Enhanced Adsorption and Recovery of Uranyl Ions by NikR Mutant-Displaying Yeast
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Surface Display of Tandemly Fused MBDs of NikR Mutant
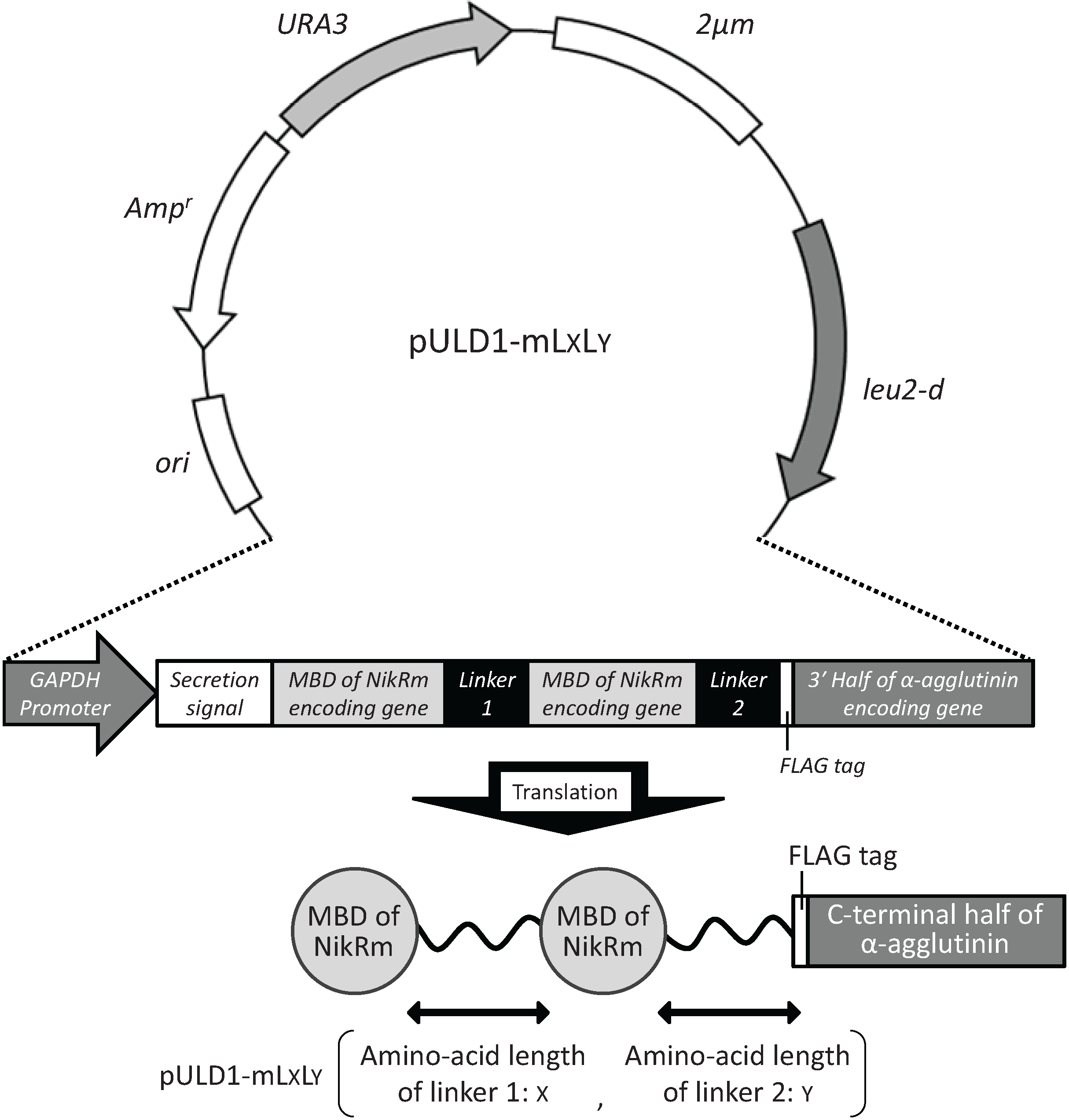
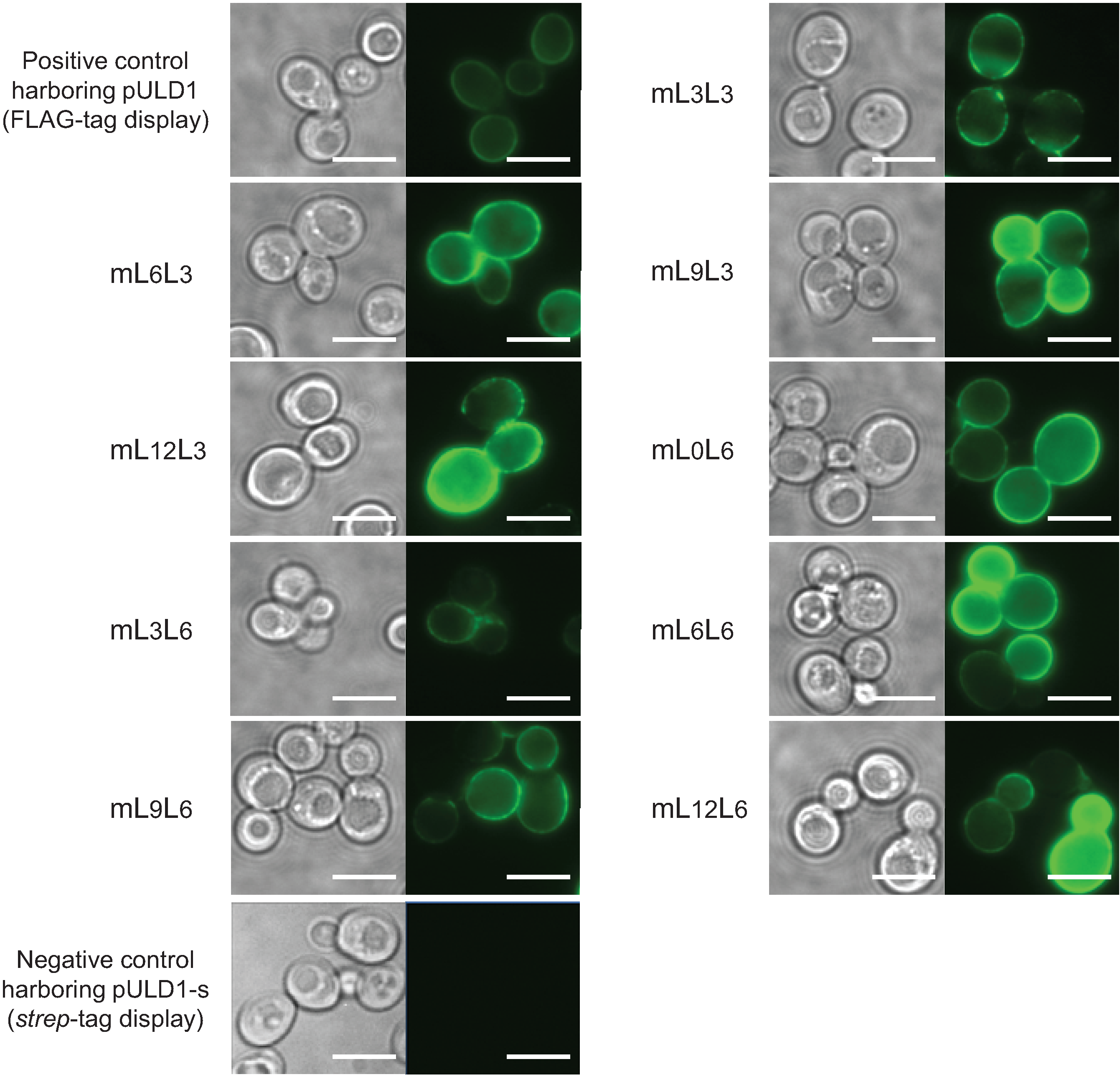
2.2. Bioadsorption of Uranyl Ions by Cell-Surface-Engineered Yeasts
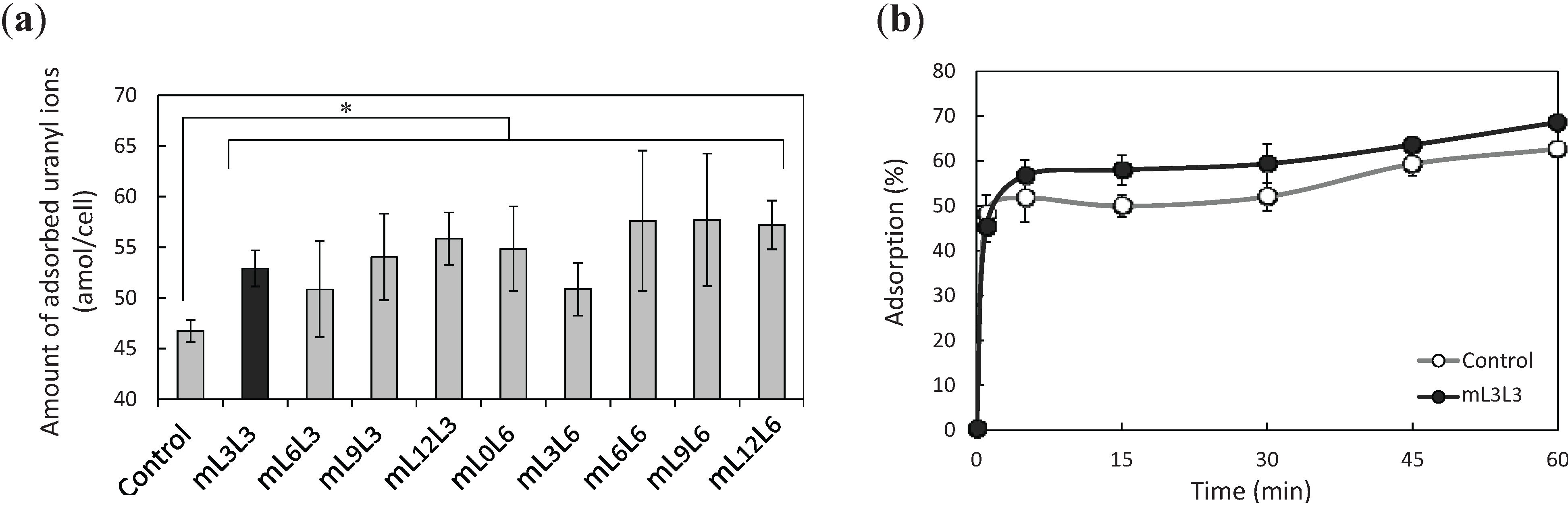
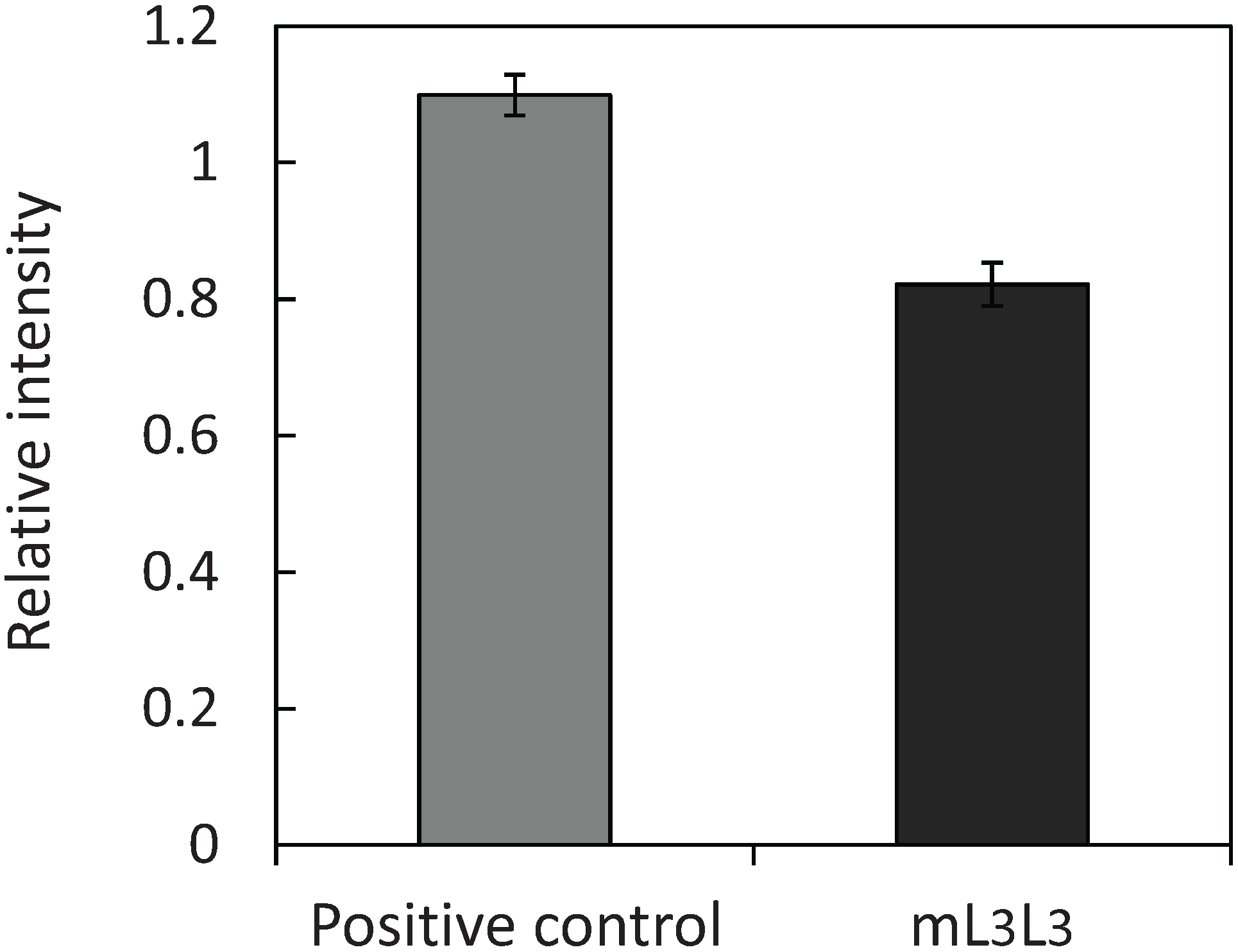
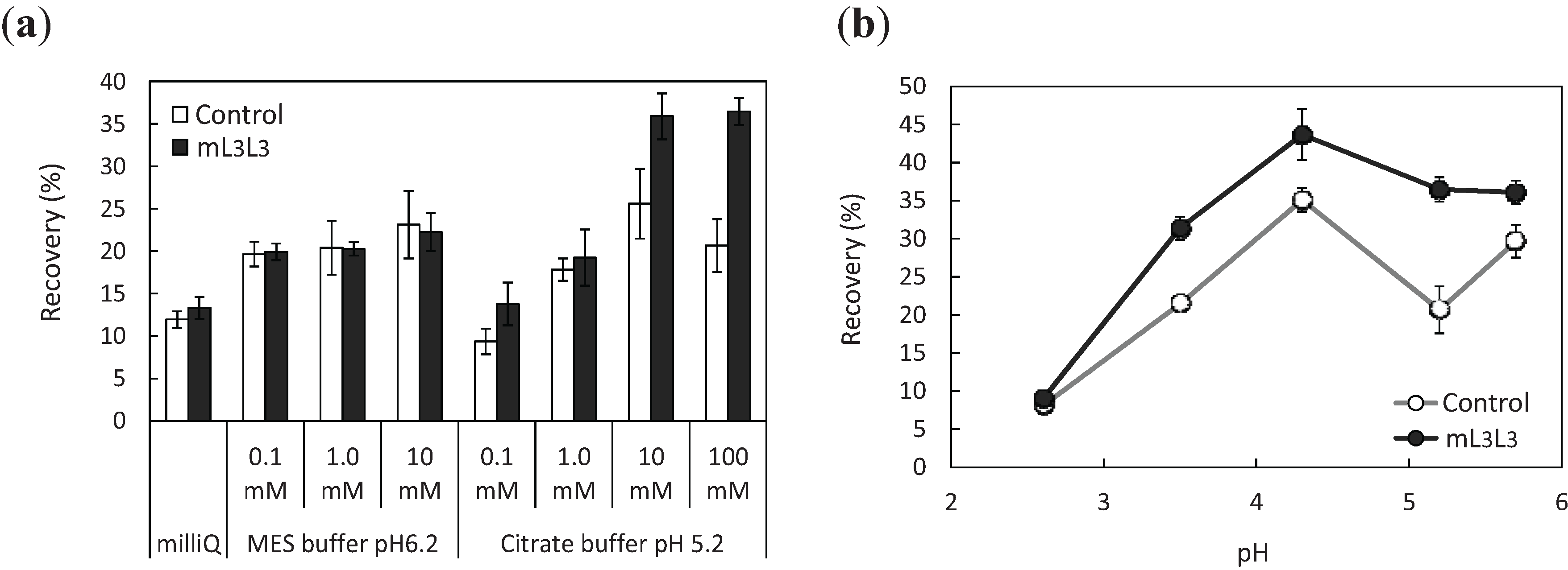
2.3. Recovery of Uranyl Ions Adsorbed on Yeast Cell Surface
3. Experimental
3.1. Strains and Media
3.2. Construction of Plasmids for Cell Surface Display
| Primer name | Sequence | Application |
|---|---|---|
| NikR-MBD-F | 5'-CGATAGATCTGGCACGCAAGGTTTCGCGGTGCTGTC-3' | Cloning of NikR-MBD |
| NikR-MBD-R | 5'-CGATCTCGAGATCTTCCTTCGGCAAGCACTGC-3' | Cloning of NikR-MBD |
| V72S-F | 5'-CGCGACTTAGCCAGCCGCATTAGCTCCACCCAGGATCATCACCACGACC-3' | Amino acid substitution (V72S) of NikR-MBD |
| V72S-R | 5'-GGTCGTGGTGATGATCCTGGGTGGAGCTAATGCGGCTGGCTAAGTCGCG-3' | Amino acid substitution (V72S) of NikR-MBD |
| H76D-F | 5'-GCATTGTCTCTCCACCCAGGATCATCACCACGACCTCTCCG-3' | Amino acid substitution (H76D) of NikR-MBD |
