Modeling Water Clarity and Light Quality in Oceans
Abstract
:1. Introduction
2. Methods
2.1. Overall Light Attenuation
2.1.1. Light Absorption by Pure Seawater, aw
2.1.2. Light Absorption by Algal Pigment, ac
2.1.3. Light Absorption by NAPs, as
2.1.4. Light Absorption by CDOM, ag
2.1.5. Light Backscattering by Phytoplankton, bp
2.1.6. Light Backscattering by NAPs (Detritus, Minerals and Others), bs
2.1.7. Light Backscattering by Pure Seawater, bw
2.2. Data
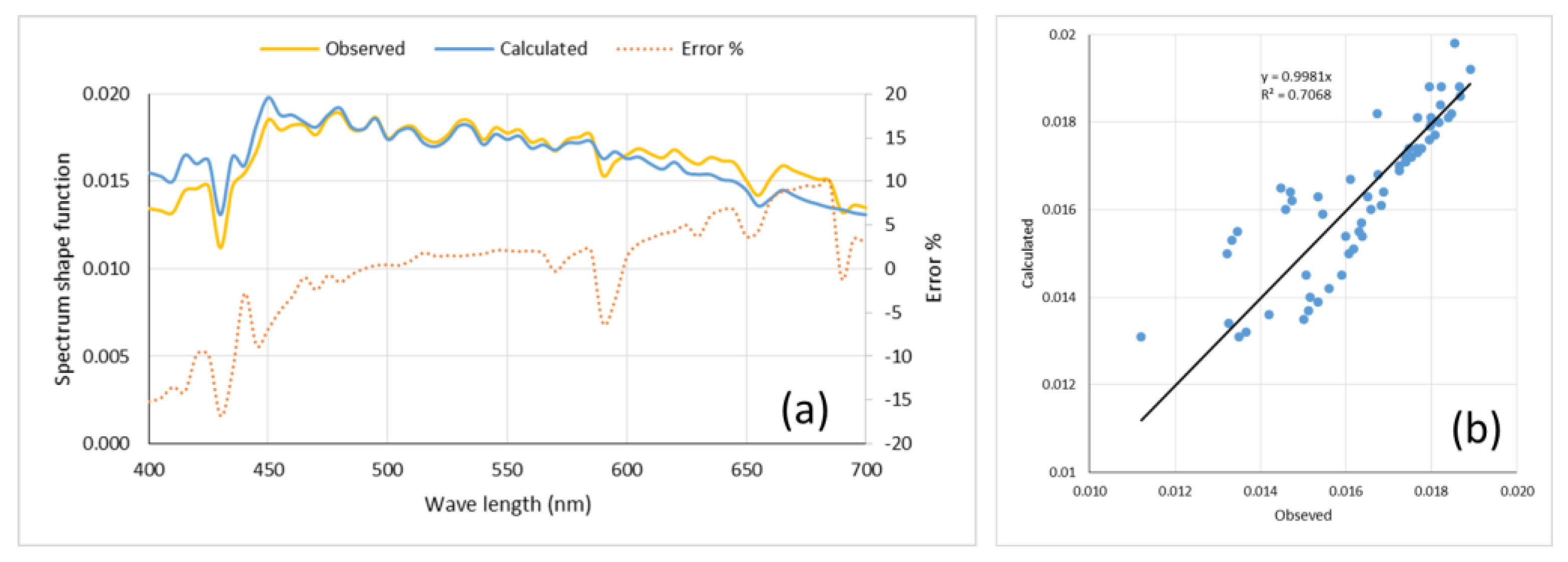
2.2.1. The Spectrum Shape Function, f
2.2.2. The Summer Dataset
2.2.3. The Fall Data Set
2.3. Calibration and Validation
2.3.1. Calibration with Fall Data
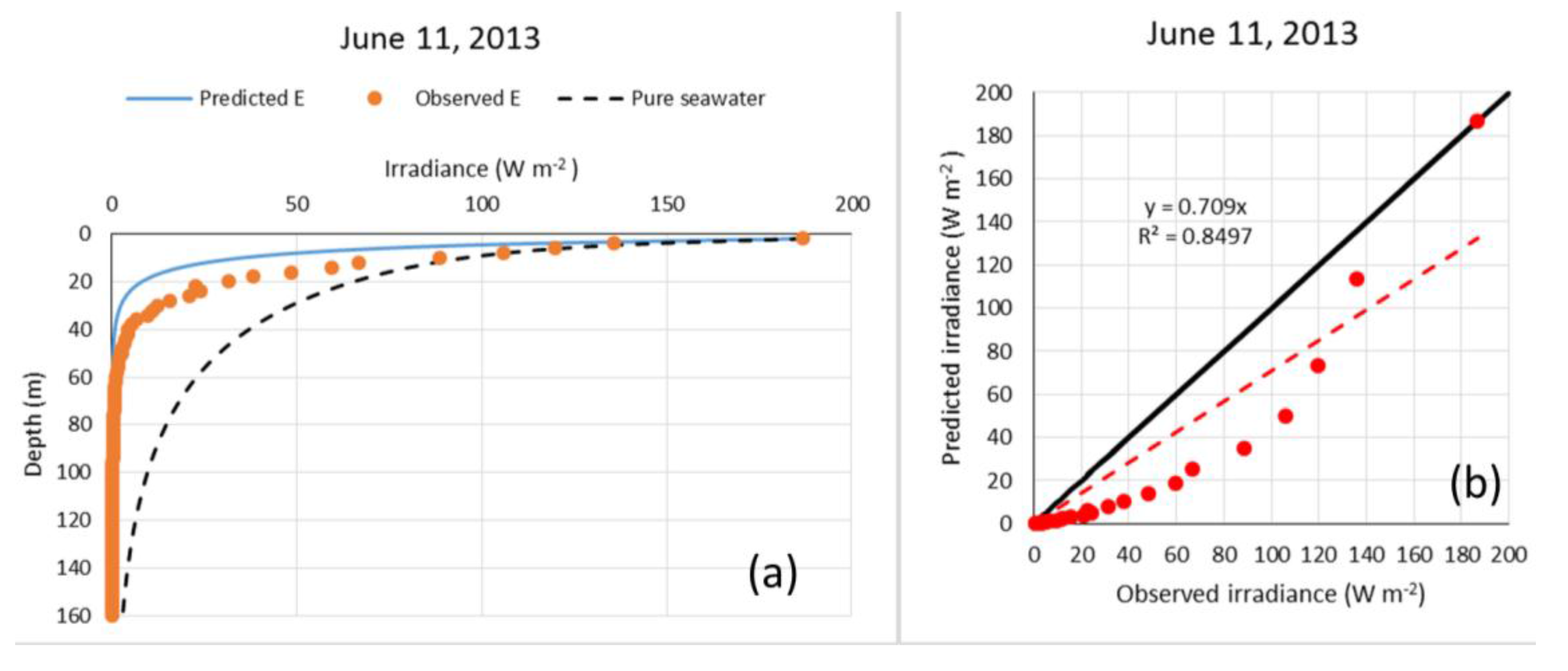
2.3.2. Validation with Summer Data
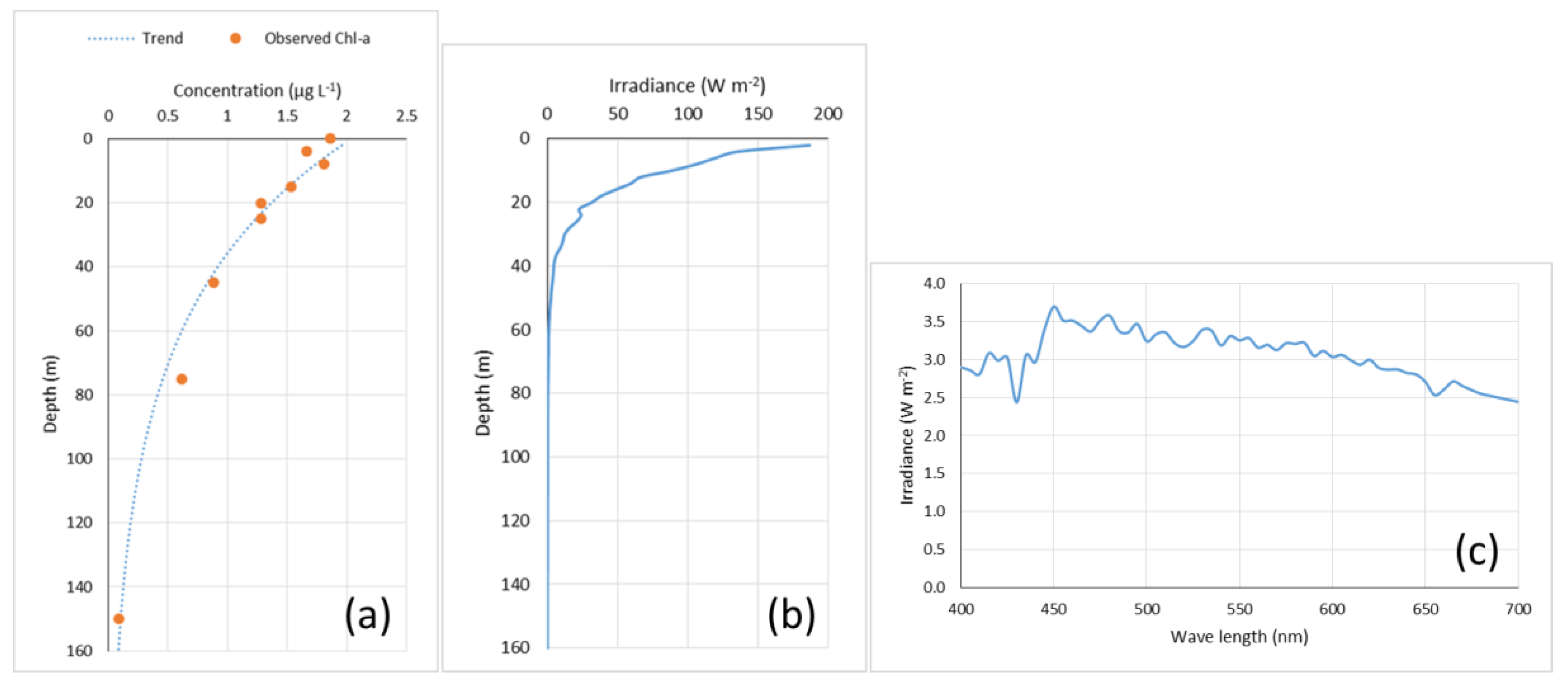
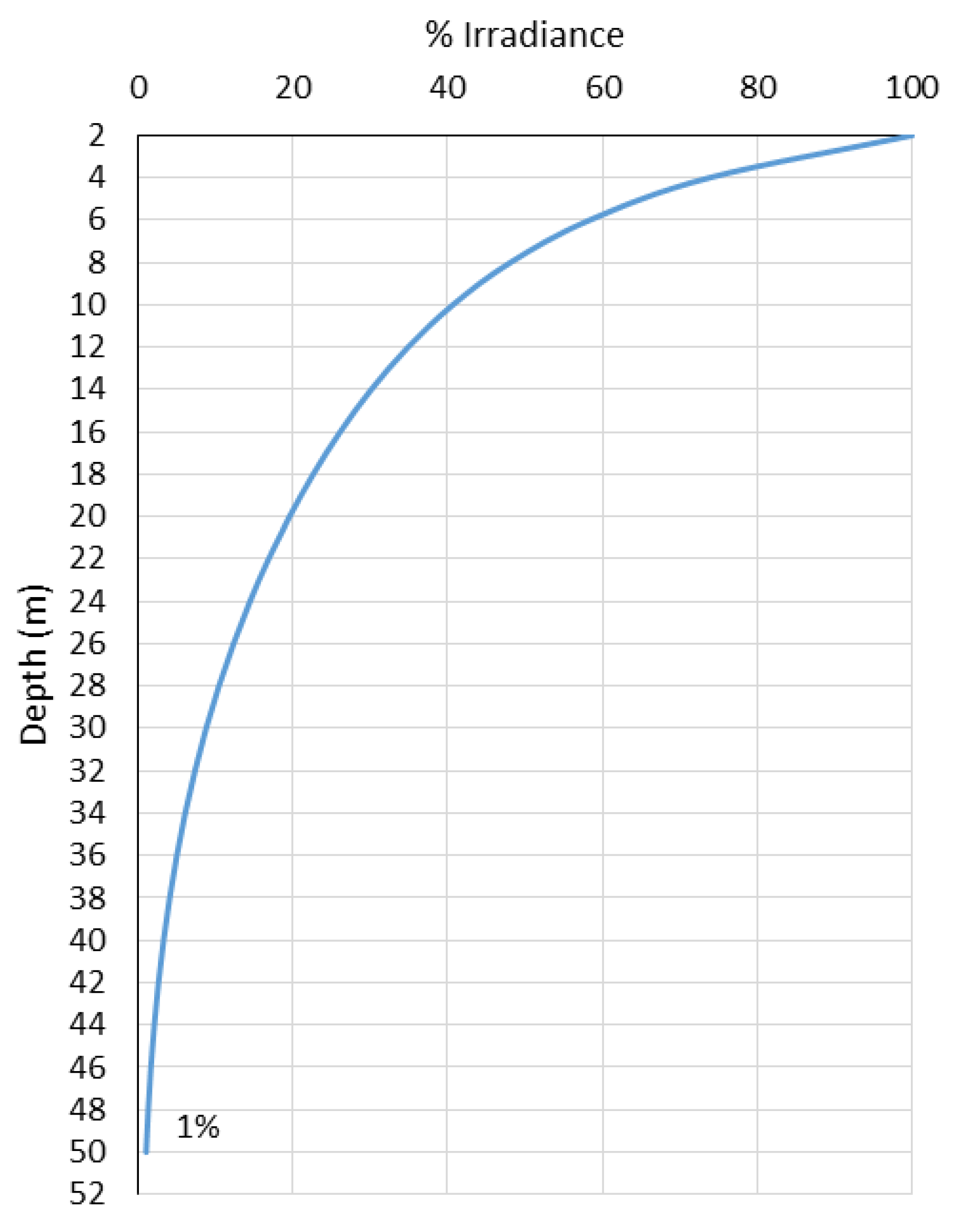
