Blood Mononuclear Cell Mitochondrial Respiratory Chain Complex IV Activity is Decreased in Multiple Sclerosis Patients: Effects of β-Interferon Treatment
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Patients
2.3. Blood Mononuclear Cell (BMNC) Preparation
2.4. Spectrophotometric Enzyme Assays
2.5. Protein Determination
2.6. Statistical Analysis
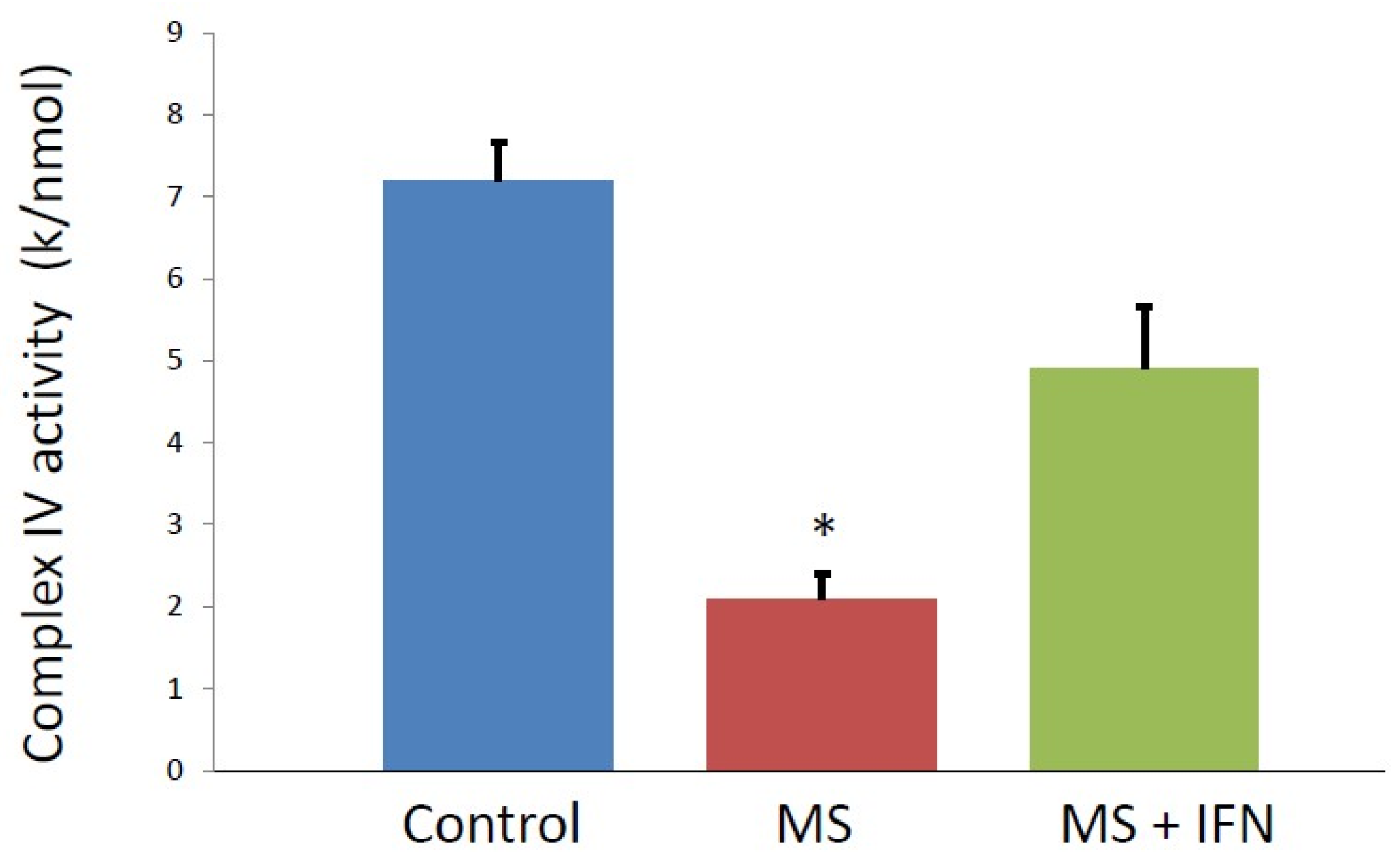
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cannella, B.; Raine, C.S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Keegan, B.M.; Noseworthy, J.H. Multiple sclerosis. Annu. Rev. Med. 2002, 53, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Wegner, C.; Bruck, W. Inflammation, demyelination, and degeneration—Recent insights from MS pathology. Biochim. Biophys. Acta 2011, 1812, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis: Where do we stand? Continuum (Minneap. Minn.) 2013, 19, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Is multiple sclerosis a mitochondrial disease? Biochim. Biophys. Acta 2010, 1802, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M.; Panegyres, P.K. Cognitive impairment in multiple sclerosis: Evidence based analysis and recommendations. J. Clin. Neurosci. 2007, 14, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Selak, M.; O’Connor, J.; Croul, S.; Lorenzana, C.; Butunoi, C.; Kalman, B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 2000, 177, 95–103. [Google Scholar] [CrossRef]
- Dutta, R.; McDonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Mastrolia, V.; Rezaei Haddad, A.; Mosley, A.; Mullali, G.; Schiza, D.; Sajic, M.; Hargreaves, I.; Heales, S.; Duchen, M.R.; et al. Mitochondrial dysfunction is an important cause of neurological deficits in an inflammatory model of multiple sclerosis. Sci Rep. 2016, 6, 33249. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Heales, S.J.; Land, J.M.; Thompson, E.J. The potential role of nitric oxide in multiple sclerosis. Mult. Scler. 1998, 4, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Bö, L.; Dawson, T.M.; Wesselingh, S.; Mörk, S.; Choi, S.; Kong, P.A.; Hanley, D.; Trapp, B.D. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann. Neurol. 1994, 36, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Jersild, C.; Fog, T.; Hansen, G.S.; Thomsen, M.; Svejgaard, A.; Dupont, B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet 1993, 2, 1121–1125. [Google Scholar] [CrossRef]
- Johnson, A.W.; Land, J.M.; Thompson, E.J.; Bolaños, J.P.; Clark, J.B.; Heales, S.J. Evidence for increased nitric oxide production in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1995, 58, 107. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, N.; Adamczyk-Sowa, M.; Niedziela, J.T. Assessment of serum nitrogen species and inflammatory parameters in relapsing remitting multiple sclerosis patients treated with different therapeutic approaches. Biomed. Res. Int. 2016, 2016, 4570351. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Maruyama, W.; Kato, Y.; Yi, H.; Shamoto-Nagai, M.; Tanaka, M.; Sato, Y.; Naoi, M. Selective nitration of mitochondrial complex I by peroxynitrite: Involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J. Neural Transm. 2002, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Heales, S.J.; Silver, N.C.; O’Riordan, J.; Miller, R.F.; Land, J.M.; Clark, J.B.; Thompson, E.J. Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J. Neurol. Sci. 1997, 145, 77–81. [Google Scholar] [CrossRef]
