3.1. Influence of Physical-Chemical Parameters on Growth and Siderophore Production
The influence of physical-chemical parameters is one of the first steps in the assessment of a new biological sensing element, particularly for the development of a portable device [
2].
The pyoverdine production in
P. fluorescens seems to be influenced by different carbon sources [
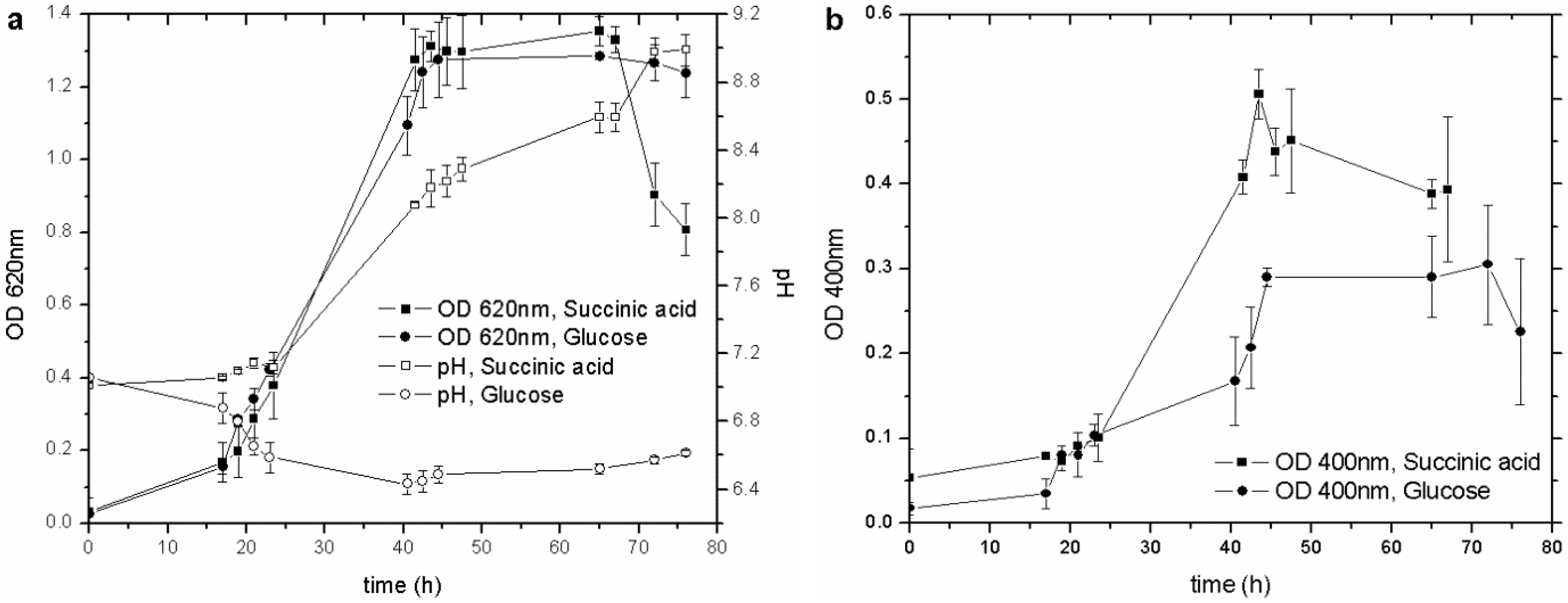
23]. Cultures with glucose and succinic acid, by far the most commonly used to culture this strain, were set up to establish the best C source for the pyoverdine synthesis. Both types of culture showed similar growth behavior (OD
620) (
Figure 1(a)), with the siderophore content increasing along with the biomass concentration, from the early exponential growth phase until the culture entered the stationary one. Nevertheless, succinic acid produced more pyoverdine (nearly double) than glucose did (
Figure 1(b)), even though the C/N ratio was unchanged (17.6 for glucose and 17.9 for succinic acid).
Figure 1.
(a) Influence of different C sources on biomass growth and pH behavior; (b) influence of different C sources on pyoverdine production. OD, optical density.
Figure 1.
(a) Influence of different C sources on biomass growth and pH behavior; (b) influence of different C sources on pyoverdine production. OD, optical density.
A possible explanation for this result is that the pyoverdine molecule is composed of a succinate moiety [
27]: this compound in the culture medium represents an advantage for the microorganism, as it saves energy for the cell metabolism and biosynthesis. In spite of the smaller amount of siderophore produced, the cultures carried out with glucose were less variable in terms of pH and OD
620. In each test conducted with succinic acid, the OD
620 fell when the pH was higher than 8.4, while the culture with glucose remained stable during the whole stationary phase, with a recorded pH of about 6.5. In other papers, it has been reported that
P. fluorescens cultures grown in succinic acid reach high pH values, of about 8.0–8.8, during the stationary phase [
4]. It is feasible that the depletion of succinic acid, buffered at pH 7.0, left an excess of OH
- in the M78 medium, with a resulting increase in pH and a detrimental effect on the viability of the microorganism.
