Influence of Iron Deficiency on HbA1c Levels in Pregnant Women: Comparison with Non-Pregnant Women
Abstract
:1. Introduction
2. Patient and Methods
2.1. Patients
2.2. Laboratory Methods
2.3.Statistical Analyses
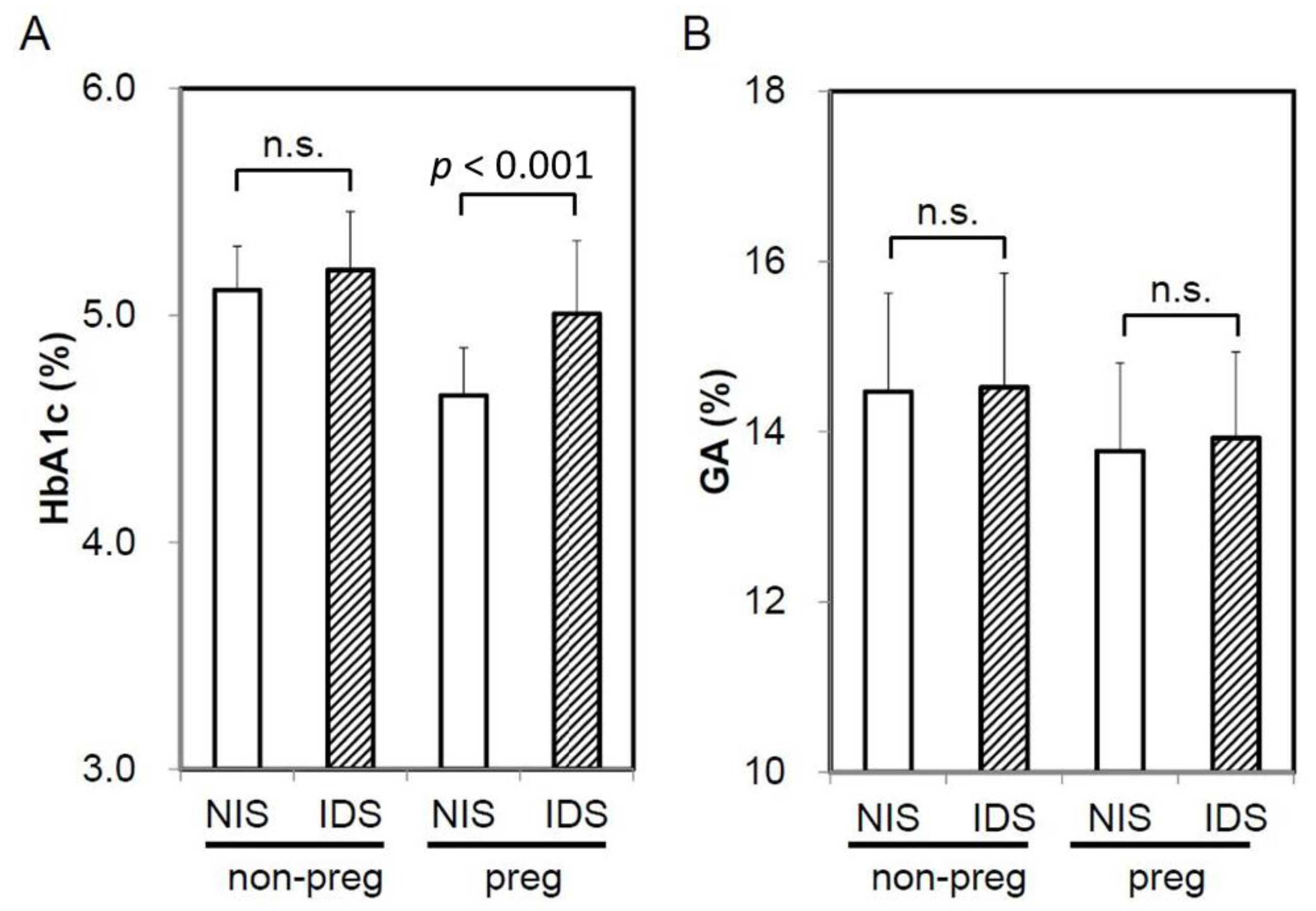
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Glycemic targets. Diabetes Care 2017, 40, S48–S56. [Google Scholar]
- Cohen, M.P.; Herman, W.H. Are glycated serum proteins ready for prime time? Lancet Diabetes Endocrinol. 2014, 2, 265–266. [Google Scholar] [CrossRef]
- Koga, M.; Hashimoto, K.; Murai, J.; Saito, H.; Mukai, M.; Ikegame, K.; Ogawa, H.; Kasayama, S. Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin. Chim. Acta 2011, 412, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Bloomgarder, Z.T.; Le Roith, D. Review of hemoglobin A1c in the management of diabetes. J. Diabetes 2009, 1, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bry, L.; Chen, P.C.; Sacks, D.B. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin. Chem. 2001, 47, 153–163. [Google Scholar] [PubMed]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Saito, H.; Mukai, M.; Matsumoto, S.; Kasayama, S. Influence of iron metabolism indices on glycated haemoglobin but not glycated albumin levels in premenopausal women. Acta Diabetol. 2010, 47, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bullard, K.M.; Herman, W.H.; Beckles, G.L. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 2010, 33, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cowie, C.C.; Li, C.; Handelsman, Y.; Bloomgarden, Z.T. Iron-deficiency anemia, non-iron- deficiency anemia and HbA1c among adults in the US. J. Diabetes 2011, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- English, E.; Idris, I.; Smith, G.; Dhatariya, K.; Kilpatrick, E.S.; John, E.S. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: A systematic review. Diabetologia 2015, 58, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Morita, S.; Saito, H.; Mukai, M.; Kasayama, S. Association of erythrocyte indices with glycated haemoglobin in pre-menopausal women. Diabetic Med. 2007, 24, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Phelps, R.L.; Honig, G.R.; Green, D.; Metzger, B.E.; Frederiksen, M.C.; Freinkel, N. Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am. J. Obstet. Gynecol. 1983, 147, 651–653. [Google Scholar] [CrossRef]
- Hiramatsu, Y.; Shimizu, I.; Omori, Y.; Nakabayashi, M. Determination of reference intervals of glycated albumin and hemoglobin A1c in healthy pregnant Japanese women and analysis of their time courses and influencing factors during pregnancy. Endocr. J. 2012, 59, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Noguchi, S.; Morimoto, Y.; Hamada, S.; Wasada, K.; Imai, S.; Murata, Y.; Kasayama, S.; Koga, M. A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care 2008, 31, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Osugi, T.; Noguchi, S.; Morimoto, Y.; Wasada, K.; Imai, S.; Waguri, M.; Toyoda, R.; Fujita, T.; Kasayama, S.; Koga, M. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care 2010, 33, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Matsubara, M.; Koga, M. Hemoglobin A1c but not glycated albumin overestimates glycemic control due to iron deficiency in pregnant women with diabetes. J. Diabetes Metab. 2014, 5, 445. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Japanese Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol. Int. 2012, 3, 8–10. [Google Scholar]
