Isolation and Characterization of Bacteria from Ancient Siberian Permafrost Sediment
Abstract
:1. Introduction
2. Materials and Methods
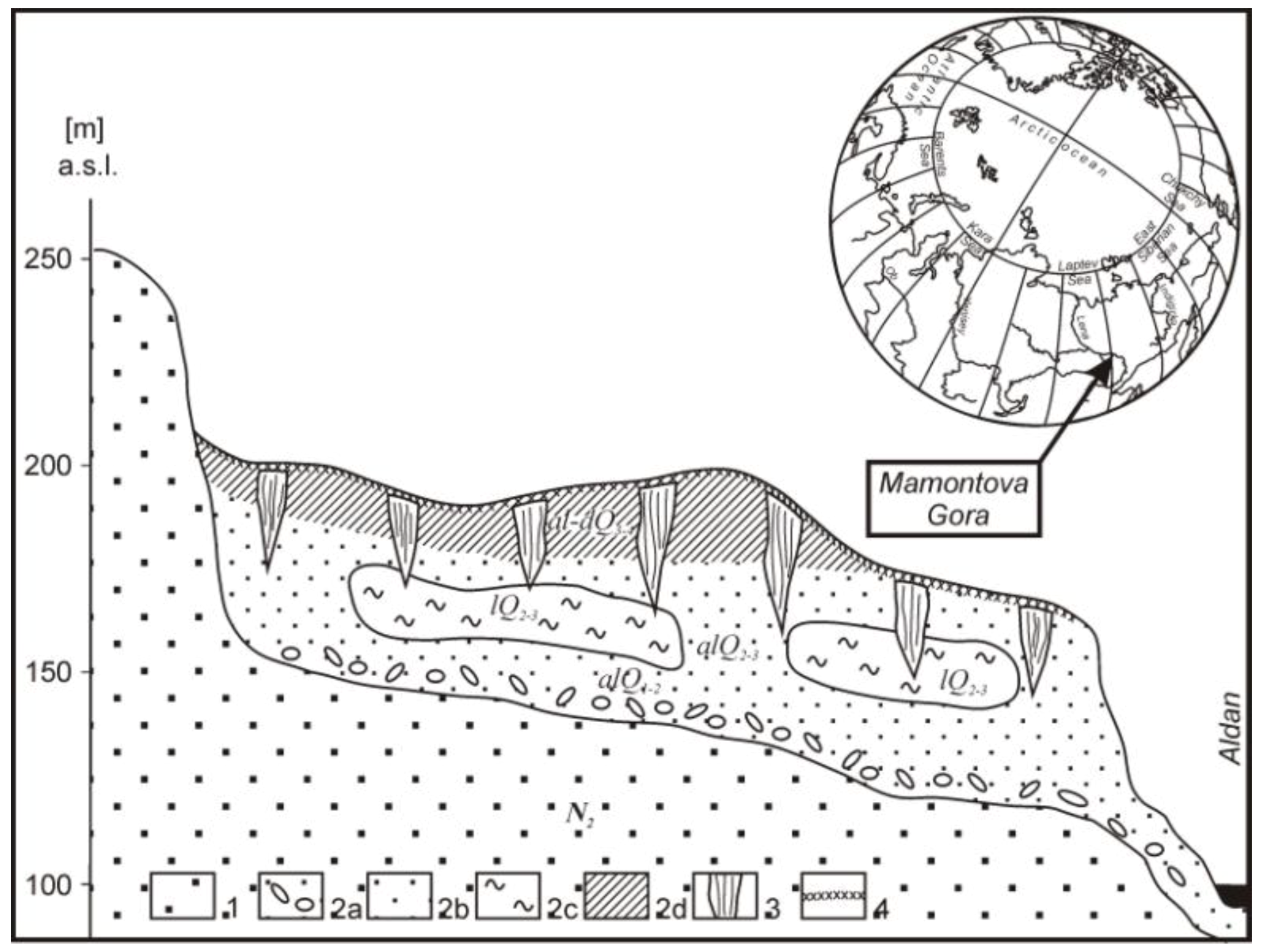
2.1. Sampling Site
2.2. Sampling
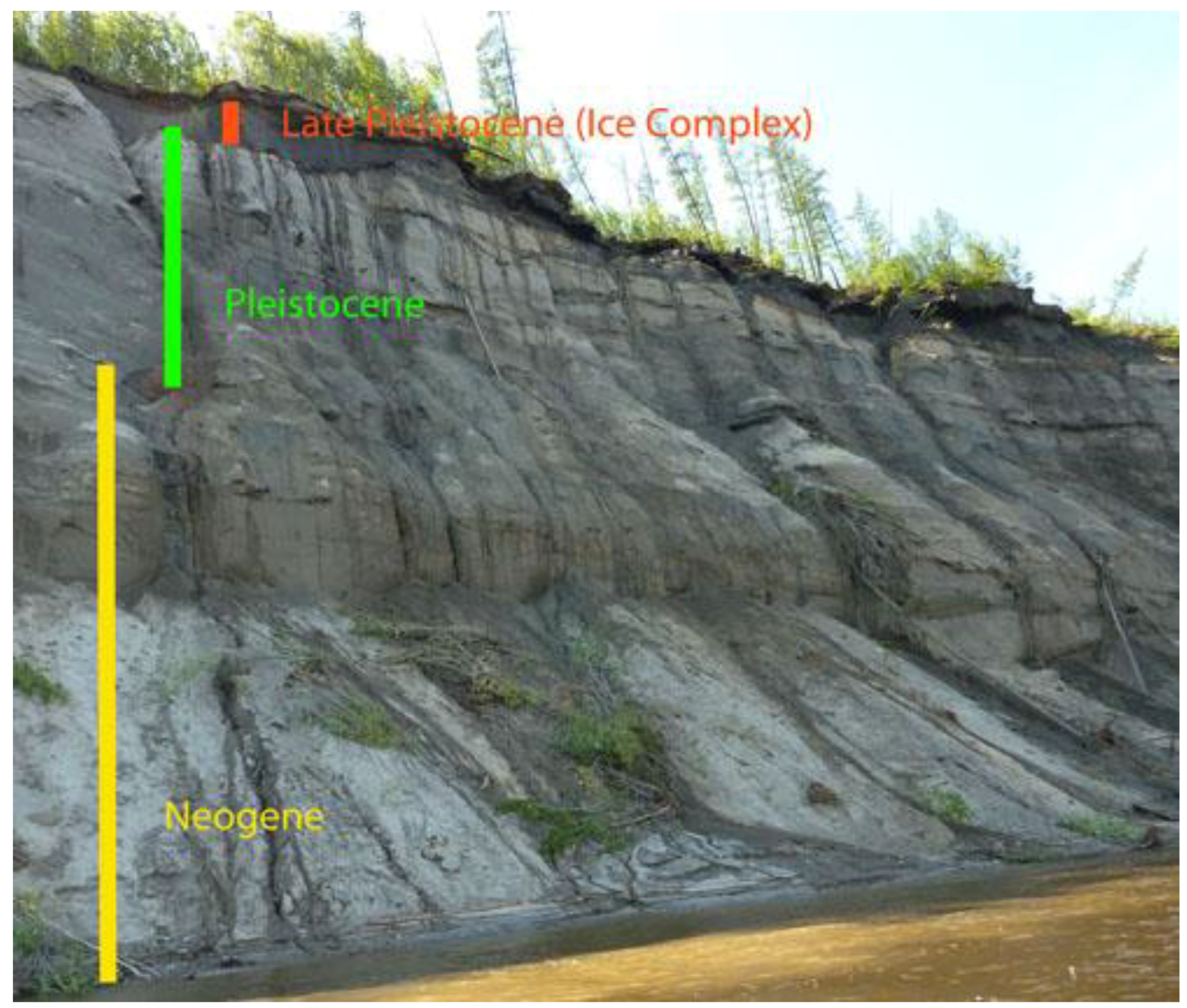


2.3. Enrichment of Microorganisms
2.4. Physical and Chemical Sediment Properties
2.5. Phospholipid Fatty Acids (PLFA)
2.6. Direct Microbial Counts
2.7. Enumeration of Culturable Heterotrophic Aerobic Sediment Microorganisms
2.8. Phylogenetic Analysis of Culturable Bacteria and Restriction Fragment Length Polymorphism (RFLP)
2.9. Characterization of Culturable Bacteria
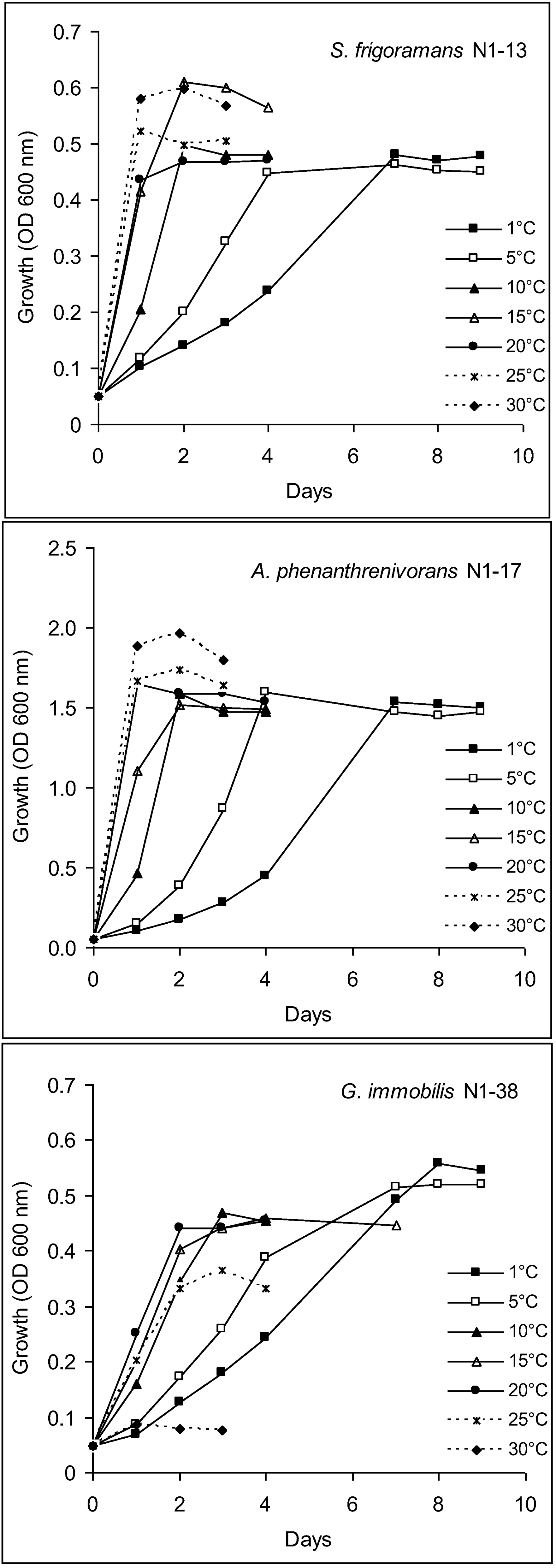
2.9.1. Growth Temperature Range
2.9.2. Growth on Different Media
2.9.3. Facultatively Anaerobic Growth
2.9.4. Salt Tolerance
2.9.5. Resistance to Antibiotics
2.9.6. Resistance to Heavy Metals
2.9.7. Biodegradation of Hydrocarbons
2.9.8. Enzyme Activities
3. Results
3.1. Sediment Properties
3.2. PLFA
3.3. Direct Microbial Counts
3.4. Enumeration of Culturable Bacteria and Fungi
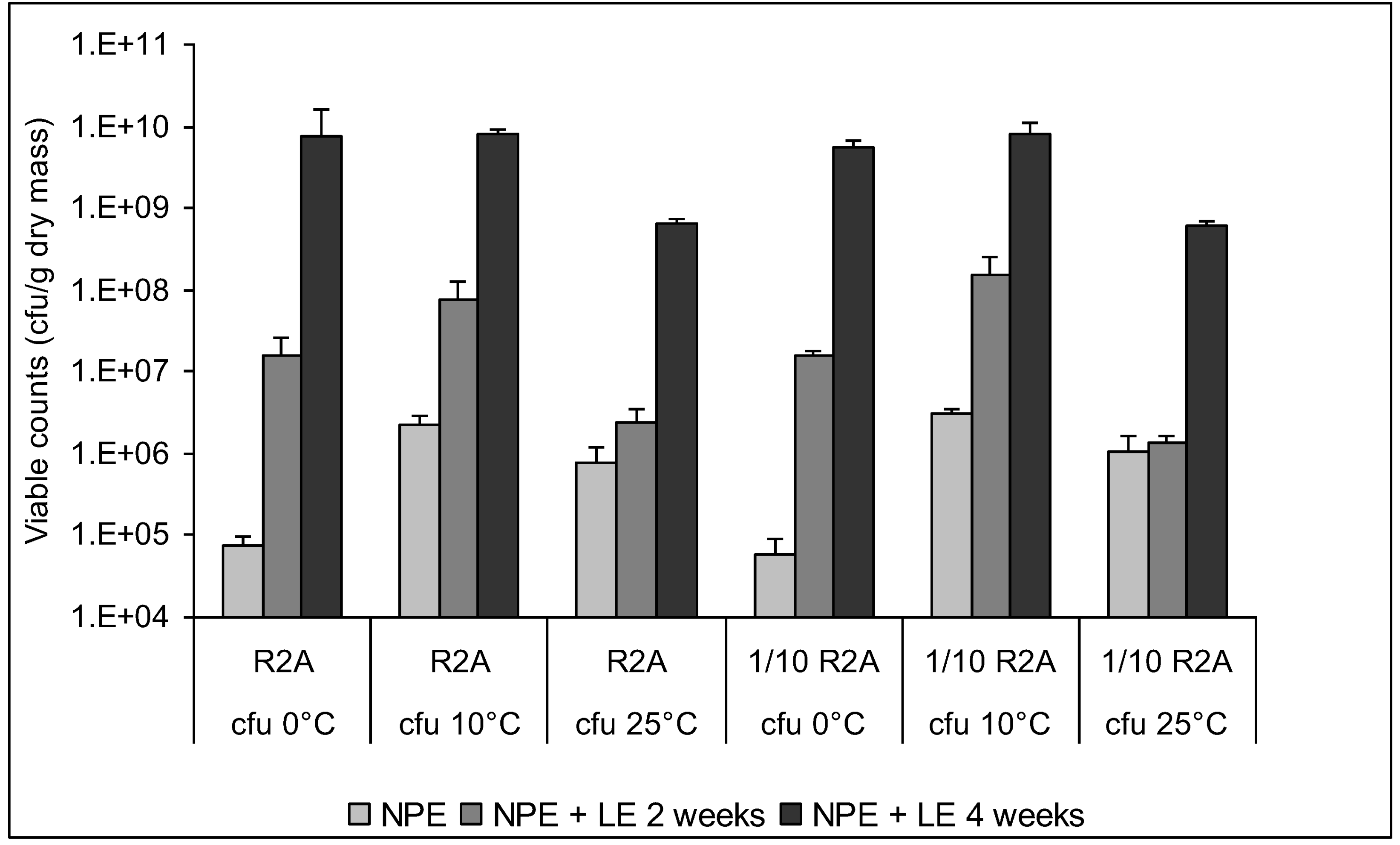
3.5. Characterization of Culturable Bacterial Isolates
| Strain properties | Subtercola frigoramans N1-13 | Arthrobacter phenanthrenivorans N1-17 | Glaciimonas immobilis N1-38 |
|---|---|---|---|
| Tmin/Tmax | |||
| R2A broth | −5 °C/30 °C | −5 °C/30 °C | −5 °C/25 °C |
| R2A agar | 1 °C/30 °C | 1 °C/30 °C | 1 °C/20 °C |
| Growth on various media (R2A, NA, LB, TSA) | |||
| 1 °C | R2A | R2A NA LB TSA | R2A |
| 10 °C | R2A NA | R2A NA LB TSA | R2A |
| 20 °C | R2A NA LB | R2A NA LB TSA | R2A |
| Growth in presence of NaCl (% w/v) | |||
| 1 °C | 0% | 1% weak | 0% |
| 10 °C | 0% (1% weak) | 2% | 0% |
| 20 °C | 1% | 3% (5% weak) | 0% |
| Growth in presence of cyclosporin A (100 µg mL−1) * | |||
| 1 °C | Weak | + | weak |
| 10 °C | + | + | + |
| 20 °C | + | + | + |
| Utilization of lignosulfonic acid | |||
| 1 °C | − | + | − |
| 10 °C | Weak | + | − |
| 20 °C | Weak | + | − |
| Resistance to heavy metals (0.1 mM Cu2+, 1 mM Pb2+) ** | |||
| 1 °C | − | − | − |
| 10 °C | (+) | + | − |
| 20 °C | + | ++ | − |
| Antibiotic (20 µg mL−1) | Relative growth (%) | ||
|---|---|---|---|
| 1 °C | 10 °C | 20 °C | |
| Without antibiotic | 100 | 100 | 100 |