These outcomes highlight the direct influence of the carbon source on microbial growth and on siderophore production in P. fluorescens. One of the most relevant results is that the higher production of pyoverdine attained with succinic acid as the C source leads to the development of a biosensor with a wider dynamic range than the one achievable with glucose. Therefore, the C source in the culture media for the subsequent trials was succinic acid.
Temperature was the second physical-chemical parameter that was evaluated. Tests were performed in BOD bottles (500 mL) and in baffled Erlenmeyer flasks (500 mL), over a 15–30 °C range, to assess the effect of temperature on growth and siderophore production, under different agitation conditions. Biomass growth and siderophore production were higher in the 15–20 °C range in both experimental devices. The pyoverdine content was three to ten times higher than in the cultures maintained at 25–30 °C. An example of the culture set-up in baffled Erlenmeyer flasks is reported in
Figure 2.
Figure 2.
Cultures performed at 20 and 30 °C: (a) pH trends and C source concentration; (b) biomass growth and pyoverdine behavior.
Figure 2.
Cultures performed at 20 and 30 °C: (a) pH trends and C source concentration; (b) biomass growth and pyoverdine behavior.
The stationary phase was reached in less than 20 h in the culture maintained at 20 °C (OD620 2.0 RU), while the duration of the exponential phase at 30 °C was longer (40 h), and the maximum OD620 attained in the stationary phase was only 1.35 RU. Likewise, a faster consumption of the carbon source was observed at 20 °C than at 30 °C: the C source in the former culture was almost depleted at 45 h of fermentation (pH 8.7), while, the stationary phase in the culture maintained at 30 °C was reached at about 40 h (pH 8.2), and at the end of the test, when the pH value was 8.6, the succinic acid concentration was still 0.7 g/L. These results also prove that the decrease in the growth rate is probably related to a C source limitation (succinic acid below 0.5 g/L).
The influence of temperature on pyoverdine production has been studied in detail in
P. aeruginosa: this strain has an optimal temperature for pyoverdine production at 30 °C [
28] and a higher optimal growth temperature, near 37 °C [
29]. The results obtained with
P. fluorescens grown at different temperatures also underline this behavior for this strain: the optimal growth temperature (20–25 °C, on OD
620 and a cell dry weight basis) was higher than the optimal temperature for pyoverdine production (15–20 °C). The temperature was therefore controlled at 20 °C for the subsequent trials.