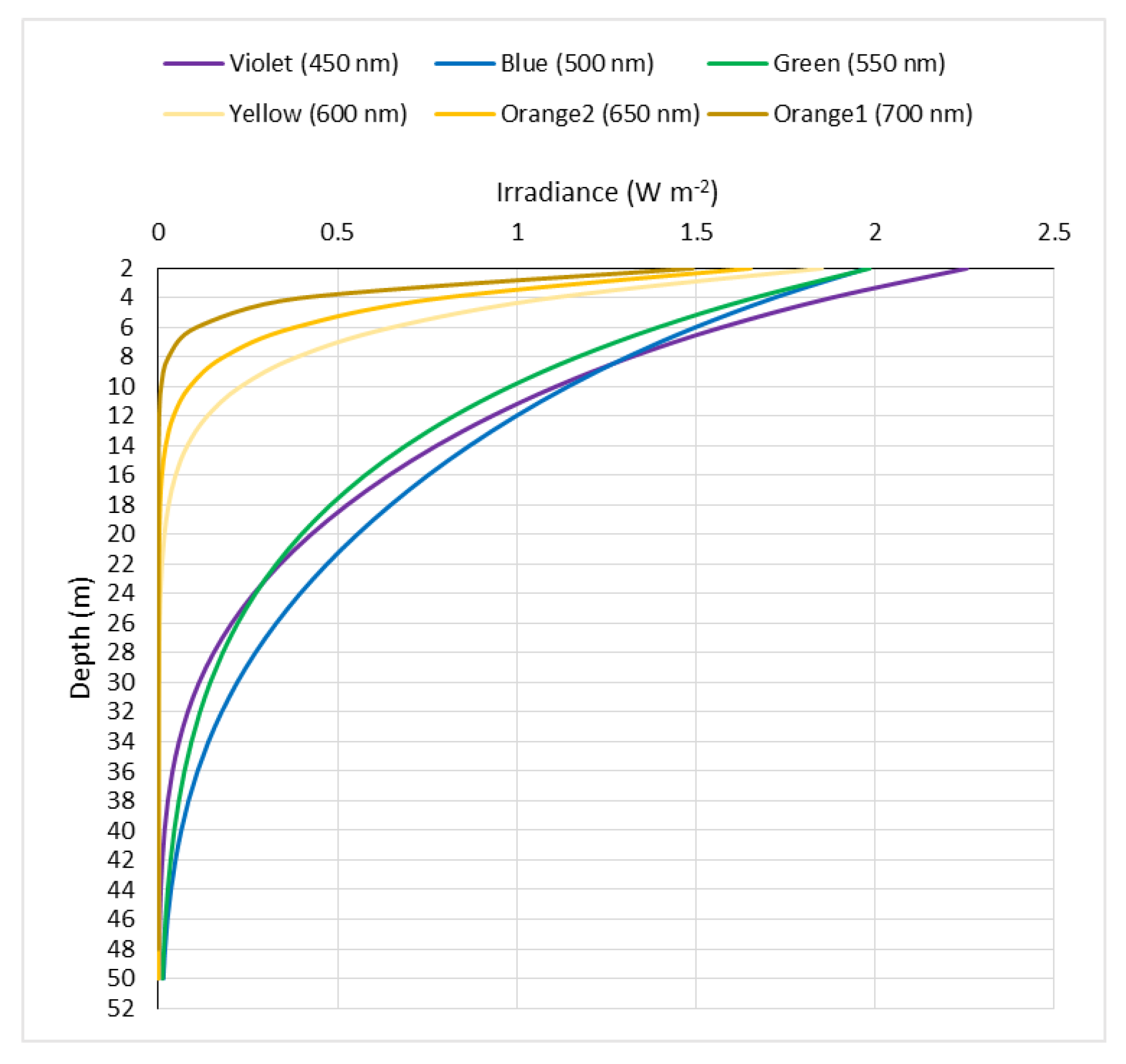
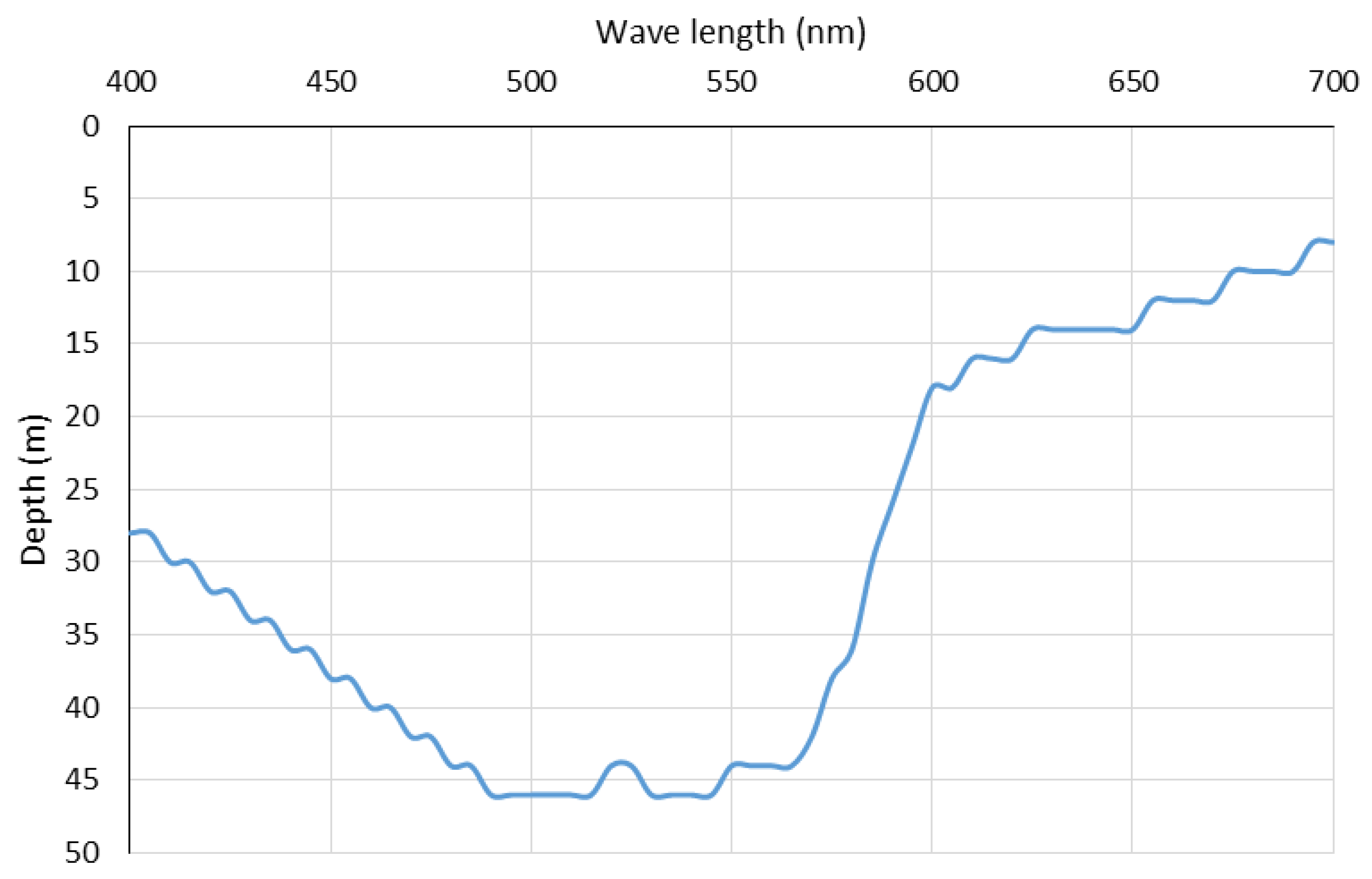
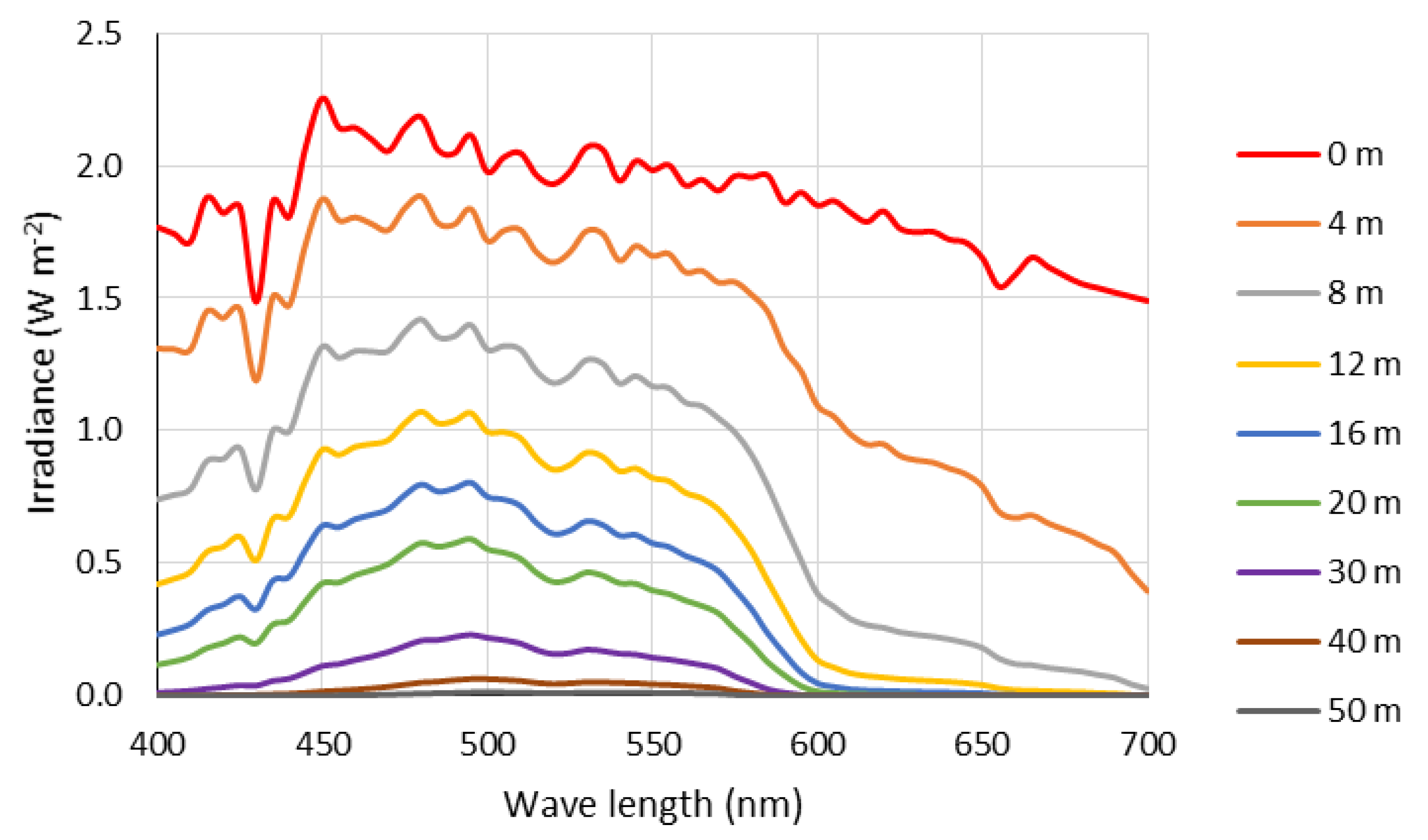
3. Results
4. Discussion
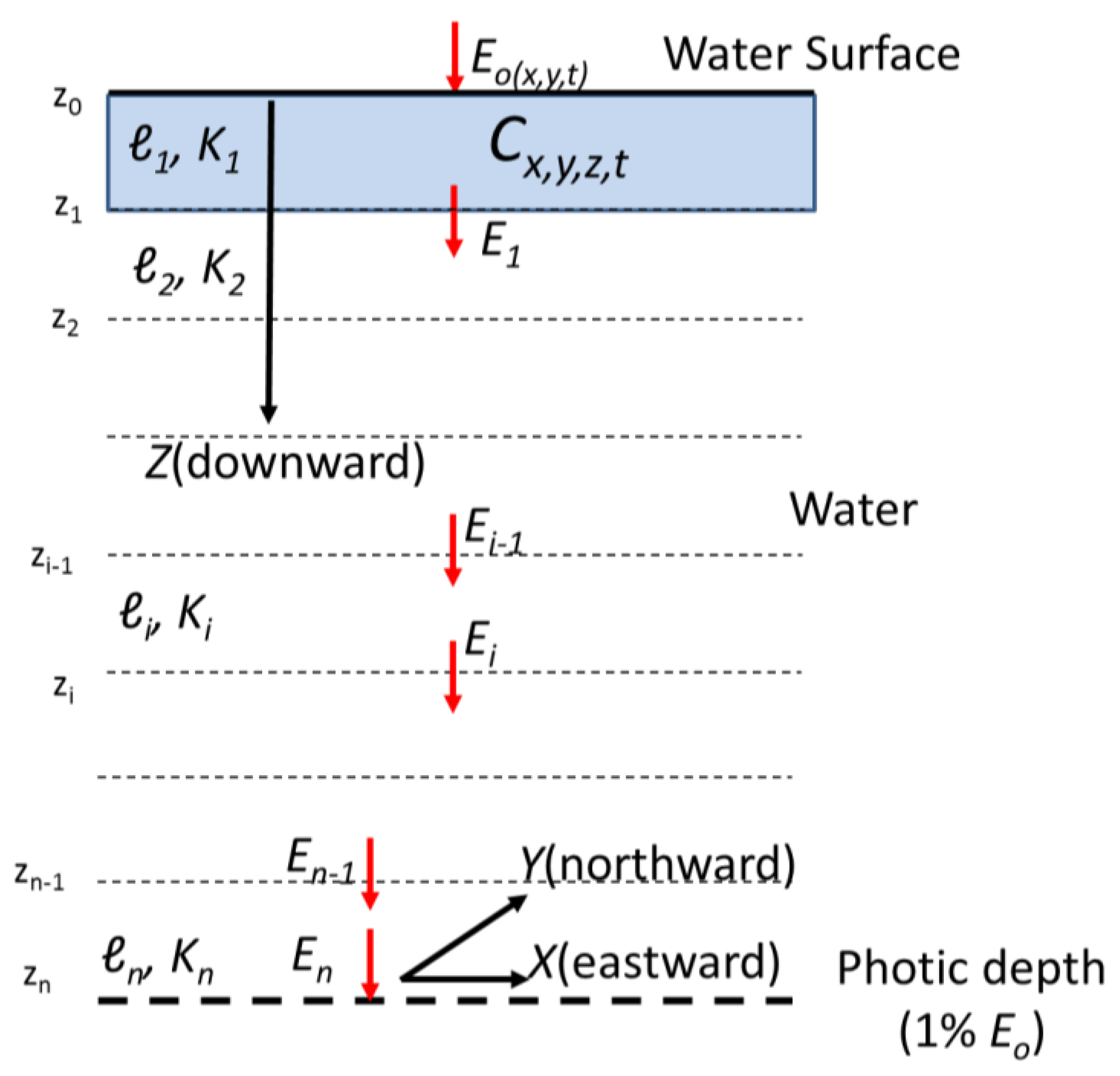
- At time t
- At location x,y
- For layer ℓi
- The numerical model provides Cx,y,z,t
- Calculate ac(440) (Equation (9))
- For every wavelength, calculate: ac(λ), as(λ), ag(λ), bp(λ), bs(λ) (Equations (8), (10), (13), (16) and (19), respectively)
- Calculate the spectral attenuation coefficient, Ki(λ), for each λ (Equation (4))
- Calculate attenuated irradiance for each λ, Ei(λ) (Equation (5))
- Calculate total irradiance, Ei, by numerical integration over all λ (Equation (6))
- Move to the next layer down (i + 1) and repeat Steps 4–9
- Move to next location and repeat Steps 3–10
