Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications
Abstract
:1. Introduction
2. Experimental
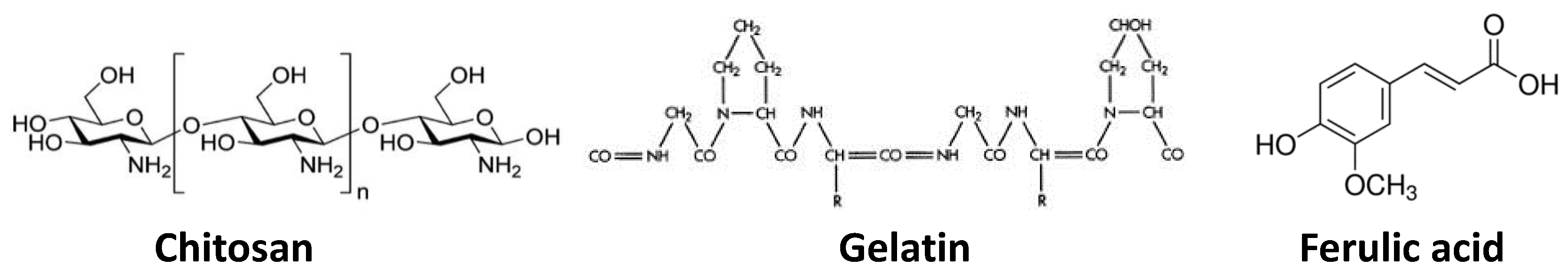
2.1. Chemicals
2.2. Film Preparation
2.3. Film Characterization
2.3.1. Film Thickness
2.3.2. Water Uptake
2.3.3. Static Water Contact Angle
2.3.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
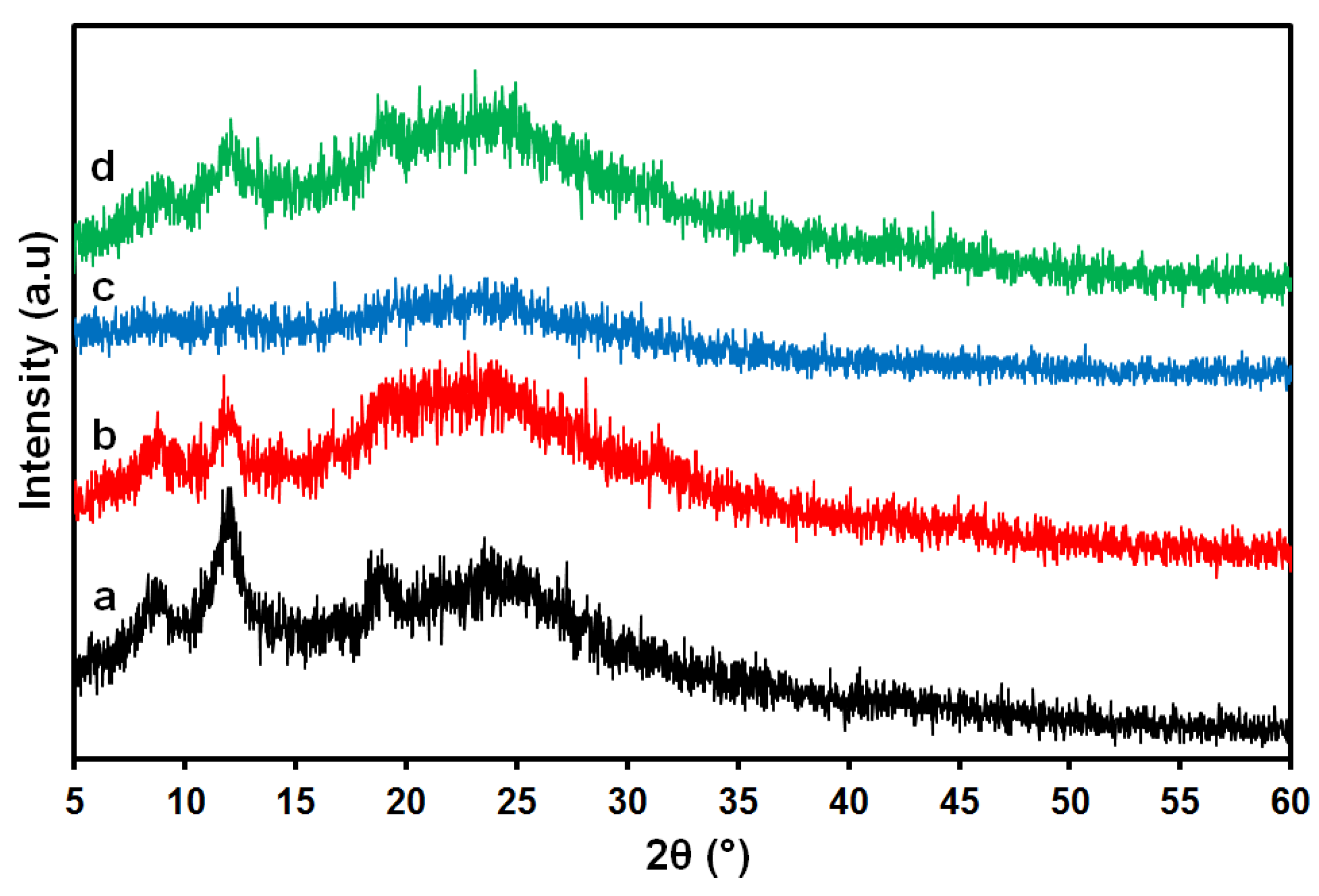
2.3.5. X-ray Diffraction (XRD) Analysis
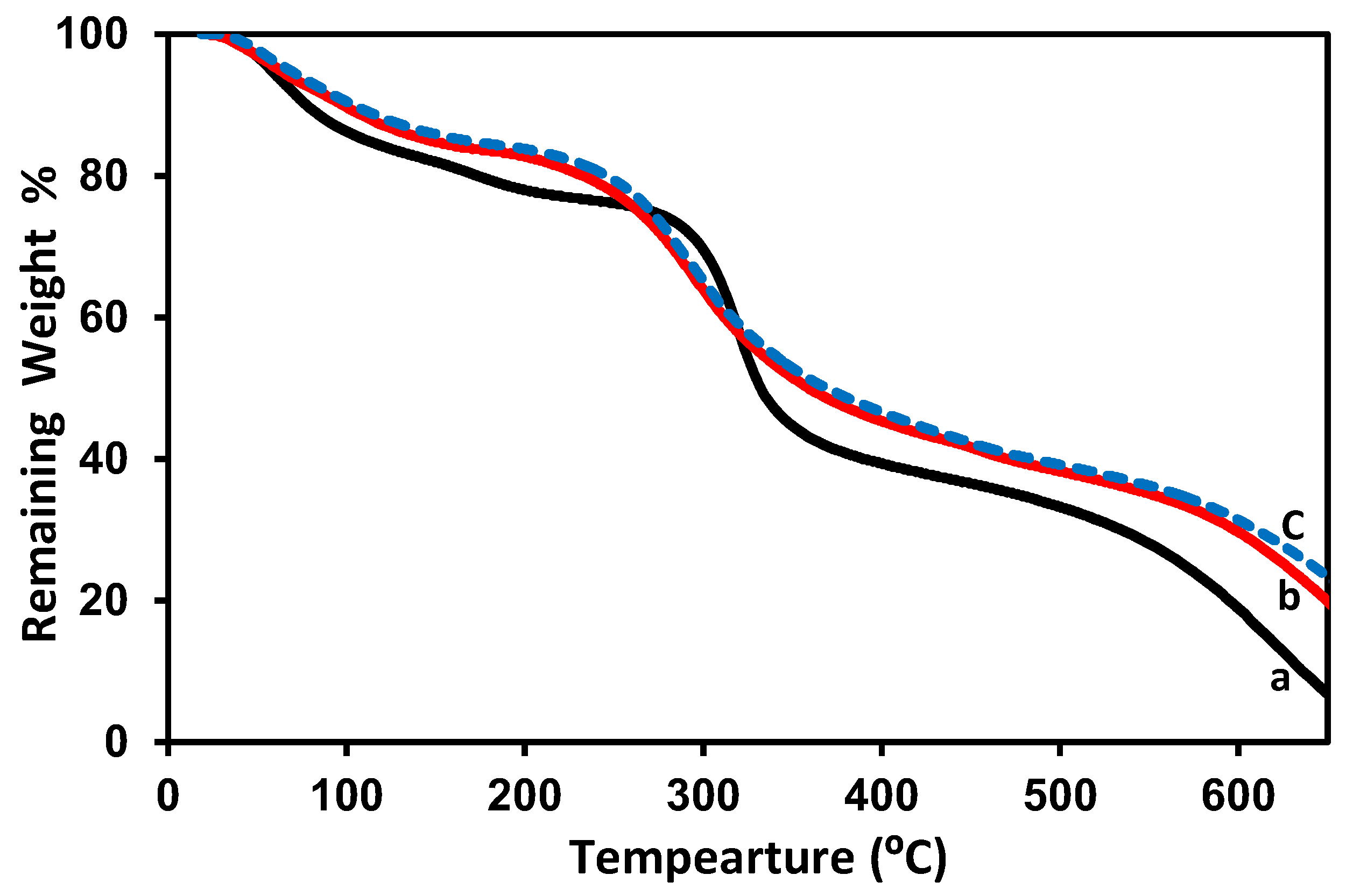
2.3.6. Thermal Gravimetric Analysis (TGA)
2.3.7. Differential Scanning Calorimetry (DSC) Analysis
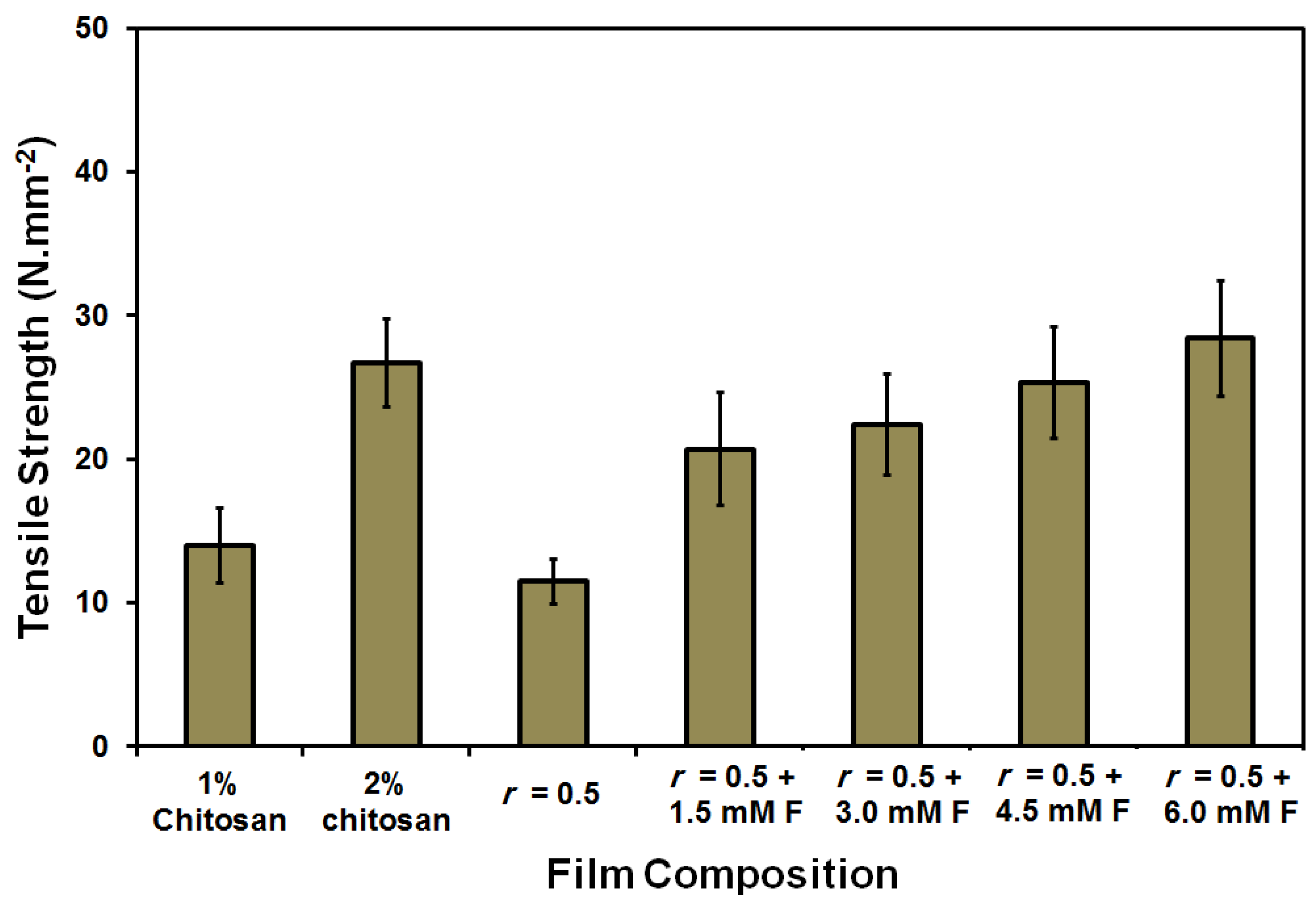
2.3.8. Tensile Strength Determination
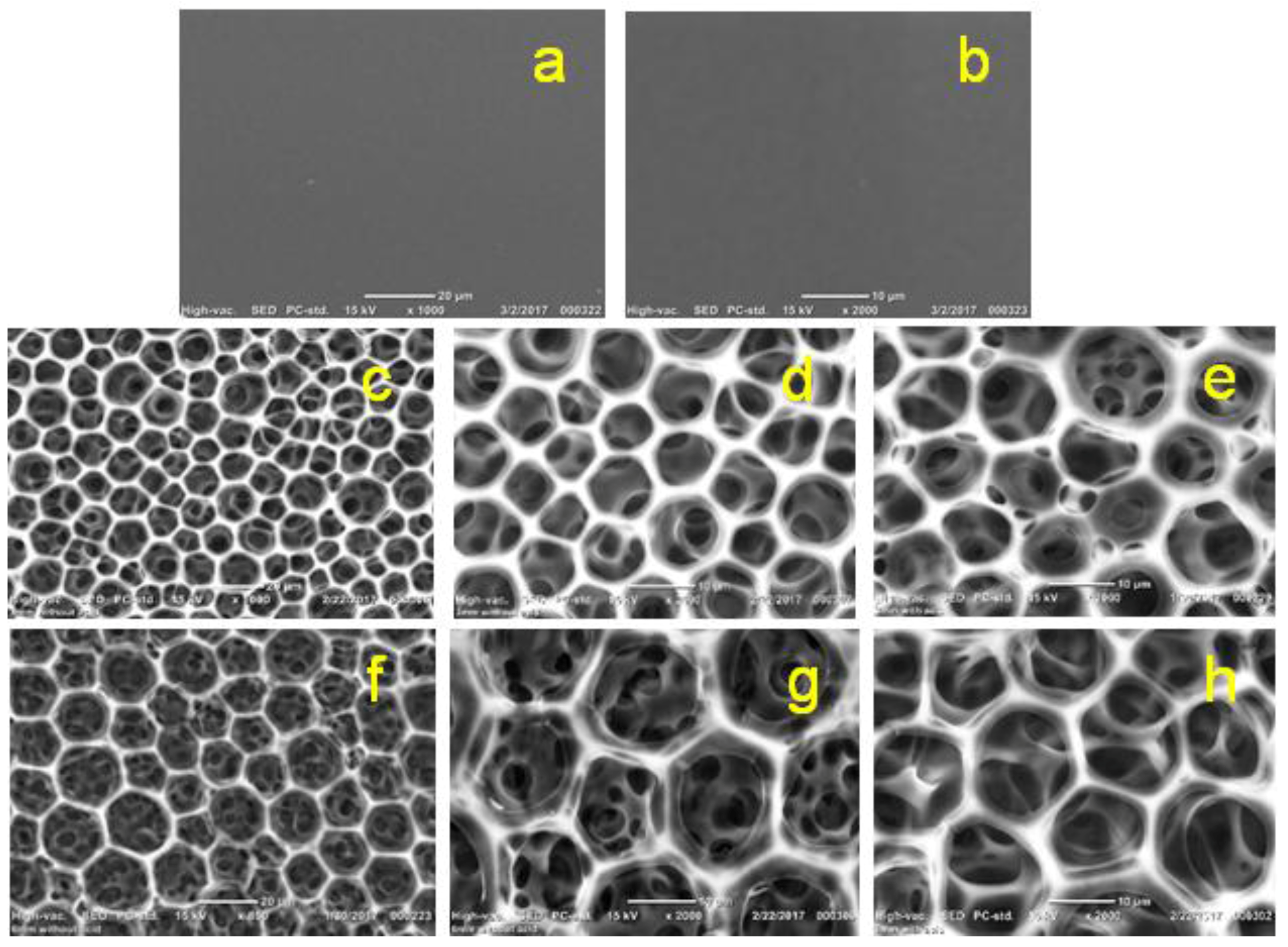
2.3.9. Scanning Electron Microscope (SEM) Imaging
3. Results and Discussion
3.1. Acetic Acid Solvent
3.2. Formic Acid Solvent
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds based bone tissue engineering: The role of chitosan. Tissue Eng. Part B 2011, 17, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Ma, L.; Mao, Z.; Gao, C. Chitosan-based biomaterials for tissue repair and regeneration. Adv. Polym. Sci. 2011, 244, 81–128. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Balau, L.; Lisa, G.; Popa, M.I.; Tura, V.; Melnig, V. Physico-chemical properties of chitosan films. Cent. Eur. J. Chem. 2004, 2, 638–647. [Google Scholar] [CrossRef]
- Albanna, M.Z.; Bou-Akl, T.H.; Walters, H.L.; Matthew, H.W.T. Improving the mechanical properties of chitosan-based heart valve scaffolds using chitosan fibers. J. Mech. Behav. Biomed. Mater. 2012, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Mao, J.; Yin, Y.; Liu, W.; Cui, Y.; Cai, K.; Zhao, F. Chitosan/Gelatin network based biomaterials in tissue engineering. Biomed. Eng. Appl. Basis Commun. 2002, 14, 115–121. [Google Scholar] [CrossRef]