| H76D-R | 5'-CGGAGAGGTCGTGGTGATGATCCTGGGTGGAGACAATCA-3' | Amino acid substitution (H76D) of NikR-MBD |
| C95D-F | 5'-GCACATCAACCACGACGACGACCTGGAAATCGCCGTG-3' | Amino acid substitution (C95D) of NikR-MBD |
| C95D-R | 5'-CACGGCGATTTCCAGGTCGTCGTCGTGGTTGATGTGC-3' | Amino acid substitution (C95D) of NikR-MBD |
| Bgl II-Sal I-NikRm-F | 5'-CGATAGATCTAGAGTCGACGGCACGCAAGGTTTCGCGGTGCTGTCG-3' | Cloning of MBD-NikRm with linker |
| NikRm-L0-R | 5'-CGATCTCGAGATCTTCCTTCGGCAAGCACTGC-3' | Cloning of MBD-NikRm with linker |
| NikRm-L3-R | 5'-CGATCTCGAGAGATCCACCATCTTCCTTCGGCAAGCACTGC-3' | Cloning of MBD-NikRm with linker |
| NikRm-L6-R | 5'-CGATCTCGAGAGATCCACCAGATCCACCATCTTCCTTCGGCAAGCACTGC-3' | Cloning of MBD-NikRm with linker |
| NikRm-L9-R | 5'-CGATCTCGAGAGATCCACCAGATCCACCAGATCCACCATCTTCCTTCGGCAAGCACTGC-3' | Cloning of MBD-NikRm with linker |
| NikRm-L12-R | 5'-CGATCTCGAGAGATCCACCAGATCCACCAGATCCACCAGATCCACCATCTTCCTTCGGCAAGCACTGC-3' | Cloning of MBD-NikRm with linker |
3.3. Transformation of S. cerevisiae
| Strain name | Signature | Length of linker 1 (a.a.) | Length of linker 2 (a.a.) |
|---|---|---|---|