| Chloramphenicol | 24 | 36 | 45 |
| Kanamycin | 28 | 41 | 52 |
| Rifampicin | 25 | 38 | 46 |
| Streptomycin | 26 | 36 | 50 |
| Tetracyclin | 28 | 37 | 48 |
4. Discussion
5. Conclusion
Acknowledgements
References and Notes
- Ershov, E.D. Foundations of Geocryology (in Russian); Moscow State University: Moscow, Russia, 1998; pp. 1–575. [Google Scholar]
- Margesin, R. Permafrost Soils; Springer Verlag: Berlin/Heidelberg, Germany, 2009; pp. 1–348. [Google Scholar]
- Johnson, S.S.; Hebsgaard, M.B.; Christensen, T.R.; Mastepanov, M.; Nielsen, R.; Munch, K.; Brand, T.B.; Gilbert, M.T.P.; Zuber, M.T.; Bunce, M.; et al. Ancient bacteria show evidence of DNA repair. Proc. Natl. Acad. Sci. USA 2007, 104, 14401–14405. [Google Scholar]
- Steven, B.; Leveille, R.; Pollard, W.H.; Whyte, L.G. Microbial ecology and biodiversity in permafrost. Extremophiles 2006, 10, 259–267. [Google Scholar] [CrossRef]
- Gilichinsky, D.; Vishnivetskaya, T.; Petrova, M.; Spirina, E.; Mamykin, V.; Rivkina, E. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2008; pp. 83–102. [Google Scholar]
- Steven, B.; Niederberger, T.D.; Whyte, L.G. Permafrost Soils; Margesin, R., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2009; pp. 59–72. [Google Scholar]
- Gilichinsky, D. Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley: New York, NY, USA, 2002; pp. 932–956. [Google Scholar]
- Bakermans, C. Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer Verlag: Berlin/Heidelberg, Germany, 2008; pp. 17–28. [Google Scholar]
- Panikov, N.S.; Sizova, M.V. Growth kinetics of microorganisms isolated from Alaskan soil and permafrost in solid media frozen down to −5 degrees C. FEMS Microbiol. Ecol. 2007, 59, 500–512. [Google Scholar]
- Amato, P.; Doyle, S.M.; Battista, J.R.; Christner, B.C. Implications of subzero metabolic activity on long-term microbial survival in terrestrial and extraterrestrial permafrost. Astrobiology 2010, 10, 789–798. [Google Scholar] [CrossRef]
- Brushkov, A.V.; Melnikov, V.P.; Sukhovei, I.G.; Griva, G.I.; Repin, V.E.; Kalenova, L.F.; Brenner, E.V.; Subbotin, A.M.; Trofimova, I.B.; Tanaka, M.; et al. Relict microorganisms of cryolithozone as possible objects of gerontology (in Russian). Adv. Gerontol. 2009, 22, 253–258. [Google Scholar]
