Applicability of PolyActive™ Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gases
2.2. Membrane Material
2.3. Free Volume Model
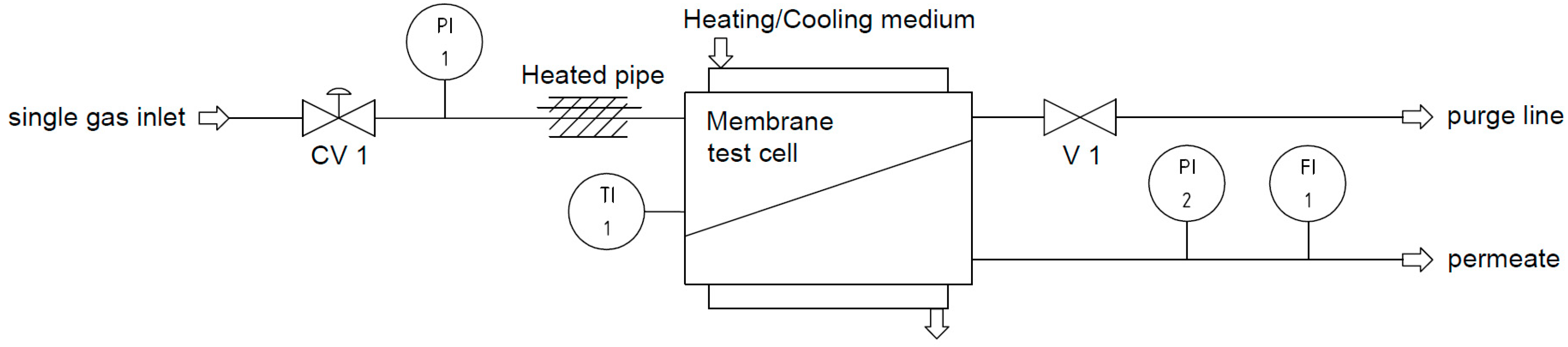
2.4. Single Gas Experiments
2.5. Mixed Gas Experiments
3. Results and Discussion
3.1. Determination of FVM Parameters Based on Results from Single Gas Experiments
3.2. Mixed Gas Experiments and Comparison with Modeled Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yave, W.; Car, A.; Wind, J.; Peinemann, K.-V. Nanometric thin film membranes manufactured on square meter scale: Ultra-thin films for CO2 capture. Nanotechnology 2010, 21, 395301. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 selective poly(ethylene oxide)-based membranes: From laboratory to pilot plant scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Tailor-made polymeric membranes based on segmented block copolymers for co2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-philic polymer membrane with extremely high separation performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Brinkmann, T.; Naderipour, C.; Pohlmann, J.; Wind, J.; Wolff, T.; Esche, E.; Müller, D.; Wozny, G.; Hoting, B. Pilot scale investigations of the removal of carbon dioxide from hydrocarbon gas streams using poly (ethylene oxide)–poly (butylene terephthalate) polyactive™) thin film composite membranes. J. Membr. Sci. 2015, 489, 237–247. [Google Scholar] [CrossRef]
- Stünkel, S.; Drescher, A.; Wind, J.; Brinkmann, T.; Repke, J.U.; Wozny, G. Carbon dioxide capture for the oxidative coupling of methane process—A case study in mini-plant scale. Chem. Eng. Res. Des. 2011, 89, 1261–1270. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R. Gas Purification; Elsevier Science: New York, NY, USA, 1997. [Google Scholar]
- Fang, S.M.; Stern, S.A.; Frisch, H.L. A “free volume” model of permeation of gas and liquid mixtures through polymeric membranes. Chem. Eng. Sci. 1975, 30, 773–780. [Google Scholar] [CrossRef]
- Ohlrogge, K.; Peinemann, K.-V.; Wind, J.; Behling, R.-D. The separation of hydrocarbon vapors with membranes. Sep. Sci. Technol. 1990, 25, 1375–1386. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Paul, D.; Peinemann, K.V.; Pereira-Nunes, S.; Scharnagl, N. Developments in membrane research: From material via process design to industrial application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Pohlmann, J.; Bram, M.; Wilkner, K.; Brinkmann, T. Pilot scale separation of CO2 from power plant flue gases by membrane technology. Int. J. Greenh. Gas Control 2016, 53, 56–64. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lillepärg, J.; Neumann, S.; Shishatskiy, S.; Abetz, V. A thermodynamic study of CO2 sorption and thermal transition of polyactive™ under elevated pressure. Polymer 2016, 93, 132–141. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of poly(ethylene glycol) segment length on CO2 permeation and stability of polyactive membranes and their nanocomposites with peg poss. ACS Appl. Mater. Interfaces 2015, 7, 12289–12298. [Google Scholar] [CrossRef] [PubMed]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; Mc-Graw Hill: New York, NY, USA, 2001; Volume 5. [Google Scholar]
- Aspen Technology. Available online: http://home.Aspentech.Com/products/pages/aspen-properties (accessed on 21 December 2017).
- Polymer Gas Separation Membranes. Available online: https://membrane-australasia.org/polymer-gas-separation-membranes/ (accessed on 29 May 2018).









| Gas | [14] | ||||||
|---|---|---|---|---|---|---|---|
| Gas | 0–2 bar | 2–30 bar | 0–2 bar | 2–30 bar | 0–2 bar | 2–30 bar | |
| CO2 | 3.29 ± 0.01 | 2.97 ± 0.15 | - | - | 0.0352 | 0.0572 | 3.94 |
| C2H4 | 1.04 ± 0.01 | 1.01 ± 0.04 | - | - | 0.0470 | 0.0470 | 4.16 |
| CH4 | - | - | 0.274 ± 0.002 | 0.281 ± 0.006 | - | - | 3.76 |
| N2 | - | - | 0.084 ± 0.001 | 0.083 ± 0.002 | - | - | 3.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuldt, K.; Pohlmann, J.; Shishatskiy, S.; Brinkmann, T. Applicability of PolyActive™ Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar. Membranes 2018, 8, 27. https://doi.org/10.3390/membranes8020027
Schuldt K, Pohlmann J, Shishatskiy S, Brinkmann T. Applicability of PolyActive™ Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar. Membranes. 2018; 8(2):27. https://doi.org/10.3390/membranes8020027
Chicago/Turabian StyleSchuldt, Karina, Jan Pohlmann, Sergey Shishatskiy, and Torsten Brinkmann. 2018. "Applicability of PolyActive™ Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar" Membranes 8, no. 2: 27. https://doi.org/10.3390/membranes8020027





