Key Stages of Fiber Development as Determinants of Bast Fiber Yield and Quality
Abstract
:1. Introduction
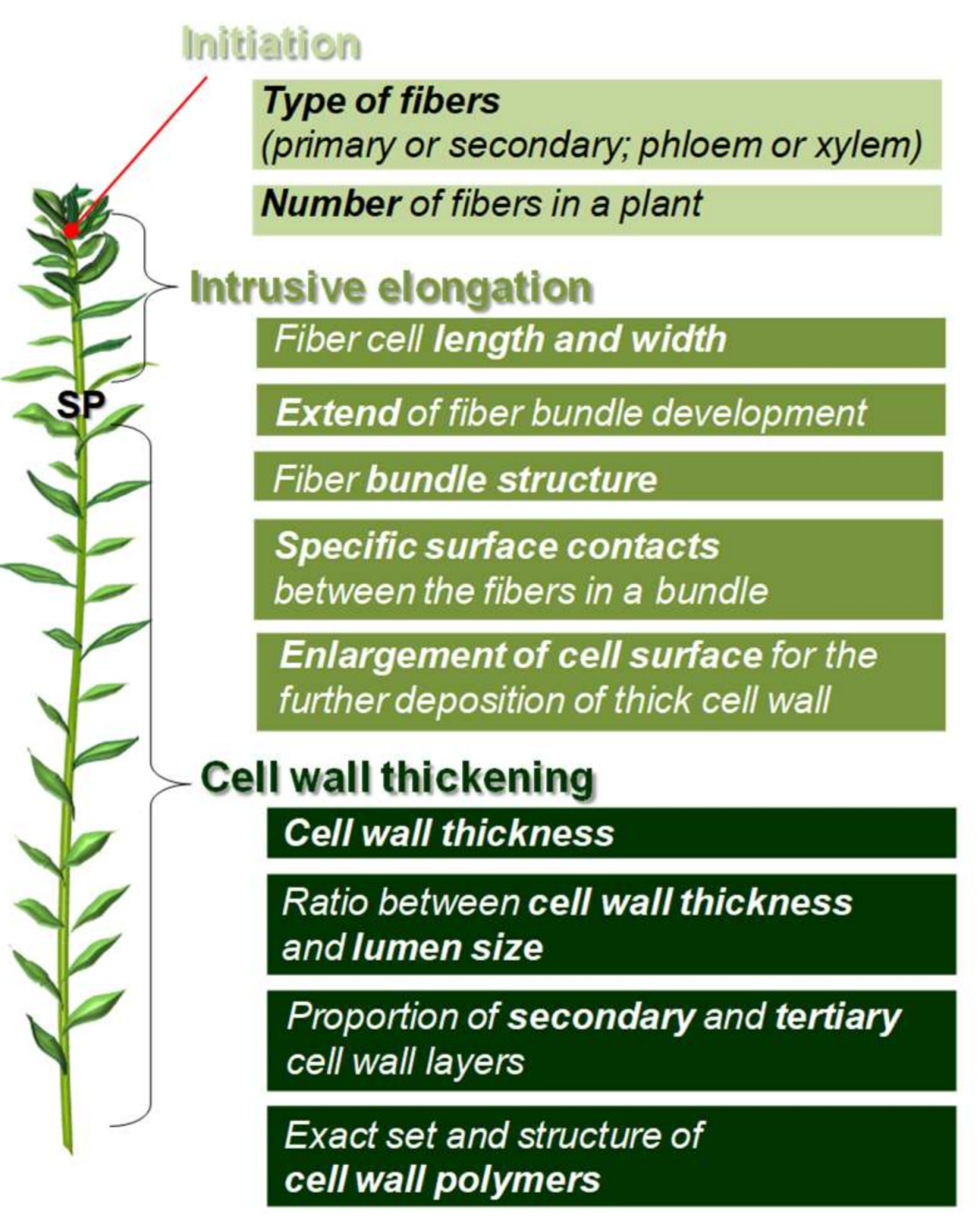
2. Fiber Initiation
3. Fiber Elongation
4. Fiber Cell Wall Thickening
5. Problems to Precisely Relate Fiber Developmental Stages to Fiber Yield and Quality
6. Perspectives of Crop Quality Regulation by Manipulation of Fiber Developmental Stages
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashik, K.P.; Sharma, R.S. A Review on Mechanical Properties of Natural Fiber Reinforced. Hybrid Polymer Composites. J. Miner. Mater. Charact. Eng. 2015, 3, 420–426. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fiber composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Namvar, F.; Jawaid, M.; Md Tahir, P.; Mohamad, R.; Azizi, S.; Khodavandi, A.; Rahman, H.S.; Nayeri, M.D. Potential use of plant fibres and their composites for biomedical applications. BioResources 2014, 9, 5688–5706. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Akampumuza, O.; Wambua, P.M.; Ahmed, A.; Li, W.; Qin, X.-H. Review of the applications of biocomposites in the automotive industry. Polym. Compos. 2017, 38, 2553–2569. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Vincent, F.; Castro, M.; Feller, J.F. Flax fibers—Epoxy with embedded nanocomposite sensors to design lightweight smart bio-composites. Nanocomposites 2016, 2, 125–134. [Google Scholar] [CrossRef]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1965; 767p. [Google Scholar]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Oxford, UK, 1990; 588p. [Google Scholar]
- Gorshkova, T.; Brutch, N.; Chabbert, B.; Deyholos, M.; Hayashi, T.; Lev-Yadun, S.; Mellerowicz, E.J.; Morvan, C.; Neutelings, G.; Pilate, G. Plant fiber formation: State of the art, recent and expected progress, and open questions. Crit. Rev. Plant Sci. 2012, 31, 201–228. [Google Scholar] [CrossRef]
- Fernandez-Tendero, E.; Day, A.; Legros, S.; Habrant, A.; Hawkins, S.; Chabbert, B. Changes in hemp secondary fiber production related to technical fiber variability revealed by light microscopy and attenuated total reflectance Fourier transforminfrared spectroscopy. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Westerhuis, W.; van Dam, J.E.G. Microscopic study on hemp bast fibre formation. J. Nat. Fibers 2007, 3, 1–12. [Google Scholar] [CrossRef]
- Snegireva, A.; Chernova, T.; Ageeva, M.; Lev-Yadun, S.; Gorshkova, T. Intrusive growth of primary and secondary phloem fibers in hemp stem determines fiber-bundle formation and structure. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Chernova, T.E.; Ageeva, M.V.; Chemikosova, S.B.; Gorshkova, T.A. The formation of primary and secondary fibers in hemp. Bull. All-Rus. Sci. Res. Inst. Bast Crops Process. 2005, 2, 6–13. [Google Scholar]
- Ageeva, M.V.; Petrovská, B.; Kieft, H.; Salnikov, V.V.; Snegireva, A.V.; van Dam, J.E.G.; Emons, A.M.C.; Gorshkova, T.A.; van Lammeren, A.A.M. Intrusive growth of flax phloem fibers is of intercalary type. Planta 2005, 222, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Snegireva, A.V.; Ageeva, M.V.; Amenitskii, S.I.; Chernova, T.E.; Ebskamp, M.; Gorshkova, T.A. Intrusive growth of sclerenchyma fibers. Rus. J. Plant Physiol. 2010, 57, 342–355. [Google Scholar] [CrossRef]