- Kumleh, H.H.; Riazi, G.H.; Houshmand, M.; Sanati, M.H.; Gharagozli, K.; Shafa, M. Complex I deficiency in Persian multiple sclerosis patients. J. Neurol. Sci. 2006, 243, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V.C.; Sharpe, M.A.; Clark, J.B.; Heales, S.J. Astrocyte-derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. J. Neurochem. 2000, 7592, 694–700. [Google Scholar] [CrossRef]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V.C.; Land, J.M.; Clark, J.B.; Heales, S.J.R. Pretreatment of astrocytes with interferon α/β prevents mitochondrial respiratory chain damage. J. Neurochem. 1998, 70, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, G.R.; Reeve, A.K.; Ziabreva, I.; Reynolds, R.; Turnbull, D.M.; Mahad, D.J. No excess of mitochondrial DNA deletions within muscle of progressive multiple sclerosis. Mult. Scler. 2013, 19, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorini, A.M.; Nociti, V.; Petzold, A.; Gasperini, C.; Quartuccio, E.; Lazzarino, G.; Di Pietro, V.; Belli, A.; Signoretti, S.; Vagnozzi, R.; et al. Serum lactate as a novel biomarker in multiple sclerosis. Biochim. Biophys. Acta 2014, 1842, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Steiniger, J.; Bock, M.; Brandt, A.U.; Haas, V.; Boschmann, M.; Paul, F. Is metabolic flexibility altered in multiple sclerosis patients? PLoS ONE 2012, 7, e43675. [Google Scholar] [CrossRef] [PubMed]
- Fonalledas Perelló, M.A.; Politi, J.V.; Dallo Lizarraga, M.A.; Cardona, R.S. The cerebrospinal fluid lactate is decreased in early stages of multiple sclerosis. P. R. Health Sci. J. 2008, 27, 171–174. [Google Scholar] [PubMed]
- Koenig, M.K. Presentation and diagnosis of mitochondrial disorders in children. Pediatr. Neurol. 2008, 38, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.P.; Sheena, Y.; Land, J.M.; Heales, S.J. Glutathione deficiency in patients with mitochondrial disease: Implications for pathogenesis and treatment. J. Inherit. Metab. Dis. 2005, 28, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, J.P.; Peuchen, S.; Heales, S.J. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J. Neurochem. 1994, 63, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Vadnal, J.; Houston, S.; Freeman, E.; McDonough, J. Impaired regulation of electron transport chain subunit genes by nuclear respiratory factor 2 in multiple sclerosis. J. Neurol. Sci. 2009, 279, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargreaves, I.; Mody, N.; Land, J.; Heales, S. Blood Mononuclear Cell Mitochondrial Respiratory Chain Complex IV Activity is Decreased in Multiple Sclerosis Patients: Effects of β-Interferon Treatment. J. Clin. Med. 2018, 7, 36. https://doi.org/10.3390/jcm7020036
Hargreaves I, Mody N, Land J, Heales S. Blood Mononuclear Cell Mitochondrial Respiratory Chain Complex IV Activity is Decreased in Multiple Sclerosis Patients: Effects of β-Interferon Treatment. Journal of Clinical Medicine. 2018; 7(2):36. https://doi.org/10.3390/jcm7020036
Chicago/Turabian StyleHargreaves, Iain, Nimesh Mody, John Land, and Simon Heales. 2018. "Blood Mononuclear Cell Mitochondrial Respiratory Chain Complex IV Activity is Decreased in Multiple Sclerosis Patients: Effects of β-Interferon Treatment" Journal of Clinical Medicine 7, no. 2: 36. https://doi.org/10.3390/jcm7020036