| mL3L3 | Displaying tandemly fused MBDs of NikRm | 3 | 3 |
| mL6L3 | Displaying tandemly fused MBDs of NikRm | 6 | 3 |
| mL9L3 | Displaying tandemly fused MBDs of NikRm | 9 | 3 |
| mL12L3 | Displaying tandemly fused MBDs of NikRm | 12 | 3 |
| mL0L6 | Displaying tandemly fused MBDs of NikRm | 0 | 6 |
| mL3L6 | Displaying tandemly fused MBDs of NikRm | 3 | 6 |
| mL6L6 | Displaying tandemly fused MBDs of NikRm | 6 | 6 |
| mL9L6 | Displaying tandemly fused MBDs of NikRm | 9 | 6 |
| mL12L6 | Displaying tandemly fused MBDs of NikRm | 12 | 6 |
| pULD1 | Negative control for bioadsorption experiment | − | − |
| pULD1-s | Negative control for immunofluorescence labeling | − | − |
3.4. Immunofluorescence Microscopy
3.5. Measurement of Fluorescence Intensity
3.6. Bioadsorption and Recovery of Uranyl Ions by Cell-Surface-Engineered Yeast Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ueda, M.; Tanaka, A. Genetic immobilization of proteins on the yeast cell surface. Biotechnol. Adv. 2000, 18, 121–140. [Google Scholar] [CrossRef]
- Ueda, M.; Tanaka, A. Cell surface engineering of yeast: Construction of arming yeast with biocatalyst. J. Biosci. Bioeng. 2000, 90, 125–136. [Google Scholar]