- Davis, J.E.; McDonald, J.M.; Jarett, L. A high-performance liquid chromatography method for hemoglobin A1C. Diabetes 1978, 27, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kouzuma, T.; Uemastu, Y.; Usami, T.; Imamura, S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin. Chim. Acta 2004, 346, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Temperley, I.J.; Sharp, A.A. The life span of erythrocytes in iron-deficiency anaemia. J. Clin. Pathol. 1962, 15, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Loría, A.; Sánchez-Medal, L.; Lisker, R.; De Rodríguez, E.; Labardini, J. Red cell life span in iron deficiency anaemia. Br. J. Haematol. 1967, 13, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Okumiya, T.; Saibara, T.; Tsubosaki, E.; Matsumura, H.; Park, K.; Sugimoto, K.; Kageoka, T.; Sasaki, M. An enzymatic assay for erythrocyte creatine as an index of the erythrocyte life time. Clin. Biochem. 1998, 31, 59–65. [Google Scholar] [CrossRef]
- Shimizu, I.; Hiramatsu, Y.; Omori, Y.; Nakabayashi, M. Glycated albumin reflects maternal and perinatal outcome in a multicenter study in Japan. Diabetes Preg. 2010, 10, 27–31. [Google Scholar]
- Sugawara, D.; Maruyama, A.; Imanishi, T.; Sugiyama, Y.; Ichihashi, K. Complications in infants of diabetic mothers related to glycated albumin and hemoglobin levels during pregnancy. Pediatr. Neonatol. 2016, 57, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Amstad, B.G.; Krafft, A.; Zimmermann, R.; Burkhardt, T. Treatment of anemia of chronic disease with true iron deficiency in pregnancy. J. Pregnancy 2017, 2017, 4265091. [Google Scholar]

| Non-Pregnant Women | Pregnant Women | P Value | |
|---|---|---|---|
| n | 42 | 42 | - |
| Age (years) | 31.6 ± 4.0 | 29.9 ± 5.9 | 0.120 |
| Pregnancy (week) | - | 27.5 ± 5.1 | - |
| RBC (×104/μL) | 429 ± 26 | 361 ± 21 | <0.0001 |
| Hb (g/dL) | 12.6 ± 1.3 | 10.8 ± 0.7 | <0.0001 |
| Ht (%) | 37.0 ± 3.3 | 33.8 ± 2.0 | <0.0001 |
| MCH (pg) | 29.4 ± 2.7 | 30.0 ± 2.1 | 0.274 |
| Tf saturation (%) | 31.2 ± 19.0 | 18.4 ± 11.8 | <0.001 |
| Ferritin (ng/mL) | 29.6 ± 26.0 | 11.4 ± 11.1 | <0.0001 |
| IDS (%) | 14 (34.1) | 27 (64.3) | 0.004 |
| HbA1c (%) | 5.14 ± 0.22 | 4.88 ± 0.33 | <0.0001 |
| Glycated albumin (%) | 14.5 ± 1.2 | 13.9 ± 1.0 | 0.013 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Koga, M. Influence of Iron Deficiency on HbA1c Levels in Pregnant Women: Comparison with Non-Pregnant Women. J. Clin. Med. 2018, 7, 34. https://doi.org/10.3390/jcm7020034
Hashimoto K, Koga M. Influence of Iron Deficiency on HbA1c Levels in Pregnant Women: Comparison with Non-Pregnant Women. Journal of Clinical Medicine. 2018; 7(2):34. https://doi.org/10.3390/jcm7020034
Chicago/Turabian StyleHashimoto, Kunihiko, and Masafumi Koga. 2018. "Influence of Iron Deficiency on HbA1c Levels in Pregnant Women: Comparison with Non-Pregnant Women" Journal of Clinical Medicine 7, no. 2: 34. https://doi.org/10.3390/jcm7020034