- For each layer, use Ei in the calculation of Cx,y,z,t+Δt, which fully couples light and phytoplankton at the next time level (t + Δt).
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Peterson, D.H.; Festa, J.F. Numerical simulation of phytoplankton productivity in partially mixed estuaries. Estuar. Coast. Shelf Sci. 1984, 19, 563–589. [Google Scholar] [CrossRef]
- Topliss, B.J. Optical monitoring of coastal waters: Photic depth estimates. Mar. Environ. Res. 1982, 7, 295–308. [Google Scholar] [CrossRef]
- Ocean Color Algorithm Working Group. Models, Parameters and Approaches That Are Used to Generate Wide Range of Absorption and Backscattering Spectra. International Ocean Color Coordinating Group, Ocean Color Algorithm Working Group. 2003, p. 11. Available online: http://www.ioccg.org/groups/lee_data.pdf (accessed on 30 July 2015).
- Gallegos, C.L.; Kenworthy, W.J. Seagrass depth limits in the Indian River Lagoon (Florida, USA): Application of an optical water quality model. Estuar. Coast. Shelf Sci. 1996, 42, 267–288. [Google Scholar] [CrossRef]
- Gallegos, C.L. Calculating optical water quality targets to restore and protect submerged aquatic vegetation: Overcoming problems in partitioning the diffuse attenuation coefficient for photosynthetically active radiation. Estuaries 2001, 24, 381–397. [Google Scholar] [CrossRef]
- Gallegos, C.L.; Neal, P.J. Partitioning spectral absorption in case 2 waters: Discrimination of dissolved and particulate components. Appl. Opt. 2002, 41, 4220–4233. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.J.; Yoder, J.A.; Freeman, S.A. Spatial and temporal distribution of colored dissolved organic matter (CDOM) in Narragansett Bay, Rhode Island: Implications for phytoplankton in coastal waters. Estuar. Coast. Shelf Sci. 2002, 55, 705–717. [Google Scholar] [CrossRef]
- Kenworthy, W.J.; Gallegos, C.L.; Costello, C.; Field, D.; di Carlo, G. Dependence of eelgrass (Zostera marina) light requirements on sediment organic matter in Massachusetts coastal bays: Implications for remediation and restoration. Mar. Pollut. Bull. 2014, 83, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Thursby, G.; Rego, S.; Keith, D. Data Report for Calibration of Bio-Optical Model for Narragansett Bay; United States Environmental Protection Agency Report Tracking Number EPA/600/R-15/211; U.S. Environmental Protection Agency: Washington, DC, USA, 2015; p. 25. Available online: https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=525010 (Accessed on 11 November 2016).
