Subjective Mood in Young Unmedicated Depressed Women under High and Low Sleep Pressure Conditions
Abstract
:1. Introduction
- Under high sleep pressure: MDD women without sleep disturbances will exhibit improvement in subjective mood during the course of a 40-h SD (high sleep pressure conditions), as compared to that of healthy control women. The SD-mood enhancement may occur due to the postulated deficiency in S-process in MDD individuals. Furthermore, the comparison to healthy age- and sex matched controls will allow us to tease apart how the SD-response is changed due to an underlying MDD (without co-morbid sleep disturbances) symptomatology.
- Under low sleep pressure conditions: subjective mood ratings will not differ between MDD and control women during the course of a 40-h multiple NAP protocol, due to a minimal SD-response in both groups, and will undergo a pronounced circadian modulation.
2. Experimental Section
2.1. Study Participants
2.2. Protocol and Study Design
2.3. Mood Ratings
2.4. Data Analyses and Statistics
3. Results
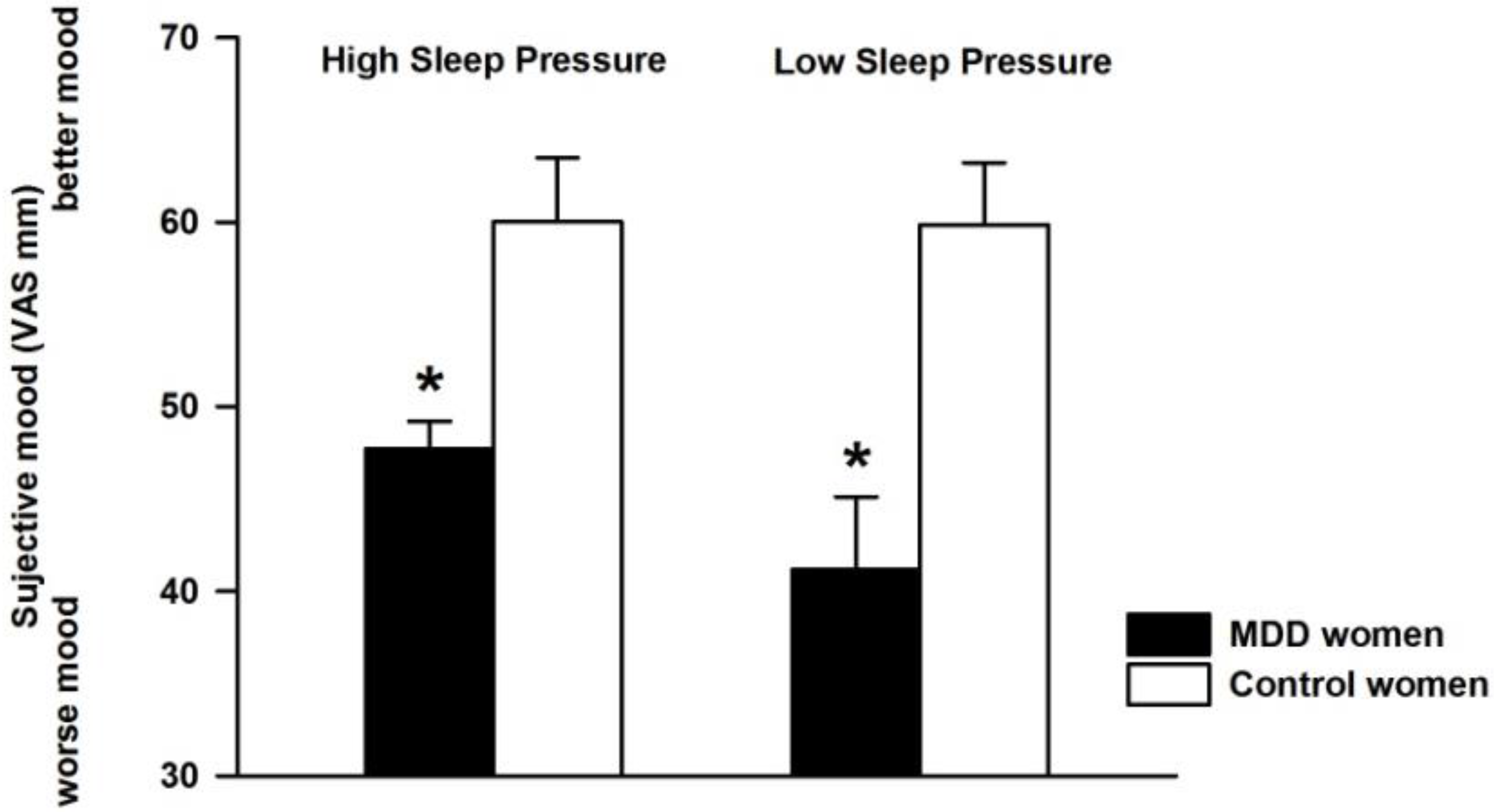
3.1. Effect of Differential Sleep Pressure Conditions on Subjective Mood
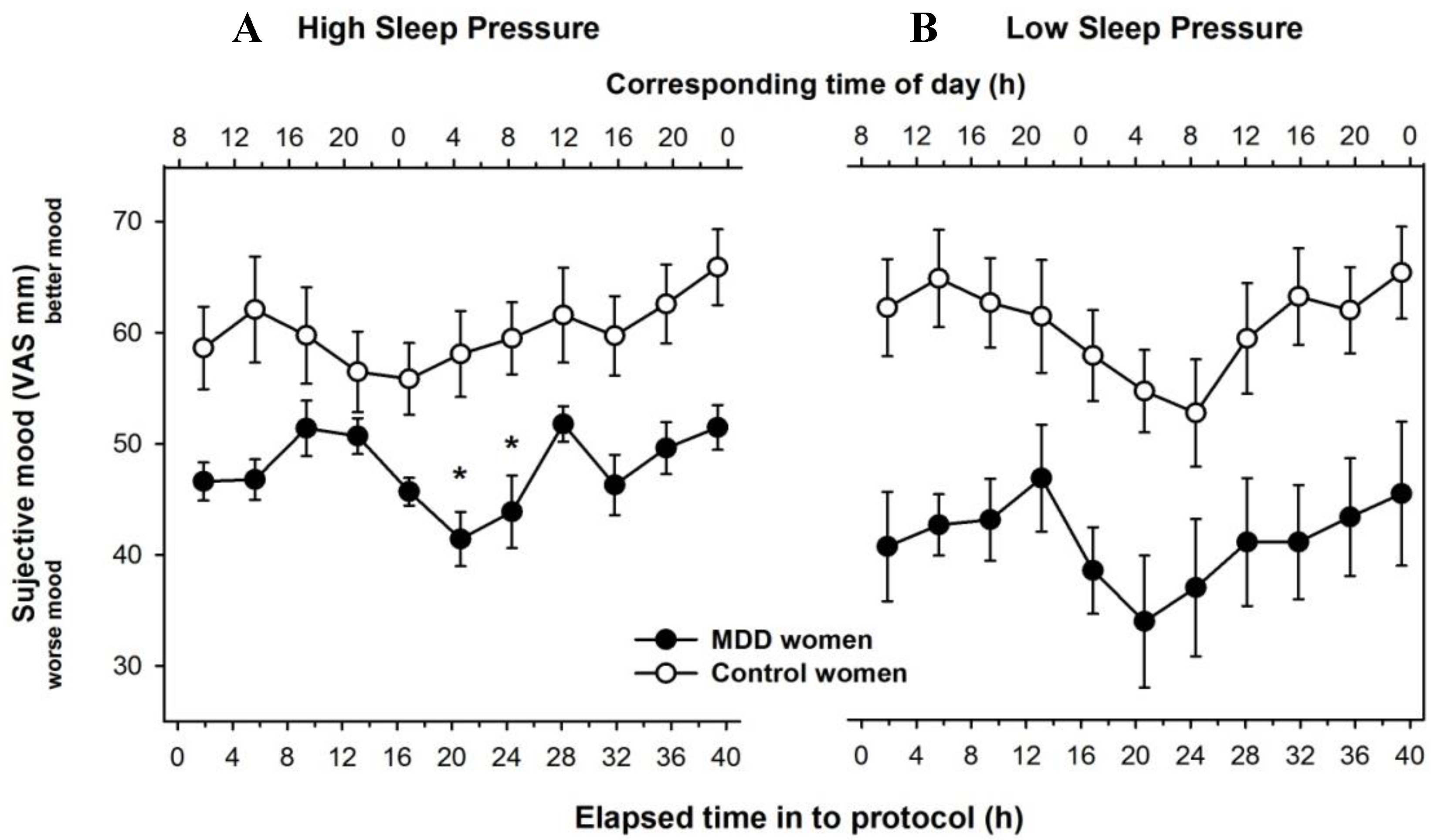
3.2. Time Course of Subjective Mood Levels under Different Sleep Pressure Conditions
3.3. Individual Time-Course of Subjective Mood
3.4. HAMD-7 Observer Ratings in MDD Women
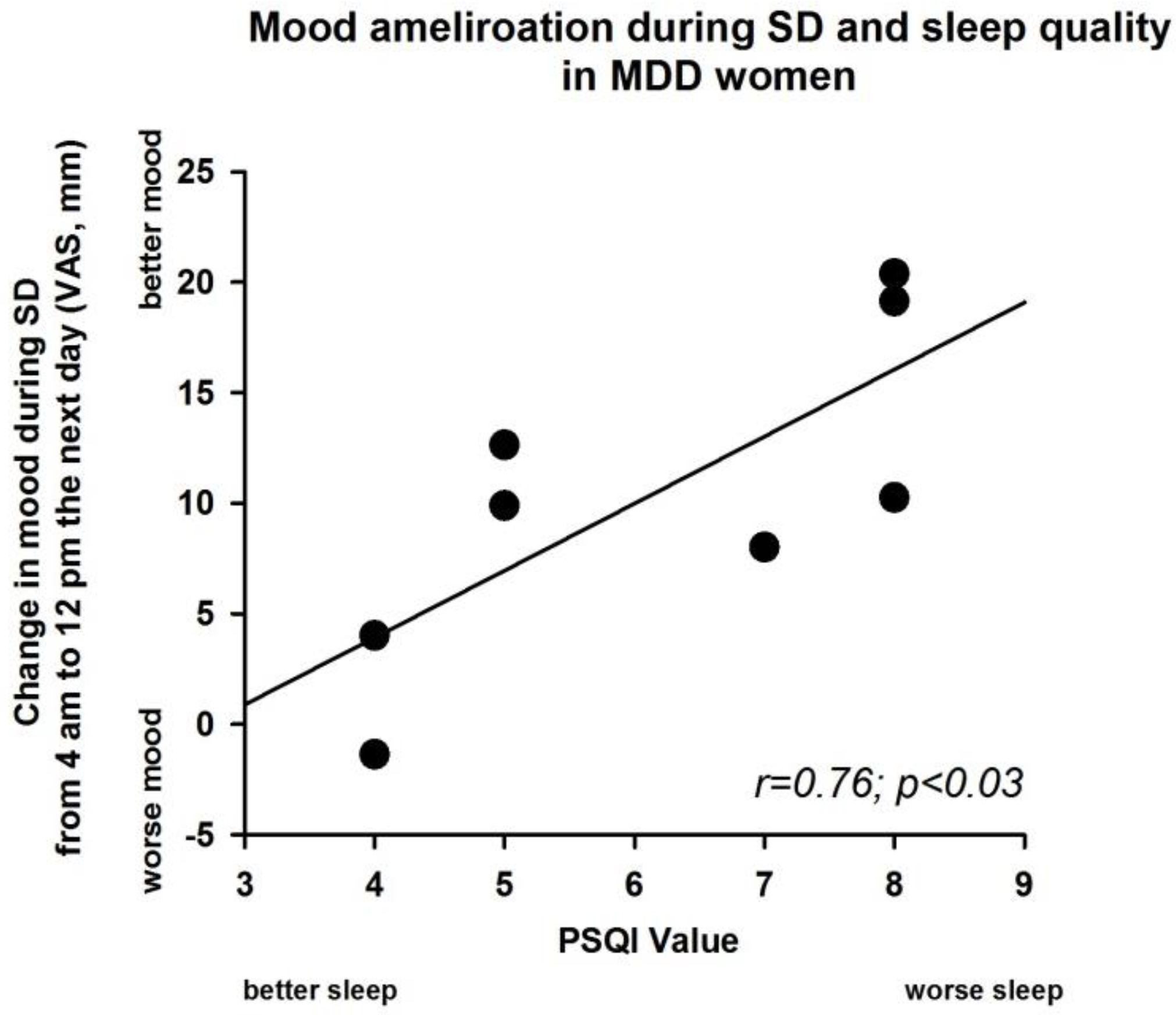
3.5. Sleep Deprivation Effect and Severity of PSQI Value in MDD Women
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wirz-Justice, A.; Van den Hoofdakker, R.H. Sleep deprivation in depression: What do we know, where do we go? Biol. Psychiatry 1999, 46, 445–453. [Google Scholar] [CrossRef]
