Prevalence of Vitamin D Deficiency Varies Widely by Season in Canadian Children and Adolescents with Sickle Cell Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Characteristics of the Studied Population
3.2. Biochemical and Clinical Markers
3.3. Factors Associated with Serum 25OHD Concentrations
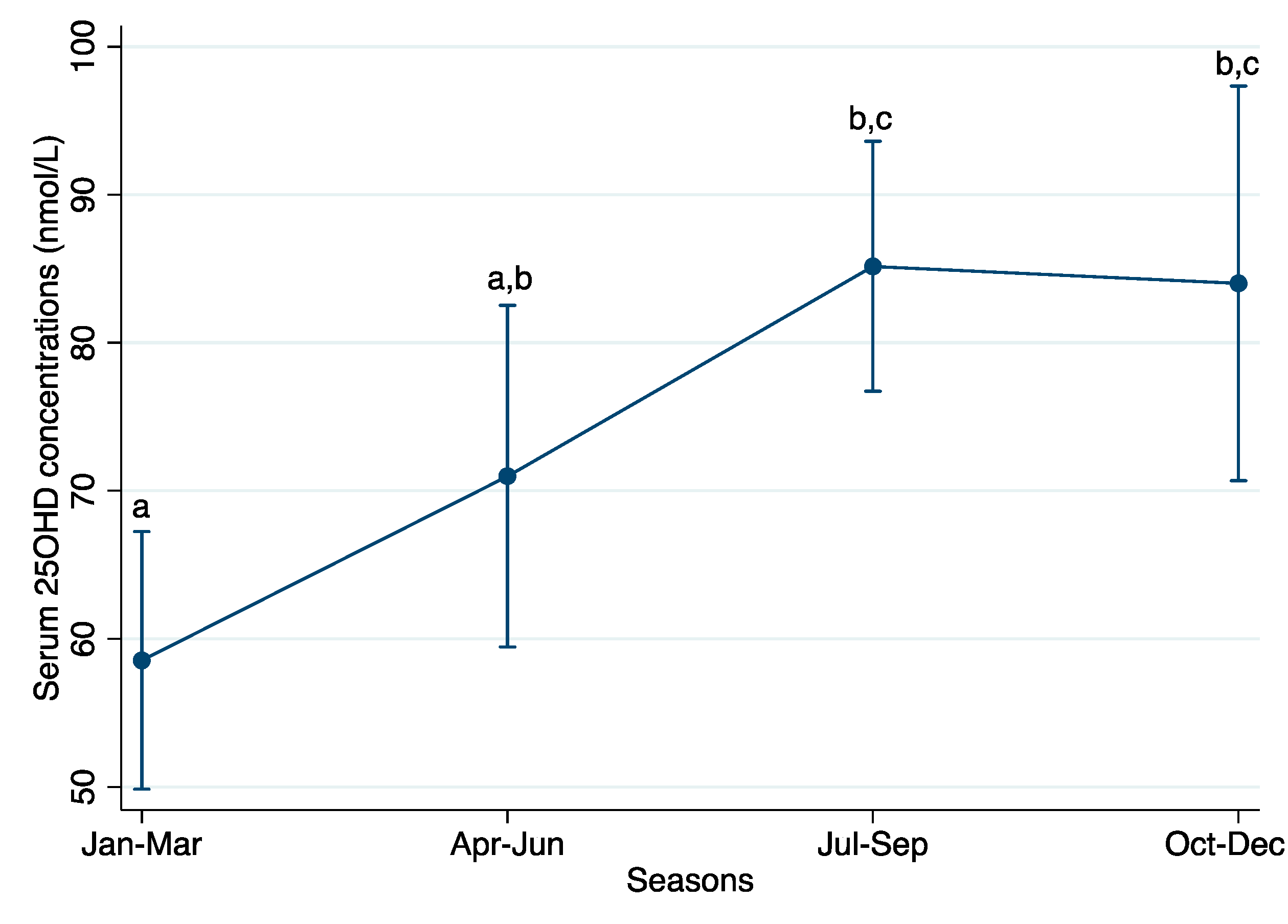
3.4. Vitamin D Concentration by Season of Blood Collection
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soe, H.H.; Abas, A.B.; Than, N.N.; Ni, H.; Singh, J.; Said, A.R.; Osunkwo, I. Vitamin D supplementation for sickle cell disease (Review). Cochrane Database Syst. Rev. 2017, 1. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Bunn, F.H. Pathogenesis and Treatment of Sickle Cell Disease. N. Engl. J. Med. 1997, 337, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.T.; Bartlett, J.M.; Kolasa, K.M.; Marcuard, S.P.; Holbrook, C.T.; Horner, R.D. Nutritional status and dietary intake of children with sickle cell anemia. Am. J. Pediatr. Hematol. Oncol. 1992, 14, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Barden, E.M.; Zemel, B.S.; Kawchak, D.A.; Goran, M.L.; Ohene-Frempong, K.; Stallings, V.A. Total and Resting Energy Expenditure in Children With Sickle Cell Disease. J. Pediatr. 2000, 136, 73–79. [Google Scholar] [CrossRef]
- Hyacinth, H.I.; Gee, B.E.; Hibbert, J.M. The Role of Nutrition in Sickle Cell Disease. Nutr. Metab. Insights 2010, 3, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.G.; Nottage, K.A.; Cole, E.W.; Hankins, J.S.; Gurney, J.G. Prevalence of Vitamin D deficiency in sickle cell disease: A systematic review. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689–1696. [Google Scholar]
- Martyres, D.J.; Vijenthira, A.; Barrowman, N.; Harris-Janz, S.; Chretien, C.; Klaassen, R.J. Nutrient Insufficiencies/Deficiencies in Children With Sickle Cell Disease and Its Association With Increased Disease Severity. Pediatr. Blood Cancer 2016, 63, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Rovner, A.J.; Stallings, V.A.; Kawchak, D.A.; Schall, J.I.; Ohene-Frempong, K.; Zemel, B.S. High Risk of Vitamin D Deficiency in Children with Sickle Cell Disease. J. Am. Diet. Assoc. 2008, 108, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Julka, R.N.; Aduli, F.; Lamps, L.W.; Olden, K.W. Ischemic duodenal ulcer, an unusual presentation of sickle cell disease. J. Natl. Med. Assoc. 2008, 100, 339–341. [Google Scholar] [CrossRef]
- Phebus, C.K.; Maciak, B.J.; Gloninger, M.F.; Paul, H.S. Zinc status of children with sickle cell disease: Relationship to poor growth. Am. J. Hematol. 1988, 29, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Flint, J.; Harding, R.M.; Boyce, A.J.; Clegg, J.B. The population genetics of the haemoglobinopathies. Baillieres Clin. Haematol. 1998, 11, 1–51. [Google Scholar] [CrossRef]