- Brushkov, A.V.; Bezrukov, V.V.; Griva, G.I.; Muradyan, K.K. The effects of the relict microorganism B. sp. on development, gas exchange, spontaneous motor activity, stress resistance, and survival of Drosophila melanogaster. Adv. Gerontol. 2012, 2, 19–26. [Google Scholar] [CrossRef]
- Kalenova, L.F.; Suhovey, U.G.; Broushkov, A.V.; Melnikov, V.P.; Fisher, T.A.; Besedin, I.M.; Novikova, M.A.; Efimova, J.A.; Subbotin, A.M. Experimental study of the effects of permafrost microorganisms on the morphofunctional activity of the immune system. Bull. Exp. Biol. Med. 2011, 151, 201–204. [Google Scholar] [CrossRef]
- Kalenova, L.F.; Sukhovei, U.G.; Brushkov, A.V.; Melnikov, V.P.; Fisher, T.A.; Besedin, I.M.; Novikova, M.A.; Efimova, J.A. Effects of permafrost microorganisms on the quality and duration of life of laboratory animals. Neurosci. Behav. Physiol. 2011, 41, 484–490. [Google Scholar] [CrossRef]
- Fursova, O.; Potapov, V.; Brouchkov, A.; Pogorelko, G.; Griva, G.; Fursova, N.; Ignatov, S. Probiotic activity of bacterial strain isolated from ancient permafrost against Salmonella infection in mice. Probiotics Antimicrob. Proteins 2012, 403, 145–153. [Google Scholar]
- Friedmann, E.I. Viable Microorganisms in Permafrost; Gilichinsky, D., Ed.; Russian Academy of Sciences: Pushchino, Russia, 1994; pp. 21–26. [Google Scholar]
- Stewart, E.J.; Madden, R.; Paul, G.; Taddei, F. Ageing and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005, 3, e45. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.R.; Mangel, M. Life histories and the evolution of aging in bacteria and other single-celled organisms. Mech. Ageing Dev. 2006, 127, 786–793. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Greenblatt, C.L.; Davis, A.; Clement, B.G.; Kitts, C.L.; Cox, T.; Cano, R.J. Diversity of microorganisms isolated from amber. Microb. Ecol. 1999, 38, 58–68. [Google Scholar] [CrossRef]
- Katayama, T.; Tanaka, M.; Moriizumi, J.; Nakamura, T.; Brouchkov, A.; Douglas, T.; Fukuda, M.; Tomita, M.; Asano, K. Phylogenetic analysis of bacteria preserved in a permafrost ice wedge for 25,000 Years. Appl. Environ. Microbiol. 2007, 73, 2360–2363. [Google Scholar] [CrossRef]
