientation distribution in nonwovens with hough transform techniques. Text. Res. J. 1997, 67, 563–571. [Google Scholar] [CrossRef]
- Wang, R.W.; Wu, X.Y.; Wang, S.Y.; Xu, B. Automatic identification of ramie and cotton fibers using characteristics in longitudinal view, Part 1: Locating capture of fiber images. Text. Res. J. 2009, 79, 1251–1261. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B.; Li, C. Accurate fiber orientation measurements in nonwovens using a multi-focus image fusion technique. Text. Res. J. 2013, 84, 115–124. [Google Scholar] [CrossRef]
- Haque, M.A.; Saif, M.T.A. A review of MEMS-based microscale and nanoscale tensile and bending testing. Exp. Mech. 2003, 43, 248–255. [Google Scholar] [CrossRef]
- Yu, M.F.; Dyer, M.J.; Skidmore, G.D.; Rohrs, H.W.; Lu, X.K.; Ausman, K.D.; Von Ehr, J.R.; Ruoff, R.S. Three-dimensional manipulation of carbon nanotubes under a scanning electron microscope. Nanotechnology 1999, 10, 244–252. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.Y.; Kim, S.D.; Kim, Y.W.; Yu, W.R. Mechanical characterization of nanofibers using a nanomanipulator and atomic force microscope cantilever in a scanning electron microscope. Polym. Test. 2010, 29, 375–380. [Google Scholar] [CrossRef]
- Lin, Y.; Clark, D.M.; Yu, X.; Zhong, Z.; Liu, K.; Reneker, D.H. Mechanical properties of polymer nanofibers revealed by interaction with streams of air. Polymer 2012, 53, 782–790. [Google Scholar] [CrossRef]
- Kolluru, P.V.; Chasiotis, I. Interplay of molecular and specimen length scales in the large deformation mechanical behavior of polystyrene nanofibers. Polymer 2015, 56, 507–515. [Google Scholar] [CrossRef]
- Tan, E.P.S.; Ng, S.Y.; Lim, C.T. Tensile testing of a single ultrafine polymeric fiber. Biomaterials 2005, 26, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Wan, Y.; Yang, L.; Cao, Q. Effect of ultrasonic vibration on structure and performance of electrospun PAN fibrous membrane. J. Nano Res. 2013, 23, 96–103. [Google Scholar] [CrossRef]
- Cao, Q.; Wan, Y.; Qiang, J.; Yang, R.; Fu, J.; Wang, H.; Gao, W.; Ko, F. Effect of sonication treatment on electrospinnability of high-viscosity PAN solution and mechanical performance of microfiber mat. Iran. Polym. J. 2014, 23, 947–953. [Google Scholar] [CrossRef]
- Cox, H.L. The elasticity and strength of paper and other fibrous materials. Br. J. Appl. Phys. 1952, 3, 72–79. [Google Scholar] [CrossRef]
- Kallmes, O.; Corte, H. The structure of paper I. The statistical geometry of an ideal two dimensional fiber network. Tappi J. 1960, 43, 737–752. [Google Scholar]
- Carlsson, L.A.; Lindstrom, T. A shear-lag approach to the tensile strength of paper. Compo. Sci. Technol. 2005, 65, 183–189. [Google Scholar] [CrossRef]
- Räisänen, V.I.; Alava, M.J.; Nieminen, R.M. Failure of planar fiber networks. J. Appl. Phys. 1997, 82, 3747–3753. [Google Scholar] [CrossRef]
- Backer, S.; Petterson, D.R. Some principles of nonwoven fabrics. Text. Res. J. 1960, 30, 704–711. [Google Scholar] [CrossRef]
- Bais-Singh, S.; Goswami, B.C. Theoretical determination of the mechanical response of spun-bonded nonwovens. J. Text. Inst. 1995, 86, 271–288. [Google Scholar] [CrossRef]
- Liao, T.; Adanur, S.; Drean, J.Y. Predicting the mechanical properties of nonwoven geotextiles with the finite element method. Text. Res. J. 1997, 67, 753–760. [Google Scholar] [CrossRef]
- Petterson, D.R. On the Nechanics of Non-Woven Fabrics. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1959. [Google Scholar]
- Zhang, X.P.; Mei, J.N.; Lu, J.; Wang, Y.Y.; Lu, S.Z. Effect of degumming solution pH value on degumming of silk fiber and molecular weight of silk fibroin. Sci. Seric. 2014, 40, 699–705. [Google Scholar]
- Inai, R.; Kotaki, M.; Ramakrishna, S. Structure and properties of electrospun PLLA single nanofibres. Nanotechnology 2005, 16, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Plastics-Determination of Tensile Properties—Part 1: General Principles. Available online: https://www.iso.org/standard/56045.html (accessed on 4 December 2017).
- Plastics-Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. Available online: https://www.iso.org/standard/4594.html (accessed on 4 December 2017).