- Mokshina, N.; Gorshkov, O.; Ibragimova, N.; Chernova, T.; Gorshkova, T. Cellulosic fibres of flax recruit both primary and secondary cell wall cellulose synthases during deposition of thick tertiary cell walls and in the course of graviresponse. Funct. Plant Biol. 2017, 44, 820–831. [Google Scholar] [CrossRef]
- Bourmaud, A.; Malvestio, J.; Lenoir, N.; Siniscalco, D.; Habrant, A.; King, A.; Legland, D.; Baley, C.; Beaugrand, J. Exploring the mechanical performance and in-planta architecture of secondary hemp fibres. Ind. Crops Prod. 2017, 108, 1–5. [Google Scholar] [CrossRef]
- Crônier, D.; Monties, B.; Chabbert, B. Structure and chemical composition of bast fibers isolated from developing hemp stem. J. Agric. Food Chem. 2005, 53, 8279–8289. [Google Scholar] [CrossRef] [PubMed]
- Placet, V.; Méteau, J.; Froehly, L.; Salut, R.; Boubakar, M.L. Investigation of the internal structure of hemp fibres using optical coherence tomography and Focused Ion Beam transverse cutting. J. Mater. Sci. 2014, 49, 8317–8327. [Google Scholar] [CrossRef]
- Fidelis, M.E.A.; Pereira, T.V.C.; Gomes, O.F.M.; Silva, F.A.; Filho, R.D.T. The effect of fiber morphology on the tensile strength of natural fibers. J. Mater. Res. Technol. 2013, 2, 149–157. [Google Scholar] [CrossRef]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fiber mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Mellerowicz, E.J.; Gorshkova, T.A. Tensional stress generation in gelatinous fibres: A review and possible mechanism based on cell-wall structure and composition. J. Exp. Bot. 2012, 63, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, T.; Chernova, T.; Mokshina, N.; Ageeva, M.; Mikshina, P. Plant “muscles”: Fibers with a tertiary cell wall. New Phytol. 2018, 218, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, O.; Mokshina, N.; Gorshkov, V.; Chemikosova, S.; Gogolev, Y.; Gorshkova, T. Transcriptome portrait of cellulose-enriched flax fibers at advanced stage of specialization. Plant Mol. Biol. 2017, 93, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Love, G.D.; Snape, C.E.; Jarvis, M.C. Determination of phenolic structures in flax fibre by solid-state 13C-NMR. Phytochemistry 1994, 35, 489–491. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Salnikov, V.V.; Pogodina, N.M.; Chemikosova, S.B.; Yablokova, E.V.; Ulanov, A.V.; Ageeva, M.V.; van Dam, J.E.G.; Lozovaya, V.V. Composition and distribution of cell wall phenolic compounds in flax (Linum usitatissimum L.) stem tissues, in annals of botany. Ann. Bot. 2000, 85, 477–486. [Google Scholar] [CrossRef]
- Chernova, T.; Mikshina, P.; Salnikov, V.; Ageeva, M.; Ibragimova, N.; Sautkina, O.; Gorshkova, T. Development of Hemp Fibers: The Key Components of Hemp Plastic Composites. In Engineering Nanocomposites—Fiber Crop Composite Production; InTech: Rijeka, Croatia, 2018; 16p, ISBN 978-953-51-5722-9. in press. [Google Scholar]