- Clein, J.S.; Schimel, J.P. Microbial activity of tundra and taiga soils at sub-zero temperatures. Soil Biol. Biochem. 1995, 27, 1231–1234. [Google Scholar] [CrossRef]
- Ashcroft, F. Life at the Extremes; HarperCollins: London, UK, 2000; pp. 1–326. [Google Scholar]
- Jaenicke, R. Stability and folding of ultrastable proteins: Eye lens crystallins and enzymes from thermophiles. FASEB J. 1996, 10, 84–92. [Google Scholar]
- Cairns, J.; Overbaugh, J.; Miller, S. The origin of mutations. Nature 1994, 335, 142–145. [Google Scholar]
- Levy, M.; Miller, S.L. The stability of the RNA bases: Implications for the origin of life. Biochemistry 1998, 95, 7933–7938. [Google Scholar]
- Rauser, C.L.; Mueller, L.D.; Rose, M.R. Evolution of late life. Ageing Res. Rev. 2005, 5, 14–32. [Google Scholar]
- Willerslev, E.; Cooper, A. Ancient DNA. Proc. Roy. Soc. B 2005, 272, 3–16. [Google Scholar] [CrossRef]
- Amann, R.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar]
- McDougald, D.; Rice, S.A.; Kjelleberg, S. New perspectives on the viable but nonculturable response. Biologia 2009, 54, 617–623. [Google Scholar]
- Spiegelman, D.; Whissell, G.; Greer, C.W. A survey of the methods for the characterization of microbial consortia and communities. Can. J. Microbiol. 2005, 51, 355–386. [Google Scholar] [CrossRef]
- Kirk, L.J.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomas, J.N.; Lee, H.; Trevors, J.T. Methods of studying soil microbiol diversity. J. Microbiol. Meth. 2004, 58, 169–188. [Google Scholar]
- Baranova, U.P.; Il'inskay, I.A.; Nikitin, V.P.; Pneva, G.P.; Fradkina, A.F.; Shvareva, N.Y. Works of Geological Institute of Russian Academy of Sciences (in Russian); Nauka: Moscow, Russia, 1976; pp. 1–284. [Google Scholar]
- Romanovsky, N.N. Basics of Croygenesis of Lithosphere (in Russian); Moscow State University: Moscow, Russia, 1993; pp. 1–336. [Google Scholar]
- Bakulina, N.T.; Spector, V.B. Climate and Permafrost (in Russian); Maksimov, G.N., Fedorov, A.N., Eds.; Permafrost Institute: Yakutsk, Russia, 2000; pp. 21–32. [Google Scholar]
- Markov, K.K. Cross-Section of the Newest Sediments; Moscow University Press: Moscow, Russia, 1973; pp. 1–198. [Google Scholar]