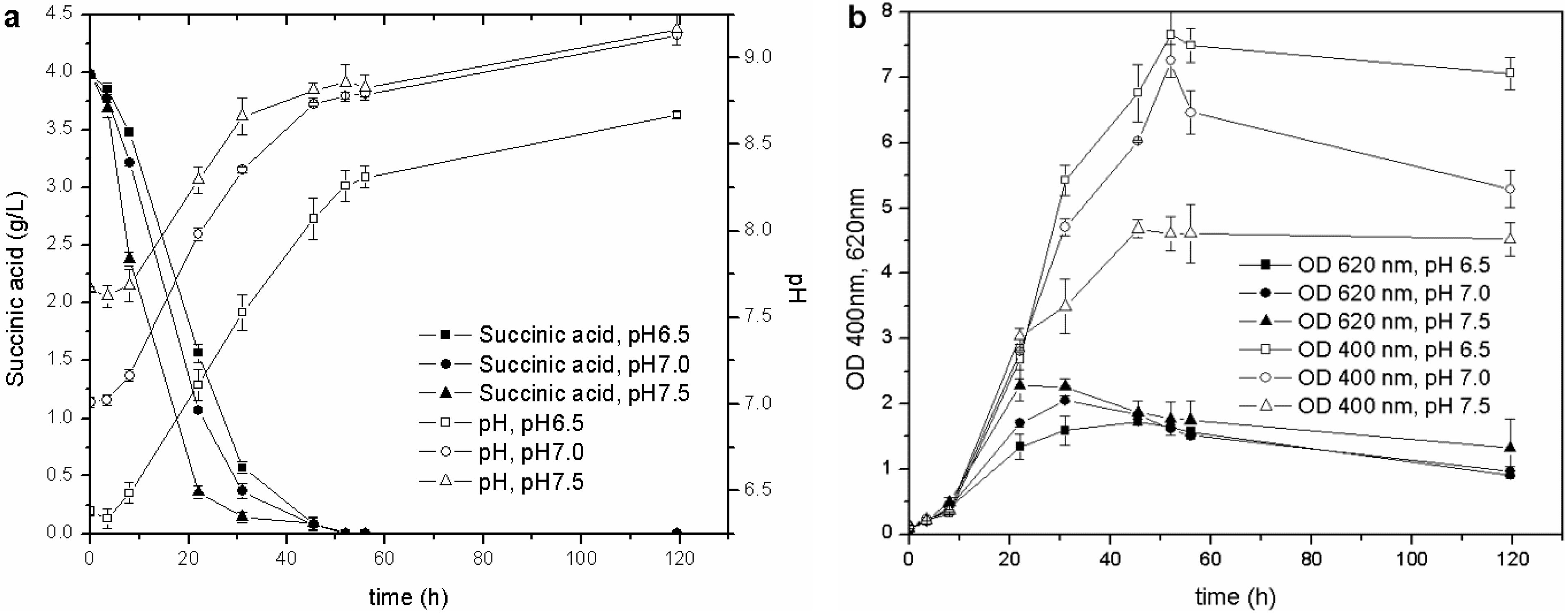
Once the optimal carbon source and temperature for growth and siderophore production were established, the influence of the initial pH was evaluated. The biomass growth, pH values, C source consumption and pyoverdine biosynthesis of the cultures started from the M78 media buffered at different pH are reported in
Figure 3. This test showed that the higher the initial pH, the faster the growth of the microorganism during the exponential phase and, consequently, the earlier the stationary phase is reached. The behavior of pH and carbon source consumption was related to the growth curve of the cultures. The stationary phase was achieved when the pH was about 8.0, and the succinic acid was almost depleted (concentration lower than 0.5 g/L) after 20, 30 and 40 h for pH 7.5, 7.0 and 6.5, respectively. This observation further confirms that the drop in OD
620 recorded during the stationary phase is probably related only to a C source limitation (succinic acid concentration <0.5 g/L): the microorganism seems to grow faster at basic pH, as can be deduced from the carbon source consumptions and OD
620 trends.
Figure 3.
Cultures carried out at different initial pH: (a) pH and C source consumption; (b) growth and pyoverdine production.
Figure 3.
Cultures carried out at different initial pH: (a) pH and C source consumption; (b) growth and pyoverdine production.
During the exponential growth phase, the siderophore production (OD
400) was very similar for the different cultures, but once the stationary phase was attained, the lower the starting pH, the higher the pyoverdine secreted by
P. fluorescens. The influence of pH on the pyoverdine production is probably related to the optimal pH value of the culture. Pyoverdine is produced by the cells over time, and its concentration depends on the amount of living cells, but also on the period of time in which they grow. In the present experiments, the cultures, which started at different pH, reached the stationary growth phase at different stages over intervals of 10 h (
Figure 3(b)), and the one that started at the lowest pH remained for a longer time in the optimal growth phase and pyoverdine production pH range than those cultures that started at a higher pH. This test has revealed that the initial pH value influences not only the microbial growth, but also the siderophore synthesis throughout the entire stationary phase.
3.2. Determination of Minimum Inhibitory Concentration (MIC) of Fe3+, Cu2+ and Zn2+ on a Solid Medium