- Wehr, T.A.; Wirz-Justice, A. Internal coincidence model for sleep deprivation and depression. In Sleep 1980: 5th European Congress on Sleep Research, Amsterdam, September 1980 Circadian Rhythms, Dreams, Noise and Sleep, Neurophysiology, 1st ed.; S. Karger: Basel, Switzerland, 1981; pp. 26–33. [Google Scholar]
- Borbely, A.A.; Wirz-Justice, A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum. Neurobiol. 1982, 1, 205–210. [Google Scholar] [PubMed]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.; Hoffmann, R.; Trivedi, M.; Rush, A.J. Slow-wave activity in NREM sleep: Sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000, 95, 201–213. [Google Scholar] [CrossRef]
- Finelli, L.A.; Baumann, H.; Borbely, A.A.; Achermann, P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience 2000, 101, 523–529. [Google Scholar] [CrossRef]
- Cajochen, C.; Knoblauch, V.; Krauchi, K.; Renz, C.; Wirz-Justice, A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport 2001, 12, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Gillin, J.C.; Duncan, W.C.; Murphy, D.L.; Post, R.M.; Wehr, T.A.; Goodwin, F.K.; Wyatt, R.J.; Bunney, W.E., Jr. Age-related changes in sleep in depressed and normal subjects. Psychiatry Res. 1981, 4, 73–78. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Foster, F.G.; Reich, L.; Thompson, S.K.; Weiss, B. EEG sleep changes as predictors in depression. Am. J. Psychiatry 1976, 133, 622–626. [Google Scholar] [PubMed]
- Kupfer, D.J.; Ulrich, R.F.; Coble, P.A.; Jarrett, D.B.; Grochocinski, V.; Doman, J.; Matthews, G.; Borbely, A.A. Application of automated REM and slow wave sleep analysis: II. Testing the assumptions of the two-process model of sleep regulation in normal and depressed subjects. Psychiatry Res. 1984, 13, 335–343. [Google Scholar] [CrossRef]