- Sajesh, K.M.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Biocompatible conducting chitosan/polypyrrole-alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 62, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gong, J.; Zeng, C.; Zhang, L. Preparation of zeolite-A/chitosan hybrid composites and their bioactivities and antimicrobial activities. Mater. Sci. Eng. C 2013, 33, 3652–3660. [Google Scholar] [CrossRef] [PubMed]
- Toskas, G.; Cherif, C.; Hund, R.D.; Laourine, E.; Mahltig, B.; Fahmi, A.; Heinemann, C.; Hanke, T. Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr. Polym. 2013, 94, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Kavya, K.C.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Casariego, A.; Souza, B.W.S.; Vicente, A.A.; Teixeira, J.A.; Cruz, L.; Díaz, R. Chitosan coating surface properties as affected by plasticizer, surfactant and polymer concentrations in relation to the surface properties of tomato and carrot. Food Hydrocoll. 2008, 22, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Shweta, A.; Sonia, P. Pharmaceutical relevance of crosslinked chitosan in microparticulate drug delivery. Int. Res. J. Pharm. 2013, 4, 45–51. [Google Scholar]
- Yua, Q.; Songa, Y.; Shia, X.; Xub, C.; Bina, Y. Preparation and properties of chitosan derivative/poly(vinyl alcohol) blend film crosslinked with glutaraldehyde. Carbohydr. Polym. 2011, 84, 465–470. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Yeh, M.K.; Cheng, K.M.; Hub, C.S.; Huang, Y.C.; Young, J.J. Novel protein-loaded chondroitin sulfate-chitosan nanoparticles: Preparation and characterization. Acta Biomater. 2011, 7, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.H.; Hassan, M.I.; Sultana, N. Electrospun Polycaprolactone (PCL) and PCL/nano-hydroxyapatite (PCL/nHA)-based nanofibers for bone tissue engineering application. In Proceedings of the 10th Asian Control Conference, Kota Kinabalu, Malaysia, 31 May–3 June 2015; pp. 1–4. [Google Scholar]
- Hsieh, C.-Y.; Tsai, S.-P.; Ho, M.-H.; Wang, D.-M.; Liu, C.-E.; Hsieh, C.-H.; Tseng, H.-C.; Hsieh, H.-J. Analysis of freeze-gelation and cross-linking processes for preparing porous chitosan scaffolds. Carbohydr. Polym. 2007, 67, 124. [Google Scholar] [CrossRef]