The interactions between the metals (Fe
3+, Cu
2+ and Zn
2+) and
P. fluorescens were then investigated applying a modified Kirby-Bauer test [
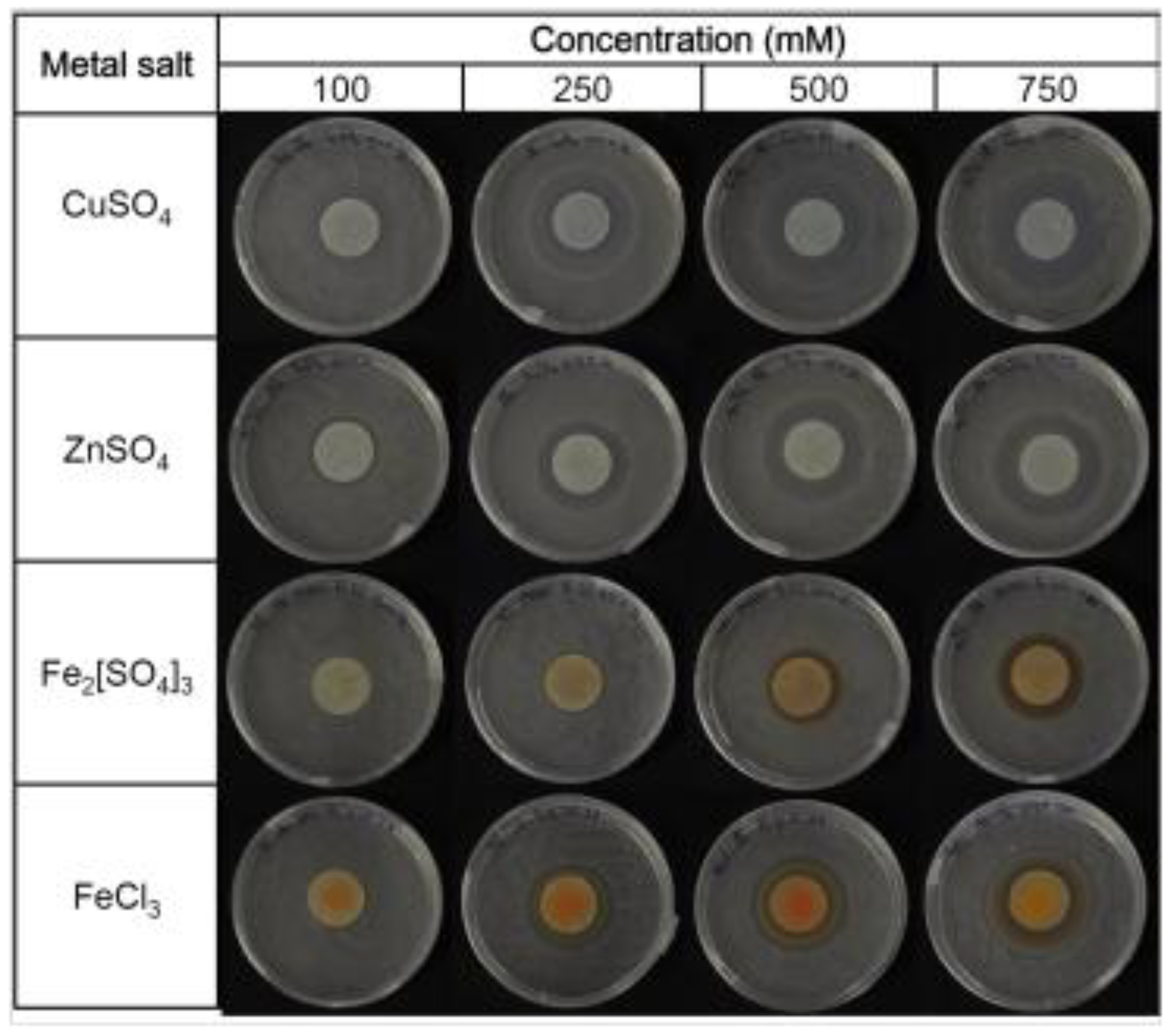
24]. The first step was to determine the minimum inhibitory concentration (MIC), which is defined as the lowest metal concentration for which the growth is inhibited after overnight incubation [
26] (
Figure 4). The area of the inhibition halo measured applying different metal concentrations allowed the values of the MICs to be calculated for CuSO
4, ZnSO
4 and Fe
2[SO
4]
3, and these resulted in 46.30, 54.40 and 74.11 mM, respectively. Two different salts were tested for Fe
3+ to evaluate the counter-ion effect: SO
42− was less toxic than Cl
− [
24].
Figure 4.
Minimum inhibitory concentrations (MICs) of Fe3+, Cu2+ and Zn2+ obtained on agar plates.
Figure 4.
Minimum inhibitory concentrations (MICs) of Fe3+, Cu2+ and Zn2+ obtained on agar plates.
The values of MIC determined on the solid media were high compared to the MIC values established in the liquid cultures, and only the order of the metal sensitivity of the strain (Cu
2+ > Zn
2+) agrees with the results reported by Poirier
et al., for the high metal resistant
Pseudomonas BA3d12 [
30], or by Teitzel and Parsek [
31]. It is reasonable to assume that mass transfer limitations, absorption and metal bioavailability affected the results obtained on the solid media more than those attained in liquid cultures. As a consequence, lower inhibitory concentrations are achieved in liquid cultures, as mentioned by other authors [
32,
33,
34], and the only advantage offered by solid media during the MIC determination is that the bioavailability of the metals is similar to that of soil [
33], where ions tend to be adsorbed to particles or complexed to organic compounds, such as organic acids (e.g., oxalic acid or humic acid) [
3]. Although the values of MIC attained with the modified Kirby-Bauer test on the solid media highlighted that the biorecognition element was fully compatible with the concentrations of metals usually found in unpolluted freshwater (e.g., 98/83/EC,
Table 2 in Subsection 3.4), it was clear that the interaction of the microorganism with metals should also be investigated in liquid cultures.
3.3. Evaluation of the Influence on Growth and Siderophore Production of Fe3+, Cu2+ and Zn2+ in Erlenmeyer Flasks
Owing to the bioavailability limitations of the tests on solid media, the interaction of P. fluorescens with Fe3+, Cu2+ and Zn2+ was investigated in metal supplemented liquid cultures, carried out in well-mixed, baffled Erlenmeyer flasks.