- Frey, S.; Birchler-Pedross, A.; Hofstetter, M.; Brunner, P.; Gotz, T.; Munch, M.; Blatter, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Young women with major depression live on higher homeostatic sleep pressure than healthy controls. Chronobiol. Int. 2012, 29, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Birchler-Pedross, A.; Hofstetter, M.; Brunner, P.; Gotz, T.; Munch, M.; Blatter, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Challenging the sleep homeostat: Sleep in depression is not premature aging. Sleep Med. 2012, 13, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Birchler-Pedross, A.; Frey, S.; Chellappa, S.L.; Gotz, T.; Brunner, P.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Higher frontal EEG synchronization in young women with major depression: A marker for increased homeostatic sleep pressure? Sleep 2011, 34, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Wunderlich, U.; Gruschwitz, S.; Zaudig, M. Strukturiertes Klinisches Interview für DSM-IV (SKID); Beltz-Test: Göttingen, Germany, 1996. [Google Scholar]
- Williams, J.B.; Terman, M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS); New York State Psychiatric Institute: New York, NY, USA, 2003. [Google Scholar]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, V.; Martens, W.L.; Wirz-Justice, A.; Cajochen, C. Human sleep spindle characteristics after sleep deprivation. Clin. Neurophysiol. 2003, 114, 2258–2267. [Google Scholar] [CrossRef]
- Birchler-Pedross, A.; Schroder, C.M.; Munch, M.; Knoblauch, V.; Blatter, K.; Schnitzler-Sack, C.; Wirz-Justice, A.; Cajochen, C. Subjective well-being is modulated by circadian phase, sleep pressure, age, and gender. J. Biol. Rhythms 2009, 24, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Torsvall, L.; Akerstedt, T. A diurnal type scale. Construction, consistency and validation in shift work. Scand. J. Work Environ. Health 1980, 6, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Driver, H.S. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Shibui, K.; Uchiyama, M.; Okawa, M.; Kudo, Y.; Kim, K.; Liu, X.; Kamei, Y.; Hayakawa, T.; Akamatsu, T.; Ohta, K.; et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol. Psychiatry 2000, 48, 1062–1068. [Google Scholar] [CrossRef]