- Kuroda, K.; Nishitani, T.; Ueda, M. Specific adsorption of tungstate by cell surface display of the newly designed ModE mutant. Appl. Microbiol. Biotechnol. 2012, 96, 153–159. [Google Scholar] [CrossRef]
- Kuroda, K.; Ueda, M. Molecular design of the microbial cell surface toward the recovery of metal ions. Curr. Opin. Biotechnol. 2011, 22, 427–433. [Google Scholar]
- Nishitani, T.; Shimada, M.; Kuroda, K.; Ueda, M. Molecular design of yeast cell surface for adsorption and recovery of molybdenum, one of rare metals. Appl. Microbiol. Biotechnol. 2010, 86, 641–648. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Sadler, P.J. Transferrin as a metal ion mediator. Chem. Rev. 1999, 99, 2817–2842. [Google Scholar] [CrossRef]
- Michon, J.; Frelon, S.; Garnier, C.; Coppin, F. Determinations of uranium(VI) binding properties with some metalloproteins (transferrin, albumin, metallothionein and ferritin) by fluorescence quenching. J. Fluoresc. 2010, 20, 581–590. [Google Scholar] [CrossRef]
- Wegner, S.V.; Boyaci, H.; Chen, H.; Jensen, M.P.; He, C. Engineering a uranyl-specific binding protein from NikR. Angew. Chem. Int. Ed. 2009, 48, 2339–2341. [Google Scholar] [CrossRef]
- Chivers, P.T.; Sauer, R.T. NikR repressor: High-affinity nickel binding to the C-terminal domain regulates binding to operator DNA. Chem. Biol. 2002, 9, 1141–1148. [Google Scholar] [CrossRef]
- Carrington, P.E.; Chivers, P.T.; Al-Mjeni, F.; Sauer, R.T.; Maroney, M.J. Nickel coordination is regulated by the DNA-bound state of NikR. Nat. Struct. Biol. 2003, 10, 126–130. [Google Scholar] [CrossRef]
- Wang, S.C.; Dias, A.V.; Bloom, S.L.; Zamble, D.B. Selectivity of metal binding and metal-induced stability of Escherichia coli NikR. Biochemistry 2004, 43, 10018–10028. [Google Scholar] [CrossRef]
- Schreiter, E.R.; Wang, S.C.; Zamble, D.B.; Drennan, C.L. NikR-operator complex structure and the mechanism of repressor activation by metal ions. Proc. Natl. Acad. Sci. USA 2006, 103, 13676–13681. [Google Scholar] [CrossRef]
- Schreiter, E.R.; Sintchak, M.D.; Guo, Y.; Chivers, P.T.; Sauer, R.T.; Drennan, C.L. Crystal structure of the nickel-responsive transcription factor NikR. Nat. Struct. Biol. 2003, 10, 794–799. [Google Scholar] [CrossRef]