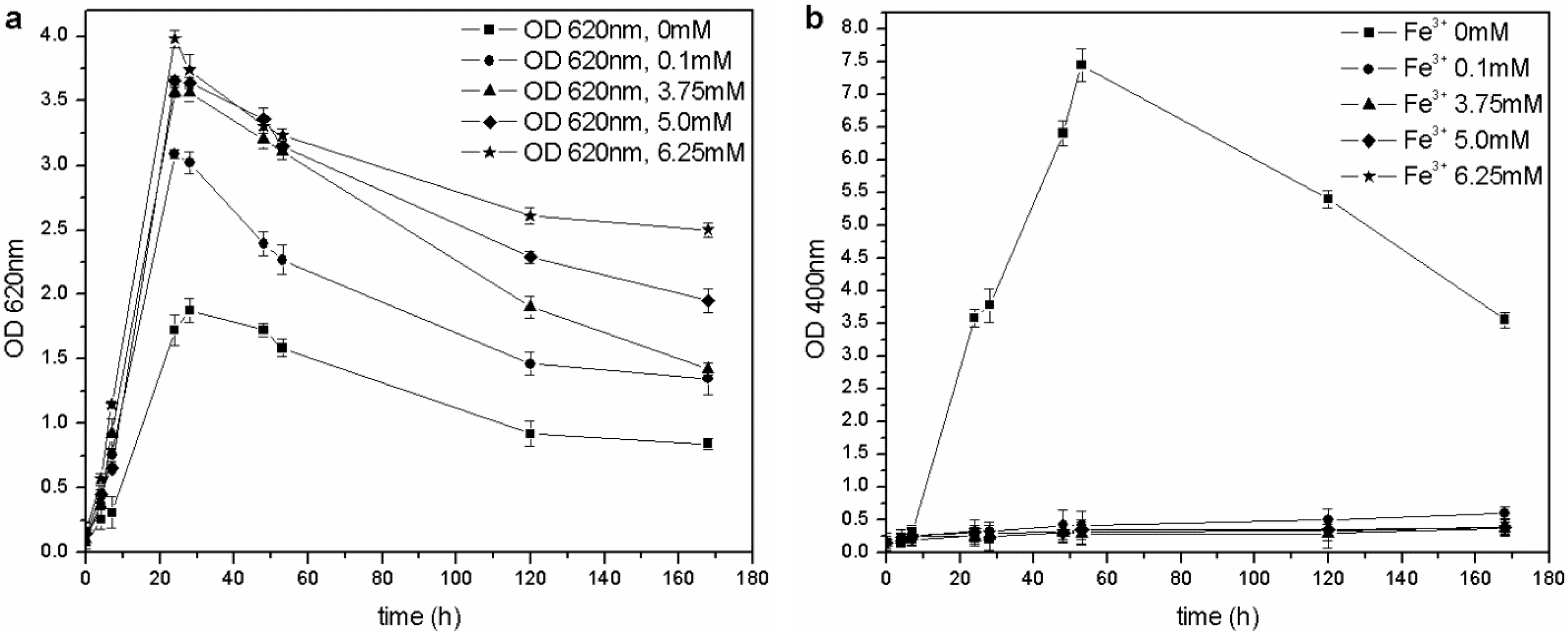
The microorganism was able to grow at each tested iron concentration, and no toxic effects were observed in the 0–6.25 mM range of Fe
3+, unlike the results by Workentine
et al. [
35] (
Table 1). Furthermore, when the concentration of ferric ion in solution was increased, faster growth and higher maximum OD
620 values were recorded, compared to the control (0 mM FeCl
3) (
Figure 5(a)): the presence of a low concentration of metals could be an advantage during microbial fermentation and, in particular, for the growth of
P. fluorescens [
14,
23]. Moreover, the pyoverdine production chiefly depends on the Fe
3+: if the concentration is above a critical value (non-limiting iron or critical iron concentration for pyoverdine production, or CICP), the siderophore synthesis is repressed by the microorganism. During this test, siderophore was only produced in the control flask (0 mM Fe
3+) and not by the other cultures (
Figure 5(b)), confirming that the CICP is lower than 0.1 mM, in agreement with the results of other authors [
23].
Table 1.
Comparison between MICs obtained in liquid cultures (mM).
Table 1.
Comparison between MICs obtained in liquid cultures (mM).
| Metal | This work | Workentine et al. [35] | Poirier et al. [30] |
|---|
| Fe3+ | >6.25 | 6.25 | / |
| Cu2+ | 0.1–0.95 | 3.13 | 0.66 |
| Zn2+ | >2.0 | 1.56 | 0.95 |
Figure 5.
(a) Biomass growth in cultures supplemented with FeCl3. (b) Pyoverdine production (OD400) in cultures supplemented with FeCl3.
Figure 5.
(a) Biomass growth in cultures supplemented with FeCl3. (b) Pyoverdine production (OD400) in cultures supplemented with FeCl3.
The same kind of experiment was carried out with CuSO
4 and ZnSO
4. As far as copper is concerned (
Figure 6(a)),
P. fluorescens was only able to grow in the 0–0.1 mM range of Cu
2+, and each flask showed similar growth behavior (OD
620 values), pH trends and carbon source consumption. The other cultures (0.95–9.5 mM of Cu
2+) were inhibited completely, and no changes were in fact observed in the pH values and C concentrations. According to these results, the MIC for CuSO
4 in liquid cultures is roughly three times less than that reported by Workentine
et al. [
35], but is similar to the one reported by Poirier
et al. [
30] for the strain, BA3d12 (
Table 1). However, the determined MIC for ZnSO
4 was higher than that reported by the previously mentioned authors: the microorganism was able to grow at each tested concentration of Zn
2+, and no toxic effects were detected in the 0–2.0 mM range (
Figure 7).