- Cajochen, C.; Khalsa, S.B.; Wyatt, J.K.; Czeisler, C.A.; Dijk, D.J. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am. J. Physiol. 1999, 277, R640–R649. [Google Scholar] [PubMed]
- McIntyre, R.S.; Konarski, J.Z.; Mancini, D.A.; Fulton, K.A.; Parikh, S.V.; Grigoriadis, S.; Grupp, L.A.; Bakish, D.; Filteau, M.J.; Gorman, C.; et al. Measuring the severity of depression and remission in primary care: Validation of the HAMD-7 scale. CMAJ 2005, 173, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Nissen, C.; Feige, B.; Konig, A.; Voderholzer, U.; Berger, M.; Riemann, D. Delta sleep ratio as a predictor of sleep deprivation response in major depression. J. Psychiatr. Res. 2001, 35, 155–163. [Google Scholar] [CrossRef]
- Landsness, E.C.; Goldstein, M.R.; Peterson, M.J.; Tononi, G.; Benca, R.M. Antidepressant effects of selective slow wave sleep deprivation in major depression: A high-density EEG investigation. J. Psychiatr. Res. 2011, 45, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.G.; Dendukuri, N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. Am. J. Psychiatry 2003, 160, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Perlis, M.L.; Giles, D.E.; Mendelson, W.B.; Bootzin, R.R.; Wyatt, J.K. Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. J. Sleep Res. 1997, 6, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Voderholzer, U. Primary insomnia: A risk factor to develop depression? J. Affect. Disord. 2003, 76, 255–259. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Roth, T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 2003, 37, 9–15. [Google Scholar] [CrossRef]