- Mikshina, P.V.; Gurjanov, O.P.; Mukhitova, F.K.; Petrova, A.A.; Shashkov, A.S.; Gorshkova, T.A. Structural details of pectic galactan from the secondary cell walls of flax (Linum usitatissimum L.) phloem fibres. Carbohyd. Polym. 2012, 87, 853–861. [Google Scholar] [CrossRef]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric. J. 2010, 4, 93–101. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Tranquet, O.; Poulain, D.; Moïse, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Hotte, N.S.C.; Deyholos, M.K. A flax fibre proteome: Identification of proteins enriched in bast fibres. BMC Plant Biol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Mokshina, N.; Gorshkova, T.; Deyholos, M.K. Chitinase-Like (CTL) and Cellulose Synthase (CESA) Gene Expression in Gelatinous-Type Cellulosic Walls of Flax (Linum usitatissimum L.) Bast Fibers. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Saito, J.A.; Emdad, E.M.; Ahmed, B.; Islam, M.M.; Halim, A.; Hossen, Q.M.; Hossain, M.Z.; Ahmed, R.; Hossain, M.S.; et al. Comparative genomics of two jute species and insight into fibre biogenesis. Nat. Plants 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Behr, M.; Legay, S.; Mangeot-Peter, L.; Zorzan, S.; Ghoniem, M.; Hausman, J.-F. Transcriptomic profiling of hemp bast fibres at different developmental stages. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Behr, M.; Backes, A.; Faleri, C.; Hausman, J.-F.; Lutts, S.; Cai, G. Bast fibre formation: Insights from Next-Generation Sequencing. Procedia Eng. 2017, 200, 229–235. [Google Scholar] [CrossRef]
- Chantreau, M.; Portelette, A.; Dauwee, R.; Kiyoto, S.; Crônier, D.; Morreel, K.; Arribat, S.; Neutelings, G.; Chabi, M.; Boerjan, W.; et al. Ectopic lignification in the flax lignified bast fiber1 mutant stem is associated with tissue-specific modifications in gene expression and cell wall composition. Plant Cell 2014, 26, 4462–4482. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.J.; Mokshina, N.Y.; Badhan, A.; Snegireva, A.V.; Hobson, N.; Deyholos, M.K.; Gorshkova, T.A. Development of cellulosic secondary walls in flax fibers requires beta-galactosidase. Plant Physiol. 2011, 156, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G. The draft genome and transcriptome of Cannabis sativa. Genome Biol. 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Mahato, A.K.; Satya, P.; Kundu, A.; Singh, S.; Jayaswal, P.K.; Singh, A.; Bahadur, K.; Pattnaik, S.; Singh, N.; et al. The draft genome of Corchorus olitorius cv. JRO-524 (Navin). Genom. Data 2017, 12, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, L.; Zhu, S.; Wu, L.; Wang, Y.; Tang, S.; Wang, H.; Zheng, X.; Zhao, J.; Chen, X.; et al. Draft genome analysis provides insights into the fiber yield, crude protein biosynthesis, and vegetative growth of domesticated ramie (Boehmeria nivea L. Gaud). DNA Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Deyholos, M.K. RNASeq analysis of the shoot apex of flax (Linum usitatissimum) to identify phloem fiber specification genes. Front Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokshina, N.; Chernova, T.; Galinousky, D.; Gorshkov, O.; Gorshkova, T. Key Stages of Fiber Development as Determinants of Bast Fiber Yield and Quality. Fibers 2018, 6, 20. https://doi.org/10.3390/fib6020020
Mokshina N, Chernova T, Galinousky D, Gorshkov O, Gorshkova T. Key Stages of Fiber Development as Determinants of Bast Fiber Yield and Quality. Fibers. 2018; 6(2):20. https://doi.org/10.3390/fib6020020
Chicago/Turabian StyleMokshina, Natalia, Tatyana Chernova, Dmitry Galinousky, Oleg Gorshkov, and Tatyana Gorshkova. 2018. "Key Stages of Fiber Development as Determinants of Bast Fiber Yield and Quality" Fibers 6, no. 2: 20. https://doi.org/10.3390/fib6020020