- Abdelrhman, M.A. Quantifying contributions to light attenuation in estuaries and coastal embayments: Application to Narragansett Bay, Rhode Island. Estuar. Coasts, 13 April 2016; under review. [Google Scholar]
- Morel, A. Optical modeling of the upper ocean in relation to its biogenous matter content (Case I Waters). J. Geophys. Res. 1988, 93, 10749–10768. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T. The spectral irradiance field at the surface and in the interior of the ocean: A model for applications in oceanography. J. Geophys. Res. 1988, 93, 9270–9280. [Google Scholar] [CrossRef]
- Prieur, L.; Sathyendranath, S. An optical classification of coastal and oceanic waters based on the specific spectral absorption curves of phytoplankton pigment, dissolved organic matter, and other particulate materials. Limnol. Oceanogr. 1981, 26, 671–689. [Google Scholar] [CrossRef]
- Jerlov, N.G. Marine Optics. In Elsevier Oceanography Series 14; Elsevier: New York, NY, USA, 1976; p. 231. [Google Scholar]
- Sathyendranath, S. Inherent optical properties of natural seawater. Defense Sci. J. 1984, 34, 1–18. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
- Fischer, J.; Fell, F. Simulation of MERIS measurements above selected ocean waters. Int. J. Remote Sens. 1999, 20, 1787–1807. [Google Scholar] [CrossRef]
- Gallegos, C.L. Refining habitat requirements of submersed aquatic vegetation: Role of optical models. Estuaries 1994, 17, 187–199. [Google Scholar] [CrossRef]
- Bricaud, A.; Stramski, D. Spectral absorption coefficients of living phytoplankton and nonalgal biogenous matter: A comparison between the Peru upwelling area and the Sargasso Sea. Limnol. Oceanogr. 1990, 35, 562–582. [Google Scholar] [CrossRef]
- Matsuoka, A.; Hill, V.; Hout, Y.; Babin, M.; Bricaud, A. Seasonal variability in the light absorption properties of western Arctic waters: Parameterization of individual components of absorption for ocean color applications. J. Geophys. Res. 2011, 116, 1–15. [Google Scholar] [CrossRef]
- Fournier, G.; Forand, J.L. Analytic phase function for ocean water. In Ocean Optics XII, Proceedings of SPIE—The International Society for Optical Engineering XII, Bergen, Norway, 13–15 June 1994; Jaffe, J.S., Ed.; Society of Photo-optical Instrumentation Engineers: Bellingham, WA, USA, 1994; Volume 2258, pp. 194–201. [Google Scholar]
- Morel, A.; Gentili, B. Diffuse reflectance of oceanic waters: Its dependence on sun angle as influenced by molecular scattering contribution. Appl. Opt. 1991, 30, 4427–4438. [Google Scholar] [CrossRef] [PubMed]
- Buiteveld, H.; Hakvoort, J.H.M.; Donze, M. The optical properties of pure water. In Ocean Optics XII, Proceedings of SPIE—The International Society for Optical Engineering XII, Bergen, Norway 13–15 June 1994; Jaffe, J.S., Ed.; Society of Photo-optical Instrumentation Engineers: Bellingham, WA, USA, 1994; Volume 2258, pp. 174–183. [Google Scholar]
- Hemsley, V.S.; University of Southampton, Southampton, UK. Personal communication, 2016.
- Hemsley, V.S.; Smyth, T.J.; Martin, A.P.; Williams, E.F.; Thompson, A.F.; Damerell, G.; Painter, S.C. Estimating oceanic primary production using vertical irradiance and chlorophyll profiles from ocean gliders in the North Atlantic. Environ. Sci. Technol. 2015, 49, 11612–11621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stomp, M.; Huisman, J.; de Jongh, F.; Veraart, A.J.; Gerla, D.; Rijkeboer, M.; Ibelings, B.W.; Wollenzien, U.I.A.; Stal, L.J. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 2004, 432, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Xiu, P.; Chai, F. Connections between physical, optical, and biogeochemical processes in the Pacific Ocean. Prog. Oceanogr. 2014, 122, 30–53. [Google Scholar] [CrossRef]


| Symbol | Definition | Dimension |