- Allison, A.C. Protection Afforded By Sickle-Cell Trait against Subtertian Malarial Infection. Br. Med. J. 1954, 1, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- The Canadian Haemoglobinopathy Association. The Canadian Haemoglobinopathy Association Consensus Statement on the Care of Patients with Sickle Cell Disease in Canada; The Canadian Haemoglobinopathy Association: Ottawa, ON, Canada, 2015; Version 2. [Google Scholar]
- Canadian Pediatric Endocrine Group (CPEG). WHO Growth Standard Charts. Available online: http://www.bcchildrens.ca/health-professionals/clinical-resources/endocrinology-diabetes/tools-calculators (accessed on 12 July 2017).
- Institute of Medicine (IOM). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011; ISBN 9780309163941. [Google Scholar]
- Godel, J.C. Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr. Child Health 2007, 12, 583–589. [Google Scholar]
- Langlois, K.; Greene-Finestone, L.; Little, J.; Hidiroglou, N.; Whiting, S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010, 21, 47–55. [Google Scholar] [PubMed]
- March, K.M.; Chen, N.N.; Karakochuk, C.D.; Shand, A.W.; Innis, S.M.; Von Dadelszen, P.; Barr, S.I.; Lyon, M.R.; Whiting, S.J.; Weiler, H.A.; et al. Maternal vitamin D3 supplementation at 50 mg/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: A randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am. J. Clin. Nutr. 2015, 102, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [PubMed]
- George, J.A.; Norris, S.A.; van Deventer, H.E.; Pettifor, J.M.; Crowther, N.J. Effect of adiposity, season, diet and calcium or vitamin D supplementation on the vitamin D status of healthy urban African and Asian-Indian adults. Br. J. Nutr. 2014, 112, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Buison, A.M.; Kawchak, D.A.; Schall, J.; Ohene-Frempong, K.; Stallings, V.A.; Zemel, B.S. Low vitamin D status in children with sickle cell disease. J. Pediatr. 2004, 145, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.C.; Krauss, M.J.; Debaun, M.R.; Strunk, R.C.; Arbeláez, A.M. Vitamin D deficiency and comorbidities in children with sickle cell anemia. Pediatr. Hematol. 2012, 29, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Arlet, J.B.; Courbebaisse, M.; Chatellier, G.; Eladari, D.; Souberbielle, J.C.; Friedlander, G.; de Montalembert, M.; Prié, D.; Pouchot, J.; Ribeil, J.A. Relationship between vitamin D deficiency and bone fragility in sickle cell disease: A cohort study of 56 adults. Bone 2013, 52, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.M.; Artz, N.; Radford, B.; Chen, I.A. Prevalence of vitamin D deficiency in adults with sickle cell disease. J. Natl. Med. Assoc. 2010, 102, 332–335. [Google Scholar] [CrossRef]
- Elder, C.J.; Bishop, N.J. Rickets. Lancet 2014, 383, 1665–1676. [Google Scholar] [CrossRef]