- Kuroda, K.; Matsui, K.; Higuchi, S.; Kotaka, A.; Sahara, H.; Hata, Y.; Ueda, M. Enhancement of display efficiency in yeast display system by vector engineering and gene disruption. Appl. Microbiol. Biotechnol. 2009, 82, 713–719. [Google Scholar] [CrossRef]
- Ito, H.; Fukuda, Y.; Murata, K.; Kimura, A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983, 153, 163–168. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuroda, K.; Ebisutani, K.; Iida, K.; Nishitani, T.; Ueda, M. Enhanced Adsorption and Recovery of Uranyl Ions by NikR Mutant-Displaying Yeast. Biomolecules 2014, 4, 390-401. https://doi.org/10.3390/biom4020390
Kuroda K, Ebisutani K, Iida K, Nishitani T, Ueda M. Enhanced Adsorption and Recovery of Uranyl Ions by NikR Mutant-Displaying Yeast. Biomolecules. 2014; 4(2):390-401. https://doi.org/10.3390/biom4020390
Chicago/Turabian StyleKuroda, Kouichi, Kazuki Ebisutani, Katsuya Iida, Takashi Nishitani, and Mitsuyoshi Ueda. 2014. "Enhanced Adsorption and Recovery of Uranyl Ions by NikR Mutant-Displaying Yeast" Biomolecules 4, no. 2: 390-401. https://doi.org/10.3390/biom4020390