- Li, C.; Han, Q.; Guan, Y.; Zhang, Y. Thermal gelation of chitosan in an aqueous alkali-urea solution. Soft Matter 2014, 10, 8245–8253. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.H.; Sultana, F.S.A.; Hutmacher, D.W.; Wong, Y.S.; Fuh, J.Y.H.; Mo, X.M.; Loh, H.T.; Burdet, E.; Teoh, S.H. Fabrication of 3D chitosan-hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2001, 20, 35. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.H. Preparations, properties and applications of chitosan based nanofibers fabricated by electrospinning. Express Polym. Lett. 2011, 5, 342–361. [Google Scholar] [CrossRef] [Green Version]
- Malafaya, P.; Pedro, A.; Peterbauer, A.; Gabriel, C.; Redl, H.; Reis, R.L. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J. Mater. Sci. Mater. Med. 2005, 16, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correlo, V.; Boesel, L.; Pinho, E.; Costa-Pinto, A.; Alves da Silva, M.; Bhattacharya, M.; Mano, J.; Neves, N.; Reis, R. Melt-Based Compression-molded scaffolds from chitosan-polyester blends and composites: Morphology and mechanical properties. J. Biomed. Mater. Res. A 2009, 91, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugind, B.M. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Correlo, V.M.; Costa-Pinto, A.R.; Sol, P.; Covas, J.A.; Bhattacharya, M.; Neves, N.M.; Reis, R.L. Melt processing of chitosan-based fibers and fiber-mesh scaffolds for the engineering of connective tissues. Macromol. Biosci. 2010, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.J.; Lee, J.Y.; Park, Y.J.; Lee, Y.M.; Young, K.; Rhyu, I.C.; Lee, S.J.; Han, S.B.; Chung, C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, W.I.; Jiang, T.; El-Bassyouni, G.E.-T.; Laurencin, C.T. Synthesis, Characterization of Chitosan and Fabrication of Sintered Chitosan Microsphere Matrices for Bone Tissue Engineering. Acta Biomater. 2007, 3, 503. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wan, Y.; Dalai, S.; Zhang, R. Response of rat osteoblasts to polycaprolactone/chitosan blend porous scaffolds. J. Biomed. Mater. Res. Part A 2009, 92, 238. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-a versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Sashikala, S.; Shafi, S. Synthesis and characterization of chitosan Schiff base derivatives. Pharm. Lett. 2014, 6, 90–97. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Van der Veen, S.; Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Boom, R.M.; Abee, T.; Schroen, K. Listeria monocytogenes repellence by enzymatically modified PES surfaces. J. Appl. Polym. Sci. 2015, 132, 41576–41582. [Google Scholar] [CrossRef]
- Nady, N. PES Surface modification using green chemistry: New generation of antifouling membranes. Membranes 2016, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ryu, K.; Kang, J.H.; Choi, A.J.; Kim, T.I.; Oh, J.M. Anticancer activity of ferulic acid-inorganic nanohybrids synthesized via two different hybridization routes, reconstruction and exfoliation-reassembly. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Characterization of ferulic acid incorporated starch-chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Biscarat, J.; Charmette, C.; Masquelez, N.; Sanchez, J.; Pochat-Bohatier, C. Crosslinking of gelatin membranes with frulic acid or glutaraldehyde: Relationship between gas permeability and renaturation lvel of gelatin triple helices. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 280–287. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Chiou, B.S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Jafri, H.; Narayan, R.; Imama, S.H.; Glenn, G.M.; Orts, W.J. Cold water fish gelatin films: Effects of cross-linking on thermal, mechanical, barrier, and biodegradation properties. Eur. Polym. J. 2008, 44, 3748–3753. [Google Scholar] [CrossRef]