- Osunkwo, I.; Ziegler, T.R.; Alvarez, J.; McCracken, C.; Cherry, K.; Osunkwo, C.E.; Ofori-Acquah, S.F.; Ghosh, S.; Ogunbobode, A.; Rhodes, J.; et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: Results of a randomized double blind pilot study. Br. J. Haematol. 2012, 159, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wykes, C.; Arasaretnam, A.; O’Driscoll, S.; Farnham, L.; Moniz, C.; Rees, D.C. Vitamin D deficiency and its correction in children with sickle cell anaemia. Ann. Hematol. 2014, 93, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Osunkwo, I. Complete resolution of sickle cell chronic pain with high dose vitamin D therapy: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2011, 33, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Adewoye, A.H.; Chen, T.C.; Ma, Q.; McMahon, L.; Mathieu, J.; Malabanan, A.; Steinberg, M.H.; Holick, M.F. Sickle cell bone disease: Response to vitamin D and calcium. Am. J. Hematol. 2008, 83, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.A.; Bertolaso, C.; Schall, J.I.; Smith-Whitley, K.; Stallings, V.A. Safety and Efficacy of High-dose Daily Vitamin D3 Supplementation in Children and Young Adults With Sickle Cell Disease. J. Pediatr. Hematol. Oncol. 2015, 37. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) |
|---|---|
| Sex | |
| Males | 21/45 (47%) |
| Females | 24/45 (53%) |
| Ethnicity | |
| African | 39/45 (87%) |
| Caribbean | 3/45 (7%) |
| South Asian | 2/45 (4%) |
| Latino | 1/45 (2%) |
| Sickle cell genotypes | |
| Homozygous sickle cell anemia (βSβS) | 35/45 (78%) |
| Hemoglobin S/β-thalassemia | 7/45 (15%) |
| Hemoglobin SC disease (βSβC) | 3/45 (7%) |
| Currently receiving disease-modifying treatments | |
| Blood transfusions | 7/45 (16%) |
| Hydroxyurea (dose ranged from 400 to 1500 mg/d) | 28/45 (62%) |
| Prophylaxis antibiotics (penicillin or amoxicillin) | 37/45 (82%) |
| Currently recommended nutritional supplements | |
| Vitamin D (dose ranged from 500 to 1000 IU/d) | 45/45 (100%) |
| Folic acid (dose ranged from 1 to 5 mg/d) | 44/45 (98%) |
| Markers | All | Male | Female |
|---|---|---|---|
| Total children and adolescents, n (%) | 45 (100%) | 21 (47%) | 24 (53%) |
| Anthropometric indicators | |||
| Weight, kg | 40.4 ± 18.4 | 37.9 ± 21.3 | 42.4 ± 16.0 |
| Height, cm | 140.6 ± 23.3 | 134.7 ± 28.1 | 145.2 ± 18.0 |
| BMI-for-age, z-score, n (%) | |||
| ≥+2 | 3/42 (7%) | 2/19 (11%) | 1/23 (4%) |
| +1 to +2 | 10/42 (24%) | 4/19 (21%) | 6/23 (26%) |
| −1 to +1 | 26/42 (62%) | 12/19 (63%) | 14/23 (61%) |
| <−1 | 3/42 (7%) | 1/19 (5%) | 2/23 (9%) |
| Nutritional indicators | |||
| 25OHD concentration, nmol/L | 79.1 ± 35.9 | 77.2 ± 38.1 | 80.7 ± 34.7 |
| Prevalence of low 25OHD, n (%) | |||
| <30 nmol/L | 2/42 (5%) | 1/19 (5%) | 1/23 (4%) |