- Vishnivetskaya, T.A.; Kathariou, S.; McGrath, J.; Gilichinsky, D.; Tiedje, J.M. Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 2000, 4, 165–173. [Google Scholar] [CrossRef]
- Schlichting, E.; Blume, H.P.; Stahr, K. Bodenkundliches Praktikum, 2. Auflage (in German); Blackwell: Wissenschafts-Verlag, Berlin, Germany, 1995; pp. 1–296. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer Lab Manual: Berlin/Heidelberg, Germany, 1996; pp. 1–426. [Google Scholar]
- Margesin, R.; Jud, M.; Tscherko, D.; Schinner, F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol. Ecol. 2009, 67, 208–218. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E.; Tunlid, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Federle, T.W. Perspectives in Microbial Ecology; Megusar, F., Gantar, M., Eds.; Slovene Society for Microbiology: Ljubljana, Slovenia, 1986; pp. 493–498. [Google Scholar]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Haubert, D.; Häggblom, M.M.; Langel, R.; Scheu, S.; Ruess, L. Trophic shift of stable isotopes and fatty acids in Collembola on bacterial diets. Soil Biol. Biochem. 2006, 38, 2004–2007. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar]
- Parkinson, D.; Gray, T.R.G.; Williams, S.T. Methods of Studying the Ecology of Soil Microorganisms; Handbooks International Biological Programme: Blackwell Sci. Publ.: Oxford/Edinburgh, UK, 1971; pp. 1–128. [Google Scholar]
- Hansen, J.F.; Thingstad, T.F.; Godsoyr, J. Evaluation of fungal lengths and hyphal biomass in soil by a membrane filter technique. Oikos 1974, 25, 102–107. [Google Scholar] [CrossRef]
- Zhang, D.C.; Liu, H.C.; Xin, Y.H.; Zhou, Y.G.; Schinner, F.; Margesin, R. Sphingopyxis bauzanensis sp. nov., a novel psychrophilic bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2618–2622. [Google Scholar] [CrossRef]
- Zhang, D.C.; Moertelmaier, C.; Margesin, R. Characterization of the bacterial and archaeal diversity in hydrocarbon-contaminated soil. Sci. Total Environ. 2012, 421-422, 184–196. [Google Scholar] [CrossRef]
- Zhang, D.C.; Schumann, P.; Redzic, M.; Zhou, Y.G.; Liu, H.C.; Schinner, F.; Margesin, R. Nocardioides alpinus sp. nov., a psychrophilic actinomycete isolated from alpine glacier cryoconite. Int. J. Syst. Evol. Microbiol. 2012, 62, 445–450. [Google Scholar] [CrossRef]