Figure 6.
(a) Biomass growth in cultures supplemented with CuSO4. (b) Pyoverdine biosynthesis (OD400) in cultures supplemented with CuSO4.
Figure 6.
(a) Biomass growth in cultures supplemented with CuSO4. (b) Pyoverdine biosynthesis (OD400) in cultures supplemented with CuSO4.
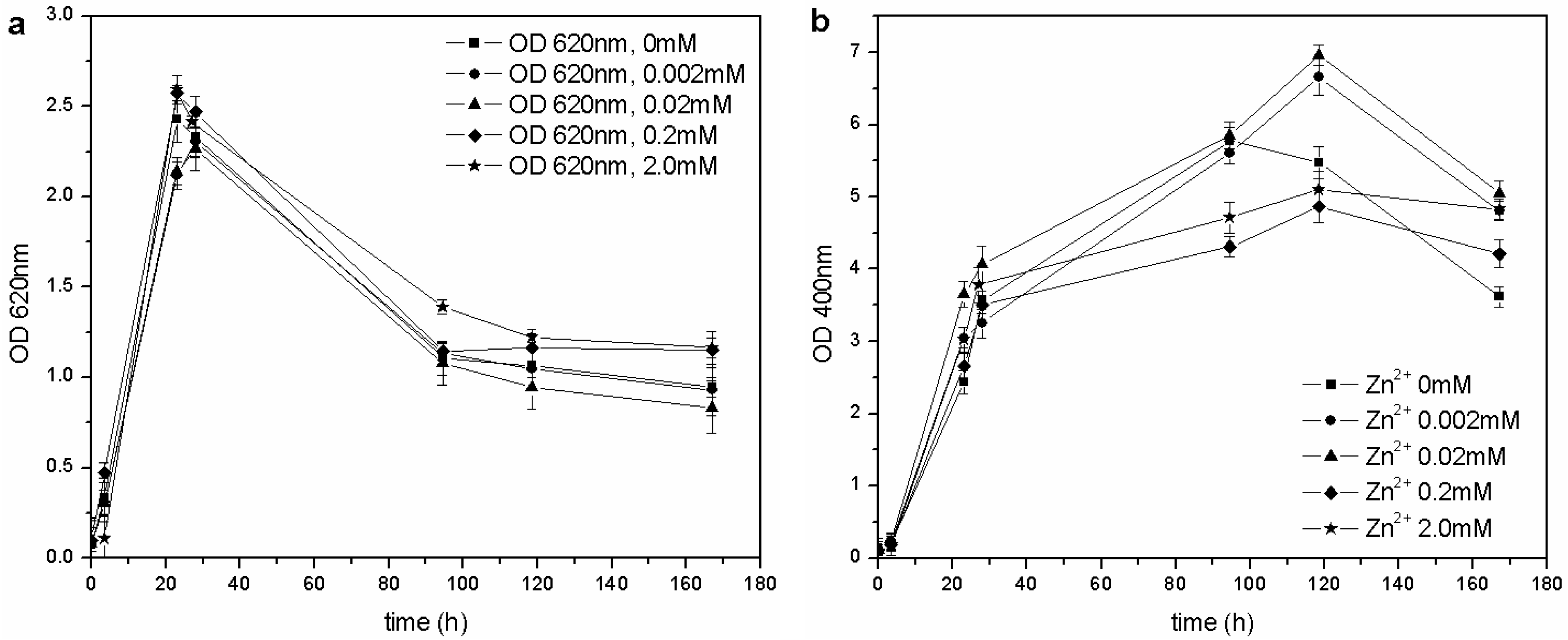
Figure 7.
(a) Biomass growth in cultures supplemented with ZnSO4. (b) Pyoverdine production (OD400) in cultures supplemented with ZnSO4.
Figure 7.
(a) Biomass growth in cultures supplemented with ZnSO4. (b) Pyoverdine production (OD400) in cultures supplemented with ZnSO4.
The same concentration of pyoverdine was obtained in those cultures supplemented with Cu
2+, in which the microorganism was able to grow (0–0.1 mM Cu
2+). A similar result was obtained in cultures supplemented with Zn
2+: the siderophore content was similar to that of the control flask (0 mM Zn
2+), except for a greater concentration in cultures supplemented with 0.02 and 0.002 mM Zn
2+ during the stationary phase of growth. As previously reported [
14,
28], it is known that 10–100 µM concentrations of Zn
2+ can improve growth and pyoverdine production in the related
Pseudomonas aeruginosa.