| <40 nmol/L | 7/42 (17%) | 3/19 (16%) | 4/23 (17%) |
| <50 nmol/L | 10/42 (24%) | 5/19 (26%) | 5/23 (22%) |
| <75 nmol/L | 21/42 (50%) | 10/19 (53%) | 11/23 (48%) |
| ALP activity, U/L, median (IQR) | 139 (84, 185) | 149 (90, 185) | 130.5 (76.5, 186) |
| Copper, μmol/L | 20.4 ± 4.9 | 20.5 ± 5.0 | 20.3 ± 4.9 |
| Selenium, μmol/L | 1.31 ± 0.17 | 1.28 ± 0.18 | 1.34 ± 0.17 |
| Zinc, μmol/L | 11.3 ± 1.8 | 12.1 ± 2.0 | 10.7 ± 1.5 |
| Hematological indicators | |||
| Hemoglobin concentration, g/L | 92.3 ± 16.5 | 93.0 ± 17.4 | 91.8 ± 16.2 |
| Anemia, <110 g/L, n (%) | 35/43 (81%) | 14/19 (74%) | 21/24 (88%) |
| Mean corpuscular volume, fL | 85.0 ± 14.9 | 81.8 ± 11.7 | 87.6 ± 16.8 |
| Red blood cell distribution width, % | 19.3 ± 5.9 | 19.9 ± 6.2 | 18.8 ± 5.7 |
| Ferritin, μg/L, median (IQR) | 69 (45, 97) | 67 (19, 95) | 69 (46, 124) |
| Associated Factors | 25OHD Concentrations (nmol/L) | ||
|---|---|---|---|
| Beta-Coefficient (95% CI) | Standardized Beta | P | |
| Age, y | −0.003 (−0.05, 0.04) | −0.01 | 0.90 |
| Season (Ref: Jan–Mar) | |||
| Apr–Jun | 10.8 (−3.7, 25.2) | 0.14 | 0.14 |
| Jul–Sep | 27.9 (15.8, 40.1) | 0.41 | <0.001 |
| Oct–Dec | 24.2 (8.3, 40.1) | 0.26 | 0.003 |
| Hemoglobin concentration, g/L | 0.4 (0.1, 0.8) | 0.20 | 0.01 |
| ALP activity, U/L | 0.1 (0.1, 0.2) | 0.17 | 0.03 |
| Receiving prophylaxis antibiotics 2 (Ref: no) | 11.5 (−1.5, 24.4) | 0.14 | 0.08 |
| Constant | −10.1 (−45.5, 25.2) | NA | 0.57 |
| Serum 25OHD Concentrations | ||||
|---|---|---|---|---|
| Season | Deficiency 2 <30 nmol/L | Deficiency <40 nmol/L | Deficiency <50 nmol/L | Insufficiency 3 <75 nmol/L |
| Jan–Mar | 3/47 (6%) | 12/47 (26%) | 17/47 (36%) | 35/47 (74%) |
| Apr–Jun | 1/28 (4%) | 4/28 (14%) | 7/28 (25%) | 19/28 (68%) |
| Jul–Sep | 3/50 (6%) | 6/50 (12%) | 10/50 (20%) | 19/50 (38%) |
| Oct–Dec | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 9/20 (45%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samson, K.L.I.; McCartney, H.; Vercauteren, S.M.; Wu, J.K.; Karakochuk, C.D. Prevalence of Vitamin D Deficiency Varies Widely by Season in Canadian Children and Adolescents with Sickle Cell Disease. J. Clin. Med. 2018, 7, 14. https://doi.org/10.3390/jcm7020014
Samson KLI, McCartney H, Vercauteren SM, Wu JK, Karakochuk CD. Prevalence of Vitamin D Deficiency Varies Widely by Season in Canadian Children and Adolescents with Sickle Cell Disease. Journal of Clinical Medicine. 2018; 7(2):14. https://doi.org/10.3390/jcm7020014
Chicago/Turabian StyleSamson, Kaitlyn L. I., Heather McCartney, Suzanne M. Vercauteren, John K. Wu, and Crystal D. Karakochuk. 2018. "Prevalence of Vitamin D Deficiency Varies Widely by Season in Canadian Children and Adolescents with Sickle Cell Disease" Journal of Clinical Medicine 7, no. 2: 14. https://doi.org/10.3390/jcm7020014