- Margesin, R.; Plaza, G.A.; Kasenbacher, S. Characterization of bacterial communities at heavy-metal-contaminated sites. Chemosphere 2011, 82, 1583–1588. [Google Scholar] [CrossRef]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar]
- Margesin, R.; Gander, S.; Zacke, G.; Gounot, A.M.; Schinner, F. Hydrocarbon degradation and enzyme activities of cold-adapted bacteria and yeasts. Extremophiles 2003, 7, 451–458. [Google Scholar] [CrossRef]
- Gratia, E.; Weekers, F.; Margesin, R.; D’Amico, S.; Thonart, P.; Feller, G. Selection of a cold-adapted bacterium for bioremediation of wastewater at low temperatures. Extremophiles 2009, 13, 763–768. [Google Scholar] [CrossRef]
- Margesin, R.; Moertelmaier, C.; Mair, J. Low-temperature biodegradation of petroleum hydrocarbons (n-alkanes, phenol, anthracene, pyrene) by four actinobacterial strains. Int. Biodeterior. Biodegradation 2012. [Google Scholar] [CrossRef]
- Vorobyova, E.; Soina, V.; Gorlenko, M.; Minkovskaya, N.; Zalinova, N.; Mamukelashvili, A.; Gilichinsky, D.A.; Rivkina, E.; Vishnivetskaya, T. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol. Rev. 1997, 20, 277–290. [Google Scholar] [CrossRef]
- Dmitriev, V.V.; Suzina, N.E.; Rusakova, T.G.; Gilichinsky, D.A.; Duda, V.I. Ultrastructural characteristics of natural forms of microorganisms isolated from permafrost grounds of Eastern Siberia by the method of low-temperature fractionation. Dokl. Biol. Sci. 2001, 378, 304–306. [Google Scholar] [CrossRef]
- Vorobyova, E.; Minkovsky, N.; Mamukelashvili, A.; Zvyagintsev, D.; Soina, V.; Polanskaya, L.; Gilichinsky, D. Permafrost Response on Economic Development, Environmental Security and Natural Resources; Paepe, R., Melnikov, V.P., Eds.; Kluwer Acedemic Publishers: Norwell, MA, USA, 2001; pp. 527–541. [Google Scholar]
- Rivkina, E.; Gilichinsky, D.; Wagener, S.; Tiedje, J.; McGrath, J. Biogeochemical activity of anaerobic microorganisms from buried permafrost sediments. Geomicrobiology 1998, 15, 87–193. [Google Scholar]
- Kepner, R.L.; Pratt, J.R. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: Past and present. Microbiol. Rev. 1994, 58, 603–615. [Google Scholar]