|---|---|---|
| Abbreviations | ||
| CDOM | Colored dissolved organic material | |
| chl-a | Chlorophyll a | |
| IOCCG | International Ocean Color Coordination Group | |
| NAPs | Non-algal particles | |
| PAR | Photosynthetically-available radiation | |
| TSS | Total suspended solids | |
| Parameters | ||
| aw | Absorption coefficient of seawater | m−1 |
| ac | Absorption coefficient of chl-a | m−1 |
| as | Absorption coefficient of NAPs | m−1 |
| ag | Absorption coefficient of CDOM | m−1 |
| bw | Backscattering a coefficient of seawater | m−1 |
| bp | Backscattering a coefficient of phytoplankton particles | m−1 |
| bs | Backscattering a coefficient of NAPs | m−1 |
| C | chl-a concentration | μg·L−1 |
| E0 | Irradiance at the water surface | W·m−2 |
| Ei, Eℓ | Irradiance at the bottom of the i-th layer, ℓi | W·m−2 |
| e | Exponentiation base (e = 2.718281828459) | dimensionless |
| f | Spectrum distribution function | dimensionless |
| g | CDOM concentration (not implemented) | μg·L−1 |
| K | Downwelling a extinction coefficient | m−1 |
| ℓi | Layer thickness (zi − zi−1) | m |
| P3,4 | Calibration coefficients | dimensionless |
| R1,2,3,4 | Calibration coefficients | dimensionless |
| RRi(λj) | Reduction ratio of wavelength λj at the i-th layer | dimensionless |
| S | NAPs concentration | mg·L−1 |
| SS | Spectral slope | nm−1 |
| λ | Wavelength | nm |
| Subscripts | ||
| i | Counter for the water layer, | |
| j | Counter for the wavelength λ | |
| t | Time | |
| x | Eastward spatial location (see Figure 1) | |
| y | Northward spatial location (see Figure 1) | |
| z | Vertical spatial location below the water surface (see Figure 1) | |
| Superscripts | ||
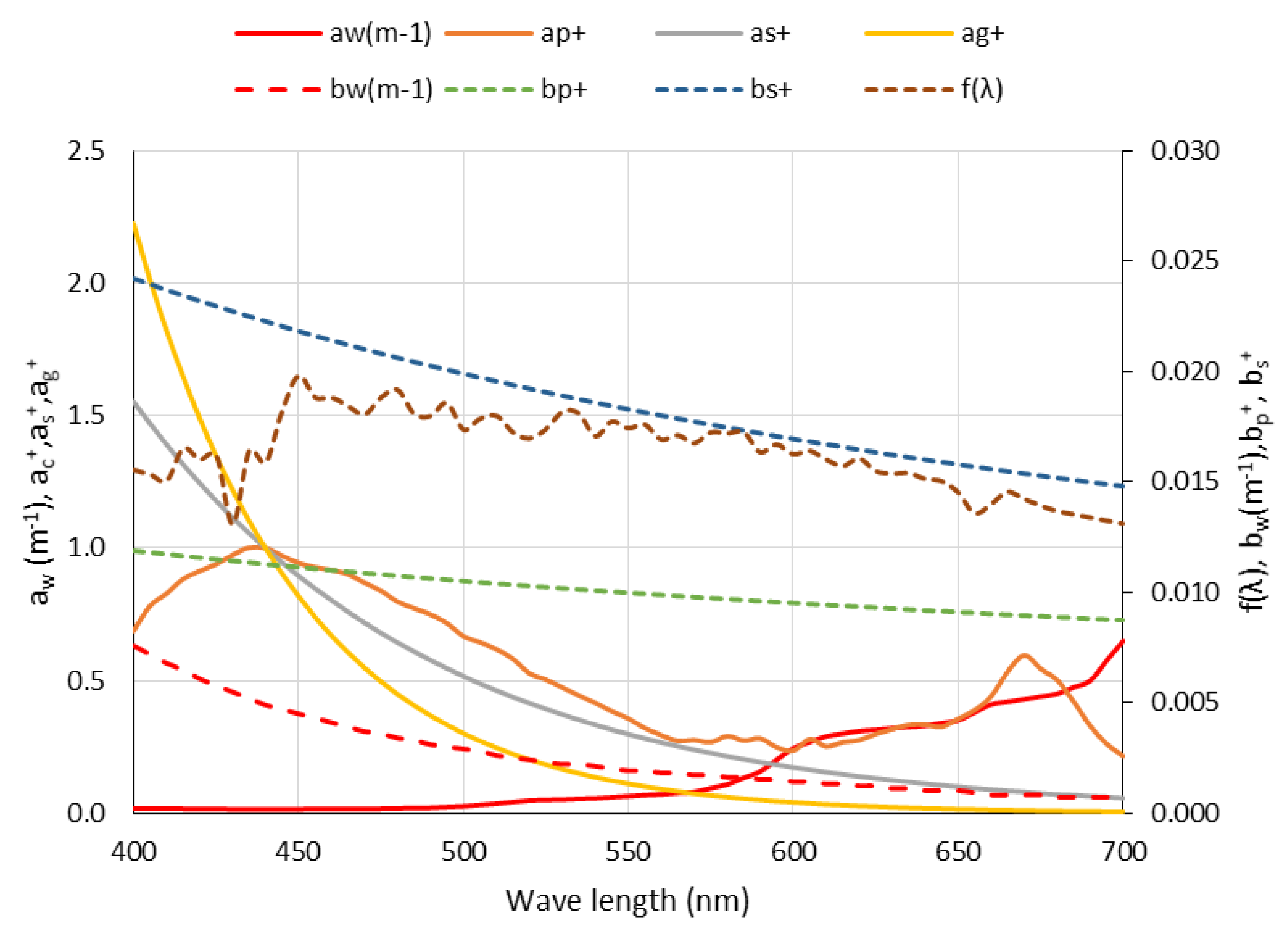
| + | Normalized value (see Table 2 and Figure 2) | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| i | λi | aw (m−1) | bw (m−1) | f(λ) | |||||
| 1 | 400 | 0.0180 | 0.6870 | 1.5530 | 2.2260 | 0.0076 | 1.1890 | 1.3250 | 0.0155 |
| 2 | 405 | 0.0180 | 0.7810 | 1.4700 | 2.0140 | 0.0072 | 1.1810 | 1.3100 | 0.0153 |
| 3 | 410 | 0.0170 | 0.8280 | 1.3910 | 1.8220 | 0.0068 | 1.1730 | 1.2960 | 0.0150 |
| 4 | 415 | 0.0170 | 0.8830 | 1.3170 | 1.6490 | 0.0065 | 1.1650 | 1.2820 | 0.0165 |
| 5 | 420 | 0.0160 | 0.9130 | 1.2460 | 1.4920 | 0.0061 | 1.1580 | 1.2690 | 0.0160 |
| 6 | 425 | 0.0160 | 0.9390 | 1.1790 | 1.3500 | 0.0058 | 1.1500 | 1.2560 | 0.0162 |
| 7 | 430 | 0.0150 | 0.9730 | 1.1160 | 1.2210 | 0.0055 | 1.1430 | 1.2430 | 0.0131 |
| 8 | 435 | 0.0150 | 1.0010 | 1.0570 | 1.1050 | 0.0052 | 1.1360 | 1.2300 | 0.0164 |
| 9 | 440 | 0.0150 | 1.0000 | 1.0000 | 1.0000 | 0.0049 | 1.1290 | 1.2180 | 0.0159 |
| 10 | 445 | 0.0150 | 0.9710 | 0.9460 | 0.9050 | 0.0047 | 1.1220 | 1.2060 | 0.0182 |
| 11 | 450 | 0.0150 | 0.9440 | 0.8960 | 0.8190 | 0.0045 | 1.1150 | 1.1940 | 0.0198 |
| 12 | 455 | 0.0160 | 0.9280 | 0.8480 | 0.7410 | 0.0043 | 1.1080 | 1.1820 | 0.0188 |
| 13 | 460 | 0.0160 | 0.9170 | 0.8030 | 0.6700 | 0.0041 | 1.1020 | 1.1710 | 0.0188 |
| 14 | 465 | 0.0160 | 0.9020 | 0.7600 | 0.6070 | 0.0039 | 1.0950 | 1.1600 | 0.0184 |
| 15 | 470 | 0.0160 | 0.8700 | 0.7190 | 0.5490 | 0.0037 | 1.0890 | 1.1490 | 0.0181 |
| 16 | 475 | 0.0170 | 0.8390 | 0.6800 | 0.4970 | 0.0036 | 1.0830 | 1.1380 | 0.0188 |
| 17 | 480 | 0.0180 | 0.7980 | 0.6440 | 0.4490 | 0.0034 | 1.0770 | 1.1280 | 0.0192 |
| 18 | 485 | 0.0190 | 0.7730 | 0.6100 | 0.4070 | 0.0033 | 1.0710 | 1.1170 | 0.0181 |
| 19 | 490 | 0.0200 | 0.7500 | 0.5770 | 0.3680 | 0.0031 | 1.0650 | 1.1070 | 0.0180 |
| 20 | 495 | 0.0230 | 0.7170 | 0.5460 | 0.3330 | 0.0030 | 1.0590 | 1.0970 | 0.0186 |
| 21 | 500 | 0.0260 | 0.6680 | 0.5170 | 0.3010 | 0.0029 | 1.0530 | 1.0880 | 0.0174 |
| 22 | 505 | 0.0310 | 0.6450 | 0.4890 | 0.2730 | 0.0028 | 1.0470 | 1.0780 | 0.0179 |
| 23 | 510 | 0.0360 | 0.6180 | 0.4630 | 0.2470 | 0.0026 | 1.0420 | 1.0690 | 0.0180 |