- Kozlov, P.V.; Burdygina, G.I. The structure and properties of solid gelatin and the principles of their modification. Polymer 1983, 24, 651–666. [Google Scholar] [CrossRef]
- Jus, S.; Stachel, I.; Fairhead, M.; Meyer, M.; Thöny-meyer, L.; Guebitz, G.M. Enzymatic cross-linking of gelatine with laccase and tyrosinase. Biocatal. Biotransform. 2012, 30, 86–95. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Osako, K. Characterisation of gelatin-fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010, 122, 1095–1101. [Google Scholar] [CrossRef]
- Madsen, E.L.; Zagzebski, J.A.; Frank, G.R. Oil-in-gelatin dispersions for use as ultrasonically tissue-mimicking materials. Ultrasound Med. Biol. 1982, 8, 277–287. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. In Recent Advances in Biopolymers; Perveen, F.K., Ed.; InTech: London, UK, 2016. [Google Scholar]
- Chang, M.C.; Douglas, W.H.; Tanaka, J. Organic-inorganic interaction and the growth mechanism of hydroxyapatite crystals in gelatin matrices between 37 °C and 80 °C. J. Mater. Sci. Mater. Med. 2006, 17, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, X.; Ma, Y.; Zhao, X. Effect of ferulic acid and its oxide on qualities of soy protein-isolate/chitosan composite films. Appl. Mech. Mater. 2015, 716–717, 24–27. [Google Scholar] [CrossRef]
- Yin, Y.; Ye, F.; Cui, J.; Zhang, F.; Li, X.; Yao, K. Preparation and characterization of macroporous chitosan–gelatin/β-tricalcium phosphate composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2003, 67, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Grayson, W.L.; Ma, T.; Bunnell, B.; Lu, W.W. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, M.H.; Shea, L.D.; Peters, M.C.; Mooney, D.J. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. J. Controll. Release 2000, 64, 91–102. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Fabrication of three-dimensional tissue. In Tissue Engineering II. Advances in Biochemical Engineering/Biotechnology; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–205. [Google Scholar]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.; Flanagan, T.C. Electrospinning of biomimetic scaffolds for tissue-engineered vascular grafts: Threading the path. Expert Rev. Cardiovasc. Ther. 2014, 12, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ma, Z.; Gao, C.; Wang, W.; Shen, J. Specially elaborated thermally induced phase separation to fabricate poly(l-lactic acid) scaffolds with ultra large pores and good interconnectivity. J. Appl. Polym. Sci. 2006, 101, 3336–3342. [Google Scholar] [CrossRef]
- Choi, J.W.; Ma, P.X. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef]
- Sallesa, T.H.C.; Lombellob, C.B.; d’Ávilaa, M.A. Electrospinning of gelatin/poly (vinylpyrrolidone) blends from water/acetic acid solutions. Mater. Res. 2015, 18, 509–518. [Google Scholar] [CrossRef]
- Garg, T.; Chanana, A.; Joshi, R. Preparation of chitosan scaffolds for tissue engineering using freeze drying technology. IOSR J. Pharm. 2012, 2, 72–73. [Google Scholar] [CrossRef]
- Lemma, S.M.; Bossard, F.; Rinaudo, M. Preparation of pure and stable chitosan nanofibers by electrospinning in the presence of poly(ethylene oxide). Int. J. Mol. Sci. 2016, 17, 1790. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Hosoya, O.; Taoka, S.; Seki, T.; Kawaguchi, T.; Sugibayashi, K.; Morimoto, Y. Relationship between solubility of chitosan in alcoholic solution and its gelation. Chem. Pharm. Bull. 1999, 47, 1044–1046. [Google Scholar] [CrossRef]
- El-heian, E.A.; Yahaya, A.H.; Misran, M. Characterization of chitosan solubilized in aqueous formic and acetic acids. Maejo Int. J. Sci. Technol. 2009, 3, 415–425. [Google Scholar]
- Chen, R.H.; Lin, W.C.; Lin, J.H. Effects of pH, ionic strength, and type of anion on the rheological properties of chitosan solutions. Acta Polym. 1994, 45, 41–46. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan-gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O. Graft copolymers of maleic anhydride and its isostructural analogues: High performance engineering materials. Int. Rev. Chem. Eng. 2011, 3, 153–215. [Google Scholar]