- Nadeau, J.L.; Perreault, N.N.; Niederberger, T.D.; Whyte, L.G.; Sun, H.J.; Leon, R. Fluorescence microscopy as a tool for in situ life detection. Astrobiology 2008, 8, 859–874. [Google Scholar] [CrossRef]
- Steven, B.; Briggs, G.; McKay, C.P.; Pollard, W.H.; Greer, C.W.; Whyte, L.G. Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods. FEMS Microbiol. Ecol. 2007, 59, 513–523. [Google Scholar] [CrossRef]
- Trolldenier, G. Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer Lab Manual: Berlin/Heidelberg, Germany, 1996; pp. 15–19. [Google Scholar]
- Gilichinsky, D.A.; Wilson, G.S.; Friedmann, E.I.; McKay, C.P.; Sletten, R.S.; Rivkina, E.M.; Vishnivetskaya, T.A.; Erokhina, L.G.; Ivanushkina, N.E.; Kochkina, G.A.; et al. Microbial populations in Antarctic permafrost: Biodiversity, state, age, and implication for astrobiology. Astrobiology 2007, 7, 275–311. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A.; Petrova, M.A.; Urbance, J.; Ponder, M.; Moyer, C.L.; Gilichinsky, D.A.; Tiedje, J.M. Bacterial community in ancient Siberian permafrost as characterized by culture and culture-independent methods. Astrobiology 2006, 6, 400–414. [Google Scholar] [CrossRef]
- Shi, T.; Reeves, R.H.; Gilichinsky, D.A.; Friedmann, E.I. Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb. Ecol. 1997, 33, 169–179. [Google Scholar] [CrossRef]
- Hinsa-Leisure, S.M.; Bhavaraju, L.; Rodrigues, J.L.M.; Bakermans, C.; Gilichinsky, D.A.; Tiedje, J.A. Characterization of a bacterial community from a northeastern Siberian seacost permafrost sample. FEMS Microbiol. Ecol. 2010, 74, 103–113. [Google Scholar] [CrossRef]
- Vandera, E.; Kavakiotis, K.; Kallimanis, A.; Kyrpides, N.C.; Drainas, C.; Koukkou, A.I. Heterologous expression and characterization of two 1-hydroxy-2-naphthoic acid dioxygenases from Arthrobacter phenanthrenivorans. Appl. Environ. Microbiol. 2012, 78, 621–627. [Google Scholar] [CrossRef]
- Mannisto, M.K.; Schumann, P.; Rainey, F.A.; Kampfer, P.; Tsitko, I.; Tiirola, M.A.; Salkinoja-Salonen, M.S. Subtercola boreus gen. nov., sp. nov. and Subtercola frigoramans sp. nov., two new psychrophilic actinobacteria isolated from boreal groundwater. Int. J. Syst. Evol. Microbiol. 2000, 50, 1731–1739. [Google Scholar]