- Rutledge, G.C.; Lowery, J.L.; Pai, C.L. Characterization by mercury porosimetry of nonwoven fiber media with deformation. J. Eng. Fibers Fabrics 2009, 4, 1–13. [Google Scholar]

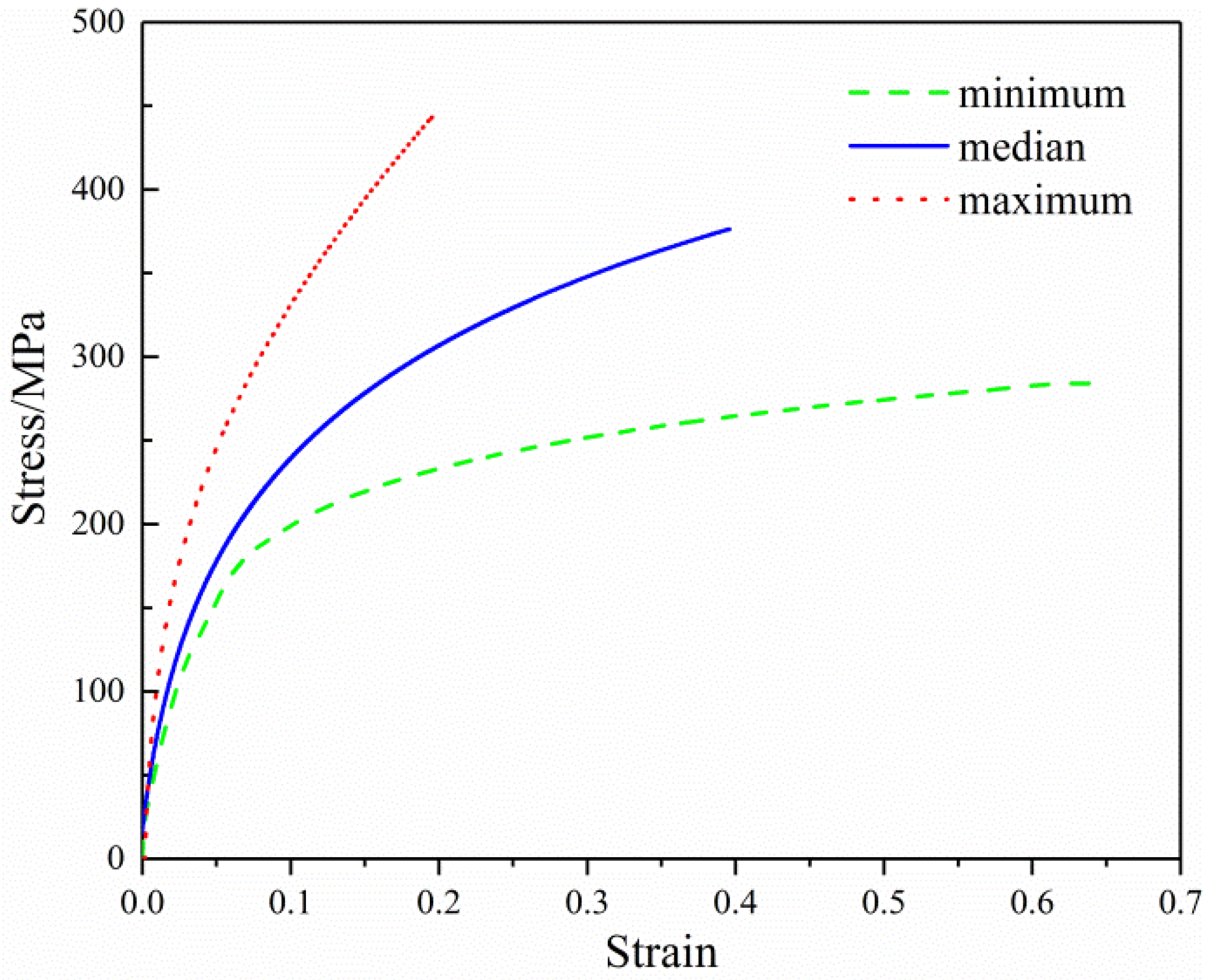
| Property (Unit) | Average | Minimum | Maximum | CV |
|---|---|---|---|---|
| Elastic modulus (MPa) | 4800 | 3500 | 6200 | 0.23 |
| Yield stress (MPa) | 190 | 170 | 212 | 0.08 |
| Yield strain | 0.040 | 0.034 | 0.048 | 0.15 |
| Break stress (MPa) | 375 | 285 | 440 | 0.17 |
| Break strain | 0.39 | 0.20 | 0.63 | 0.40 |
| Property (Unit) | Average | Minimum | Maximum | CV |
|---|---|---|---|---|
| Elastic modulus (MPa) | 98 | 86 | 110 | 0.10 |
| Yield stress (MPa) | 4.11 | 3.88 | 4.33 | 0.05 |
| Yield strain | 0.049 | 0.044 | 0.052 | 0.07 |
| Post-yield slope (MPa) | 2.60 | 2.29 | 3.25 | 0.15 |
| Break stress (MPa) | 5.53 | 5.38 | 6.02 | 0.05 |
| Break strain | 0.543 | 0.378 | 0.725 | 0.25 |
| Sample | Elastic Modulus (MPa) | Yield Stress (MPa) | Yield Strain | Break Stress (MPa) | Break Strain |
|---|---|---|---|---|---|
| Tested membranes | 98 | 4.11 | 0.049 | 5.53 | 0.543 |
| Tested nanofibers | 5500 | 242.58 | 0.044 | 376.36 | 0.396 |
| Predicted nanofibers | 6280 | 295.24 | 0.047 | 397.25 | 0.516 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Pu, D.; Xiong, J. Analysis of the Comprehensive Tensile Relationship in Electrospun Silk Fibroin/Polycaprolactone Nanofiber Membranes. Membranes 2017, 7, 67. https://doi.org/10.3390/membranes7040067
Yin Y, Pu D, Xiong J. Analysis of the Comprehensive Tensile Relationship in Electrospun Silk Fibroin/Polycaprolactone Nanofiber Membranes. Membranes. 2017; 7(4):67. https://doi.org/10.3390/membranes7040067
Chicago/Turabian StyleYin, Yunlei, Dandan Pu, and Jie Xiong. 2017. "Analysis of the Comprehensive Tensile Relationship in Electrospun Silk Fibroin/Polycaprolactone Nanofiber Membranes" Membranes 7, no. 4: 67. https://doi.org/10.3390/membranes7040067