| 24 | 515 | 0.0420 | 0.5820 | 0.4380 | 0.2230 | 0.0025 | 1.0360 | 1.0600 | 0.0172 |
| 25 | 520 | 0.0480 | 0.5280 | 0.4150 | 0.2020 | 0.0024 | 1.0310 | 1.0510 | 0.0170 |
| 26 | 525 | 0.0500 | 0.5040 | 0.3930 | 0.1830 | 0.0023 | 1.0260 | 1.0420 | 0.0174 |
| 27 | 530 | 0.0510 | 0.4740 | 0.3720 | 0.1650 | 0.0022 | 1.0200 | 1.0330 | 0.0182 |
| 28 | 535 | 0.0540 | 0.4440 | 0.3520 | 0.1500 | 0.0022 | 1.0150 | 1.0250 | 0.0181 |
| 29 | 540 | 0.0560 | 0.4160 | 0.3330 | 0.1350 | 0.0021 | 1.0100 | 1.0160 | 0.0171 |
| 30 | 545 | 0.0600 | 0.3840 | 0.3150 | 0.1220 | 0.0020 | 1.0050 | 1.0080 | 0.0177 |
| 31 | 550 | 0.0640 | 0.3570 | 0.2980 | 0.1110 | 0.0019 | 1.0000 | 1.0000 | 0.0174 |
| 32 | 555 | 0.0680 | 0.3210 | 0.2820 | 0.1000 | 0.0019 | 0.9950 | 0.9920 | 0.0176 |
| 33 | 560 | 0.0710 | 0.2940 | 0.2670 | 0.0910 | 0.0018 | 0.9900 | 0.9840 | 0.0169 |
| 34 | 565 | 0.0760 | 0.2730 | 0.2530 | 0.0820 | 0.0018 | 0.9860 | 0.9770 | 0.0171 |
| 35 | 570 | 0.0800 | 0.2760 | 0.2390 | 0.0740 | 0.0017 | 0.9810 | 0.9690 | 0.0168 |
| 36 | 575 | 0.0940 | 0.2680 | 0.2270 | 0.0670 | 0.0017 | 0.9760 | 0.9620 | 0.0172 |
| 37 | 580 | 0.1080 | 0.2910 | 0.2140 | 0.0610 | 0.0016 | 0.9720 | 0.9540 | 0.0172 |
| 38 | 585 | 0.1330 | 0.2740 | 0.2030 | 0.0550 | 0.0016 | 0.9670 | 0.9470 | 0.0173 |
| 39 | 590 | 0.1570 | 0.2820 | 0.1920 | 0.0500 | 0.0015 | 0.9630 | 0.9400 | 0.0163 |
| 40 | 595 | 0.2010 | 0.2490 | 0.1820 | 0.0450 | 0.0015 | 0.9580 | 0.9330 | 0.0167 |
| 41 | 600 | 0.2450 | 0.2360 | 0.1720 | 0.0410 | 0.0014 | 0.9540 | 0.9260 | 0.0163 |
| 42 | 605 | 0.2680 | 0.2790 | 0.1630 | 0.0370 | 0.0014 | 0.9500 | 0.9190 | 0.0164 |
| 43 | 610 | 0.2900 | 0.2520 | 0.1540 | 0.0330 | 0.0013 | 0.9450 | 0.9130 | 0.0160 |
| 44 | 615 | 0.3000 | 0.2680 | 0.1460 | 0.0300 | 0.0013 | 0.9410 | 0.9060 | 0.0157 |
| 45 | 620 | 0.3100 | 0.2760 | 0.1380 | 0.0270 | 0.0012 | 0.9370 | 0.9000 | 0.0161 |
| 46 | 625 | 0.3150 | 0.2990 | 0.1310 | 0.0250 | 0.0012 | 0.9330 | 0.8930 | 0.0155 |
| 47 | 630 | 0.3200 | 0.3170 | 0.1240 | 0.0220 | 0.0011 | 0.9290 | 0.8870 | 0.0154 |
| 48 | 635 | 0.3250 | 0.3330 | 0.1170 | 0.0200 | 0.0011 | 0.9250 | 0.8810 | 0.0154 |
| 49 | 640 | 0.3300 | 0.3340 | 0.1110 | 0.0180 | 0.0010 | 0.9210 | 0.8750 | 0.0151 |
| 50 | 645 | 0.3400 | 0.3260 | 0.1050 | 0.0170 | 0.0010 | 0.9170 | 0.8690 | 0.0150 |
| 51 | 650 | 0.3500 | 0.3560 | 0.0990 | 0.0150 | 0.0010 | 0.9130 | 0.8630 | 0.0145 |
| 52 | 655 | 0.3800 | 0.3890 | 0.0940 | 0.0140 | 0.0009 | 0.9100 | 0.8570 | 0.0136 |
| 53 | 660 | 0.4100 | 0.4410 | 0.0890 | 0.0120 | 0.0008 | 0.9060 | 0.8510 | 0.0140 |
| 54 | 665 | 0.4200 | 0.5340 | 0.0840 | 0.0110 | 0.0008 | 0.9020 | 0.8460 | 0.0145 |
| 55 | 670 | 0.4300 | 0.5950 | 0.0800 | 0.0100 | 0.0008 | 0.8980 | 0.8400 | 0.0142 |
| 56 | 675 | 0.4400 | 0.5440 | 0.0750 | 0.0090 | 0.0008 | 0.8950 | 0.8350 | 0.0139 |
| 57 | 680 | 0.4500 | 0.5020 | 0.0710 | 0.0080 | 0.0007 | 0.8910 | 0.8290 | 0.0137 |
| 58 | 685 | 0.4750 | 0.4200 | 0.0680 | 0.0070 | 0.0007 | 0.8880 | 0.8240 | 0.0135 |
| 59 | 690 | 0.5000 | 0.3290 | 0.0640 | 0.0070 | 0.0007 | 0.8840 | 0.8190 | 0.0134 |
| 60 | 695 | 0.5750 | 0.2620 | 0.0610 | 0.0060 | 0.0007 | 0.8810 | 0.8130 | 0.0132 |
| 61 | 700 | 0.6500 | 0.2150 | 0.0570 | 0.0060 | 0.0007 | 0.8770 | 0.8080 | 0.0131 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrhman, M.A. Modeling Water Clarity and Light Quality in Oceans. J. Mar. Sci. Eng. 2016, 4, 80. https://doi.org/10.3390/jmse4040080
Abdelrhman MA. Modeling Water Clarity and Light Quality in Oceans. Journal of Marine Science and Engineering. 2016; 4(4):80. https://doi.org/10.3390/jmse4040080
Chicago/Turabian StyleAbdelrhman, Mohamed A. 2016. "Modeling Water Clarity and Light Quality in Oceans" Journal of Marine Science and Engineering 4, no. 4: 80. https://doi.org/10.3390/jmse4040080