- Zhang, D.C.; Redzic, M.; Schinner, F.; Margesin, R. Glaciimonas immobilis gen. nov., sp. nov., a novel member of the family Oxalobacteraceae isolated from alpine glacier cryoconite. Int. J. Syst. Evol. Microbiol. 2011, 61, 2186–2190. [Google Scholar] [CrossRef]
- Yergeau, E.; Hogues, H.; Whyte, L.G.; Greer, C.W. The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses. ISME J. 2010, 4, 1206–1214. [Google Scholar] [CrossRef]
- Ozerskaya, S.; Kochkina, G.; Ivanushhkina, N.; Gilichinsky, D.A. Permafrost Soils; Margesin, R., Ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2009; pp. 85–95. [Google Scholar]
- Steven, B.; Pollard, W.H.; Greer, C.W.; Whyte, L.G. Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian high Arctic. Environ. Microbiol. 2008, 10, 3388–3403. [Google Scholar] [CrossRef]
- Franks, F. Nucleation of ice and its management in ecosystems. Phil. Trans. Roy. Soc. Lond. A 2003, 361, 557–574. [Google Scholar] [CrossRef]
- Petrova, M.; Gorlenko, Z.; Mindlin, S. Tn5045, a novel integron-containing antibiotic and chromate resistance transposon isolated from a permafrost bacterium. Res. Microbiol. 2011, 162, 337–345. [Google Scholar] [CrossRef]
- Margesin, R.; Miteva, V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 2011, 162, 346–361. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar]
- Ponder, M.A.; Gilmour, S.J.; Bergholz, P.W.; Mindock, C.A.; Hollingsworth, R.; Thomashow, M.F.; Tiedje, J.M. Characterization of potential stress responses in ancient Siberian permafrost psychroactive bacteria. FEMS Microbiol. Ecol. 2005, 53, 103–115. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, D.-C.; Brouchkov, A.; Griva, G.; Schinner, F.; Margesin, R. Isolation and Characterization of Bacteria from Ancient Siberian Permafrost Sediment. Biology 2013, 2, 85-106. https://doi.org/10.3390/biology2010085
Zhang D-C, Brouchkov A, Griva G, Schinner F, Margesin R. Isolation and Characterization of Bacteria from Ancient Siberian Permafrost Sediment. Biology. 2013; 2(1):85-106. https://doi.org/10.3390/biology2010085
Chicago/Turabian StyleZhang, De-Chao, Anatoli Brouchkov, Gennady Griva, Franz Schinner, and Rosa Margesin. 2013. "Isolation and Characterization of Bacteria from Ancient Siberian Permafrost Sediment" Biology 2, no. 1: 85-106. https://doi.org/10.3390/biology2010085