- Santos-Esteve, L.; Berna, A. Compreeibility of woven textiles on the architecture of a high ordered cylindrical, orthogonal, hollow-channeled scaffolds. J. Appl. Polym. Sci. 2017, 134, 11. [Google Scholar] [CrossRef]
- Chen, F.; Gällstedt, M.; Olsson, R.T.; Gedde, U.W.; Hedenqvist, M.S. Unusual effects of monocarboxlic acid on the structure and on the transport and mechanical properties of chitosan films. Carbonhydr. Polym. 2015, 12, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.H. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Wanga, X.Y.; Heuzey, M.C. Pickering emulsion gels based on insoluble chitosan/gelatin electrostatic complexes. RSC Adv. 2016, 6, 88916–89983. [Google Scholar] [CrossRef]
- Chandra Roy, J.; Salaün, F.; Giraud, S.; Ferri, A.; Guan, J. Surface behavior and bulk properties of aqueous chitosan and type-B gelatin solutions for effective emulsion formulation. Carbohydr. Polym. 2017, 173, 202–214. [Google Scholar] [CrossRef]
- Maleknia, L.; Majdi, Z.R. Electrospinning of gelatin nanofiber for biomedical application. Orient. J. Chem. 2014, 30, 2043–2048. [Google Scholar] [CrossRef]
- Cota-Arriola, O.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Robles-Sánchez, R.M.; Ezquerra-Brauer, J.M.; Ruíz-García, J. Preparation of chitosan matrices with ferulic acid: Physicochemical characterization and relationship on the growth of Aspergillus parasiticus. CyTA J. Food 2017, 15, 65–74. [Google Scholar] [CrossRef]
- Simionatto, G.L.; Gomes, C.E.T. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Oo, H.H.; Naing, K.; Naing, K.M.; Aye, T.T.; Wynn, N. Electrochemical and thermal studies of prepared conducting chitosan biopolymer film. J. Myan. Acad. Arts Sci. 2005, 3, 62–72. [Google Scholar]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC flms: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Rahman, M.M.; Pervez, S.; Nesa, B.; Khan, M.A. Preparation and characterization of porous scaffold composite films by blending chitosan and gelatin solutions for skin tissue engineering. Polym. Int. 2013, 62, 79–86. [Google Scholar] [CrossRef]