These experiments carried out in Erlenmeyer flasks have proven that the MICs obtained in liquid cultures are lower than those attained on solid media. Moreover, higher (for Fe
3+ and Zn
2+) or lower (for Cu
2+) concentrations inhibited the growth of the microorganism compared to the MICs determined by other authors [
30,
35] (
Table 1). These discrepancies could be due to the effect of different culture conditions, such as the use of diverse experimental devices (96-well plates instead of flasks), higher or lower inoculum percentages and rich or minimal culture media. Owing to the large number of variables, and in order to use the same small-scale applied by other authors [
31,
35], the tests that were carried out in Erlenmeyer flasks were also performed in 96-well plates.
3.4. Evaluation of the Influence on Growth and Siderophore Production of Fe3+, Cu2+ and Zn2+ in 96-Well Plates
The MICs in the 96-well plates were assessed by means of the serial dilution method [
26], initially using two different culture media: a complex medium (DSM1) and a minimal one (M78). The obtained results clearly confirmed that microbial growth is influenced by the presence of the metals and also by the type of culture media: the MIC was higher for Cu
2+ and Zn
2+ in the complex medium (DSM1), while the MIC for Fe
3+ was higher in the minimal medium (M78). Generally, the highest MIC values are obtained for a complex growth medium, as reported by other authors [
31,
32]: metal bioavailability is probably influenced because of a higher level of ion complexation by the medium components. This hypothesis is appropriate for copper and zinc, but not for iron: the concentration of Fe
3+ required for microbial growth, which was not sufficient in the minimal medium [
23], was probably re-established by supplementing the M78 medium. Instead, only a toxic effect was recorded for Cu
2+ and Zn
2+.
Some solubility problems were encountered in these experimental tests: with the addition of a concentrated metal solution to both culture media, which were already rich in salts, the solubility product was reached easily, insoluble precipitates were formed and the nominal concentration of bioavailable metal was reduced. In order to confirm the precipitate production in the absence of biomass, a control test was conducted with both media, at different metal concentrations in non-inoculated 96-well plates, and the optical density was evaluated (OD600 and OD400). As soon as the solutions were mixed, a precipitate formed as the metal concentration increased, especially in the presence of chloride salts, and the solubility problems were much more evident in the presence of iron and copper.
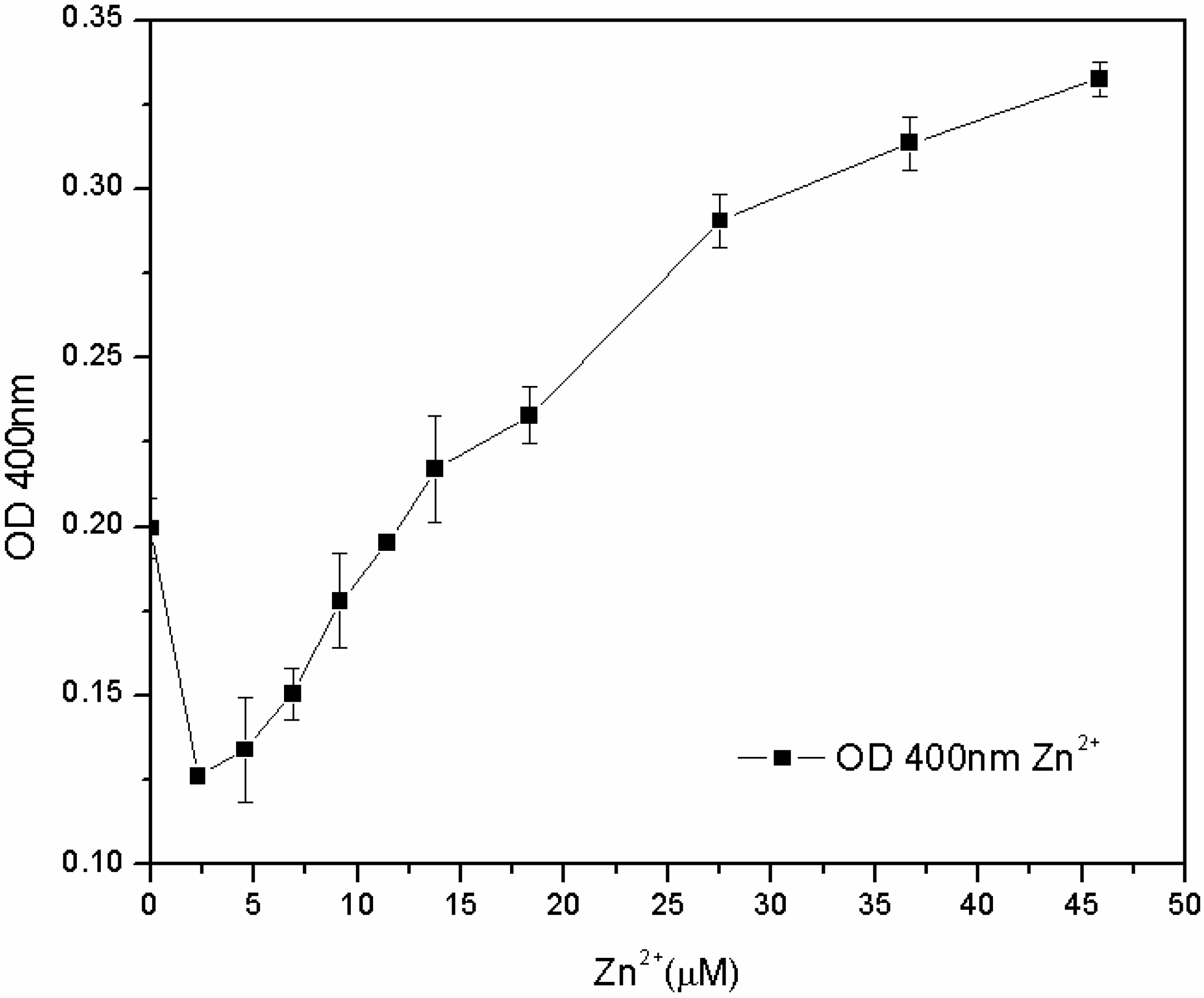
The subsequent tests, carried out in the M78 medium with a lower range of metal concentration and with different inoculum percentages, showed that the susceptibility of the microorganism for Fe
3+ and Cu
2+ depends not only on the concentration of metal, but also on the inoculum percentage: the lower the biomass in the inoculum, the lower the MIC. On the other hand, the microorganism grew for each zinc concentration, and after 24 h, the pyoverdine content was directly proportional to the concentration of Zn
2+ in the 7.6–46 µM range (
Figure 8). The reasons for this effect are not clear. One hypothesis is that the siderophore content secreted by the microorganism from zero to 7.6 μM of Zn