- Breslau, N.; Roth, T.; Rosenthal, L.; Andreski, P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol. Psychiatry 1996, 39, 411–418. [Google Scholar] [CrossRef]
- Dryman, A.; Eaton, W.W. Affective symptoms associated with the onset of major depression in the community: Findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr. Scand. 1991, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mallon, L.; Broman, J.E.; Hetta, J. Relationship between insomnia, depression, and mortality: A 12-year follow-up of older adults in the community. Int. Psychogeriatr. 2000, 12, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Frank, E.; Lowe, K.K.; Cherry, C.R.; Kupfer, D.J. Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol. Psychiatry 1997, 41, 406–418. [Google Scholar] [CrossRef]
- Koorengevel, K.M.; Beersma, D.G.; den Boer, J.A.; van den Hoofdakker, R.H. Mood regulation in seasonal affective disorder patients and healthy controls studied in forced desynchrony. Psychiatry Res. 2003, 117, 57–74. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birchler-Pedross, A.; Frey, S.; Götz, T.; Brunner, P.; Knoblauch, V.; Wirz-Justice, A.; Chellappa, S.L.; Cajochen, C. Subjective Mood in Young Unmedicated Depressed Women under High and Low Sleep Pressure Conditions. Biology 2016, 5, 52. https://doi.org/10.3390/biology5040052
Birchler-Pedross A, Frey S, Götz T, Brunner P, Knoblauch V, Wirz-Justice A, Chellappa SL, Cajochen C. Subjective Mood in Young Unmedicated Depressed Women under High and Low Sleep Pressure Conditions. Biology. 2016; 5(4):52. https://doi.org/10.3390/biology5040052
Chicago/Turabian StyleBirchler-Pedross, Angelina, Sylvia Frey, Thomas Götz, Patrick Brunner, Vera Knoblauch, Anna Wirz-Justice, Sarah L. Chellappa, and Christian Cajochen. 2016. "Subjective Mood in Young Unmedicated Depressed Women under High and Low Sleep Pressure Conditions" Biology 5, no. 4: 52. https://doi.org/10.3390/biology5040052