- Jiankang, H.; Dichen, L.; Yaxiong, L.; Bo, Y.; Hanxiang, Z.; Qin, L.; Bingheng, L.; Yi, L. Preparation of chitosan-gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009, 5, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.S.; Zhao, L.G.; Yin, Y.J.; Yao, K.D. Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 2003, 24, 1067–1074. [Google Scholar] [CrossRef]

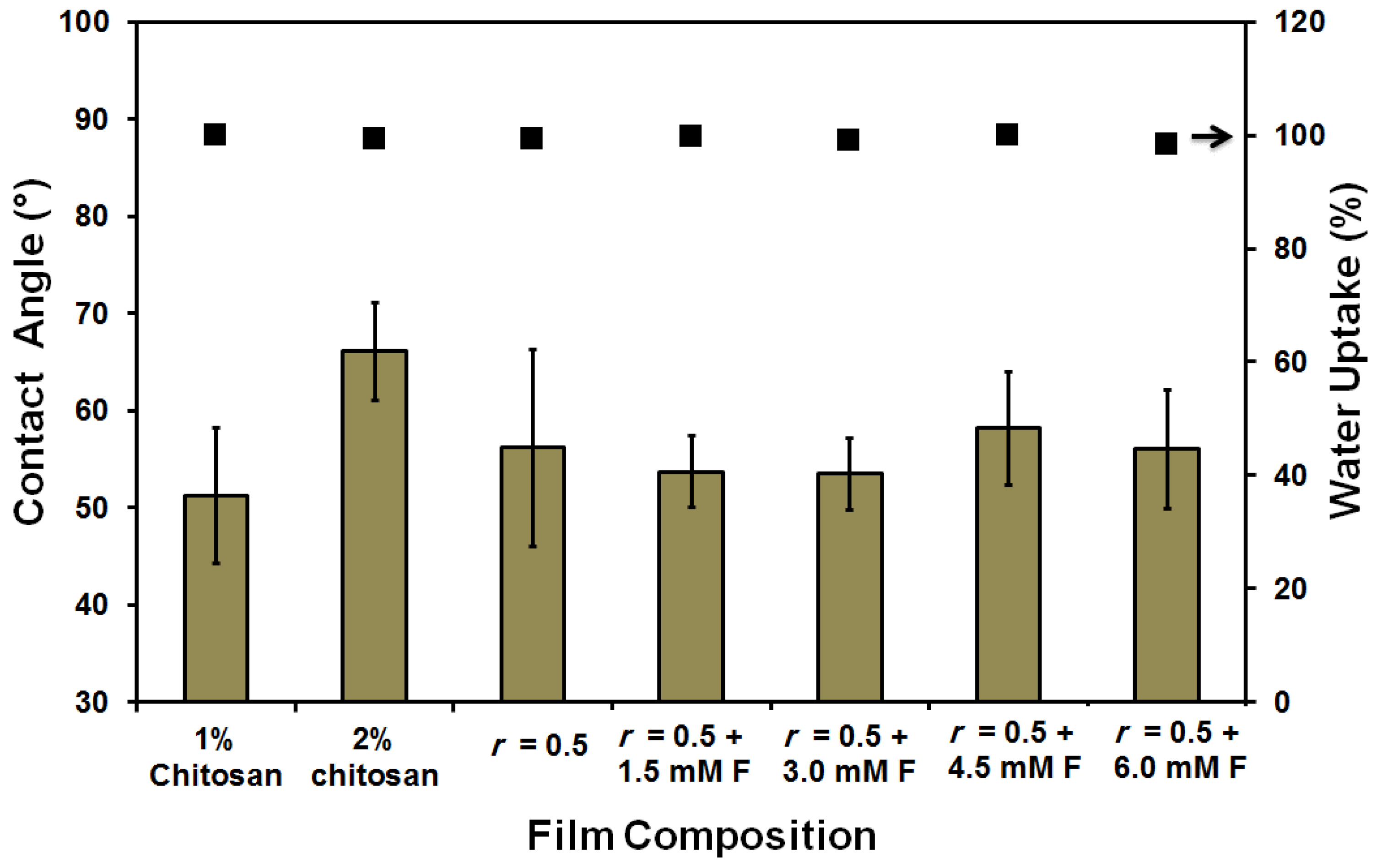
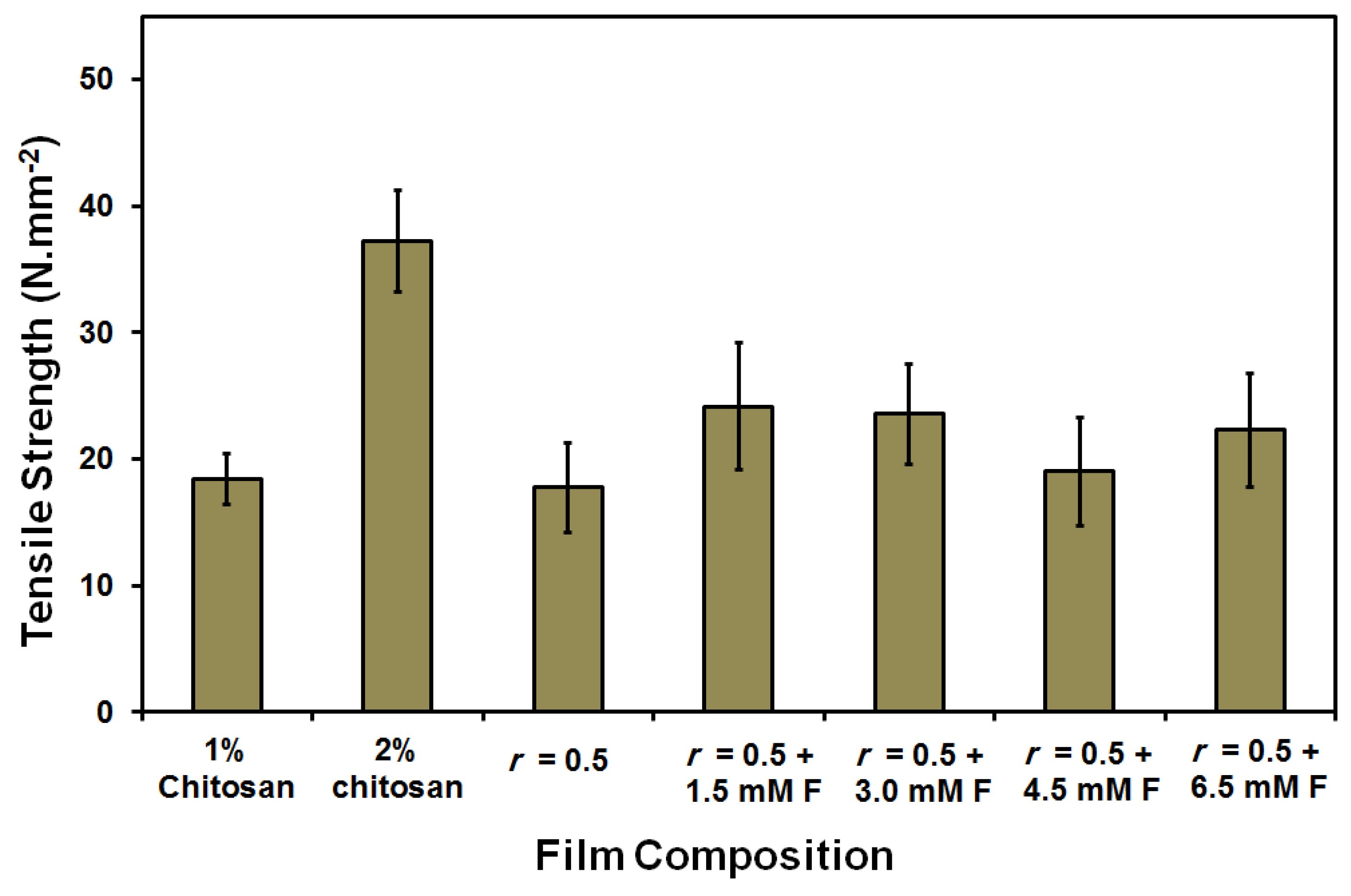

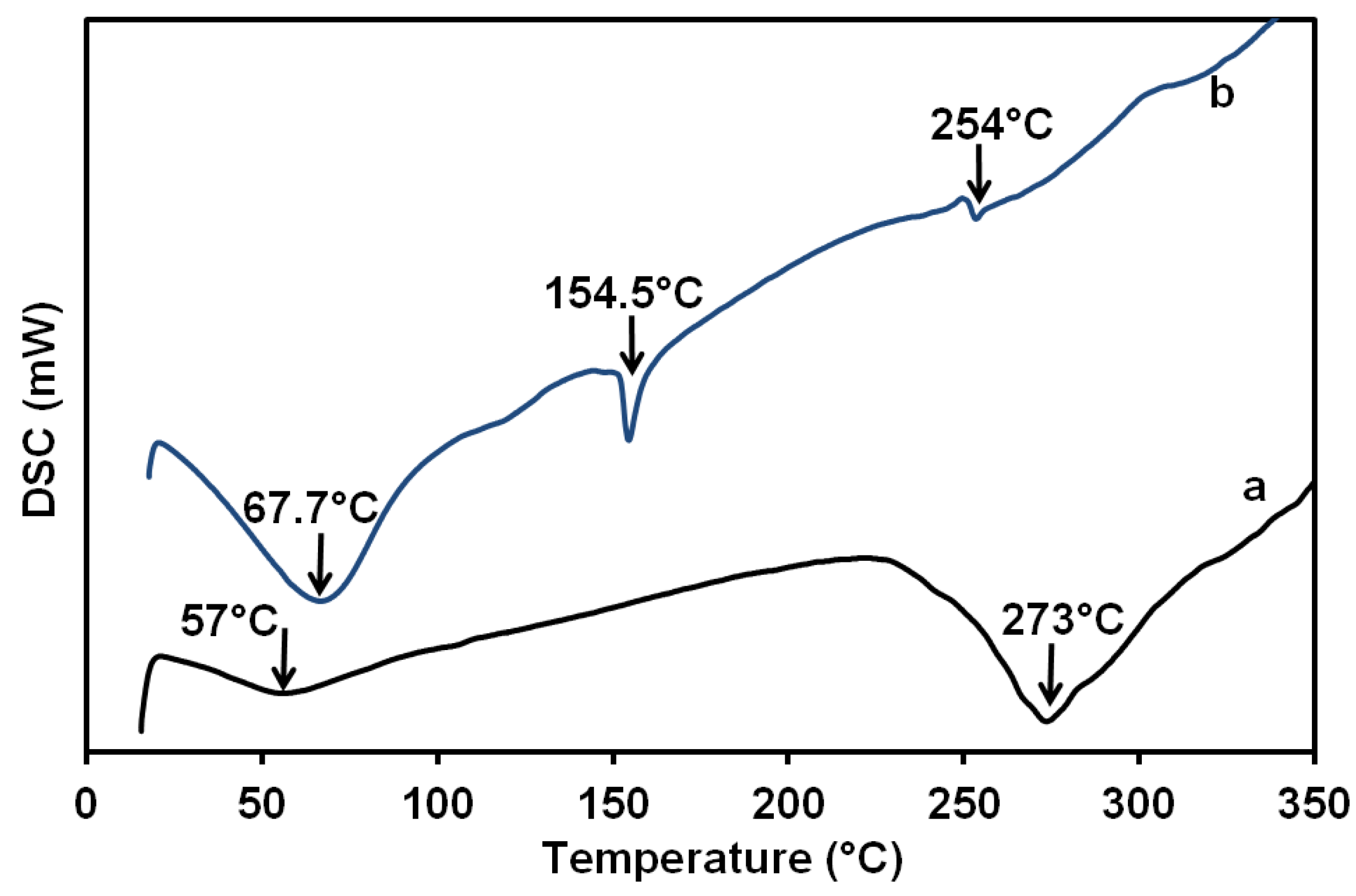
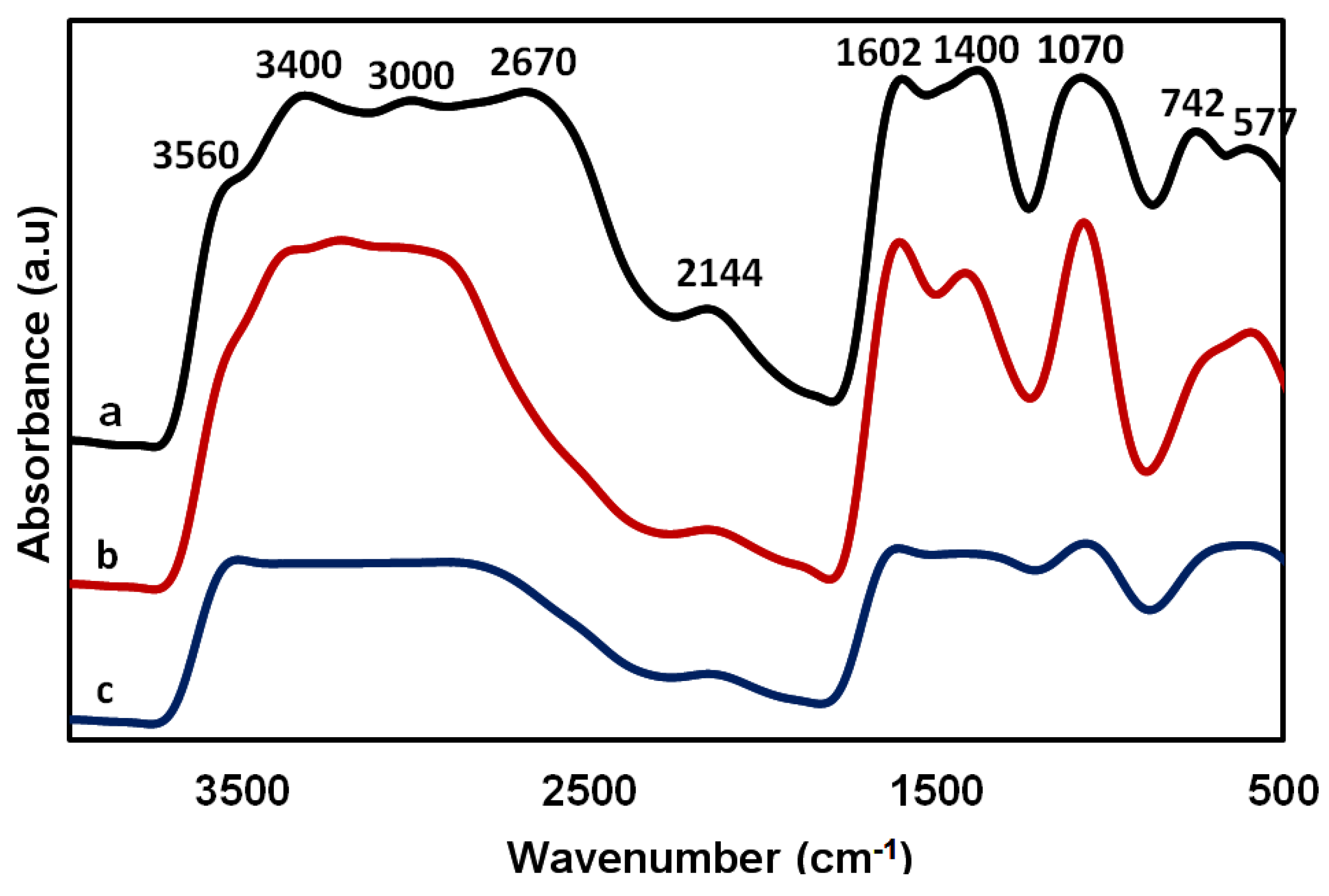
| Film/Membrane Composition | Film/Membrane Thickness (mm) |
|---|---|
| 1 wt % Chitosan | 0.06 ± 0.002 |
| 2 wt % chitosan | 0.08 ± 0.001 |
| r = 0.5 | 0.08 ± 0.001 |
| r = 0.5 + 1.5 mM F | 0.07 ± 0.001 |
| r = 0.5 + 3.0 mM F | 0.09 ± 0.001 |
| r = 0.5 + 4.5 mM F | 0.09 ± 0.001 |
| r = 0.5 + 6.0 mM F | 0.08 ± 0.001 |
| r = 0.5 + 3.0 mM F plasticized | 0.08 ± 0.001 |
| Film/Membrane Composition | Film/Membrane Thickness (mm) |
|---|---|
| 1 wt % Chitosan | 0.058 ± 0.001 |
| 2 wt % chitosan | 0.093 ± 0.001 |
| r = 0.5 | 0.058 ± 0.001 |
| r = 0.5 + 1.5 mM F | 0.051 ± 0.002 |
| r = 0.5 + 3.0 mM F | 0.051 ± 0.001 |
| r = 0.5 + 4.5 mM F | 0.052 ± 0.001 |
| r = 0.5 + 6.0 mM F | 0.052 ± 0.001 |
| r = 0.5 + 3.0 mM F plasticized | 0.051 ± 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nady, N.; Kandil, S.H. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes 2018, 8, 2. https://doi.org/10.3390/membranes8010002
Nady N, Kandil SH. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes. 2018; 8(1):2. https://doi.org/10.3390/membranes8010002
Chicago/Turabian StyleNady, Norhan, and Sherif H. Kandil. 2018. "Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications" Membranes 8, no. 1: 2. https://doi.org/10.3390/membranes8010002