2+ is sufficient to sustain growth, without any toxic effect. At 7.6 μM, Zn
2+ became toxic to the microorganism, which increased its pyoverdine production as a protective mechanism. It is known that pyoverdine is effective in shielding the microbial cell of related
Pseudomonads from Cu
2+ and Zn
2+ toxicity [
10,
15]. Unfortunately, in this work this effect was only observed for zinc and not for copper; further investigations are required to have a clearer picture of the interactions between
P. fluorescens and these different metal ions.
Figure 8.
Pyoverdine (OD400) at 24 hours in 96-well plates cultures treated with low Zn2+ concentrations.
Figure 8.
Pyoverdine (OD400) at 24 hours in 96-well plates cultures treated with low Zn2+ concentrations.
From these results, it is clear that the MICs of metals depend on several variables (e.g. the medium, the inoculum percentage), and this makes it difficult to compare data reported by different authors and obtained in diverse experimental conditions. For instance, regarding Cu
2+, Teitzel and Parsek [
31] found a MIC of 127 mg/L for
Pseudomonas aeruginosa, Chen
et al. [
36] established 190 mg/L for
Pseudomonas putida, whereas Tom-Petersen
et al. [
37] encountered 3 mg/L for
P. fluorescens. These data highlight the heterogeneity of MIC, which is closely related to the tested strain and conditions. This complexity of iron uptake is further influenced by the simultaneous presence of different siderophores: pyochelin synthesis in
P. aeruginosa is repressed by the same concentration that induces pyoverdine synthesis [
14,
38]. Teitzel
et al. [
39], through 2D-electrophoresis, verified that exposure to Cu
2+ upregulates the genes involved in the synthesis of pyoverdine and downregulates those involved in the synthesis of pyochelin [
10]. A similar experimental approach should be used to investigate this effect in
P. fluorescens ATCC 13525.
Table 2.
Comparison between MICs, critical iron concentrations for pyoverdine (CICPs) and EU and WHO drinking water regulations (μM).
Table 2.
Comparison between MICs, critical iron concentrations for pyoverdine (CICPs) and EU and WHO drinking water regulations (μM).
| Metal | MIC | EU | WHO | CICP |
|---|
| Fe3+ | 1,500.0 | 3.6 | 5.4 | 3.6 |
| Cu2+ | 100.0–500.0 | 31.5 | 31.5 | 25.0 |
| Zn2+ | 46.0–500.0 | / | 46.0 | / |
These tests performed in 96-well plates have also been useful to investigate the CICP for Fe
3+, Cu
2+ and Zn
2+ at different concentrations. By comparing the attained values with those indicated in different international regulations for drinking water (
Table 2), it is possible to see that the MICs of Fe
3+, Cu
2+ and Zn
2+ are always above the threshold specified in the EU drinking water directive (98/83/EC) and in the WHO guidelines for drinking water quality. At the same time, the concentration of metal ions required to inhibit pyoverdine production (CICP) is very close to the recommended thresholds for iron and copper. Instead, CICP was not determined for zinc in the tested concentration range (0–46 μM) and conditions.
This last experiment has highlighted that P. fluorescens is well-suited to grow in the range of allowed concentrations of metals in water, and then, the correlation between the environmental quality standards and the CICP of Fe3+ and Cu2+ can be used to build a colorimetric biosensor, with the hopeful prospects of the in situ application of P. fluorescens pyoverdine.