Diverse Mechanisms of Sulfur Decoration in Bacterial tRNA and Their Cellular Functions
Abstract
:1. Introduction
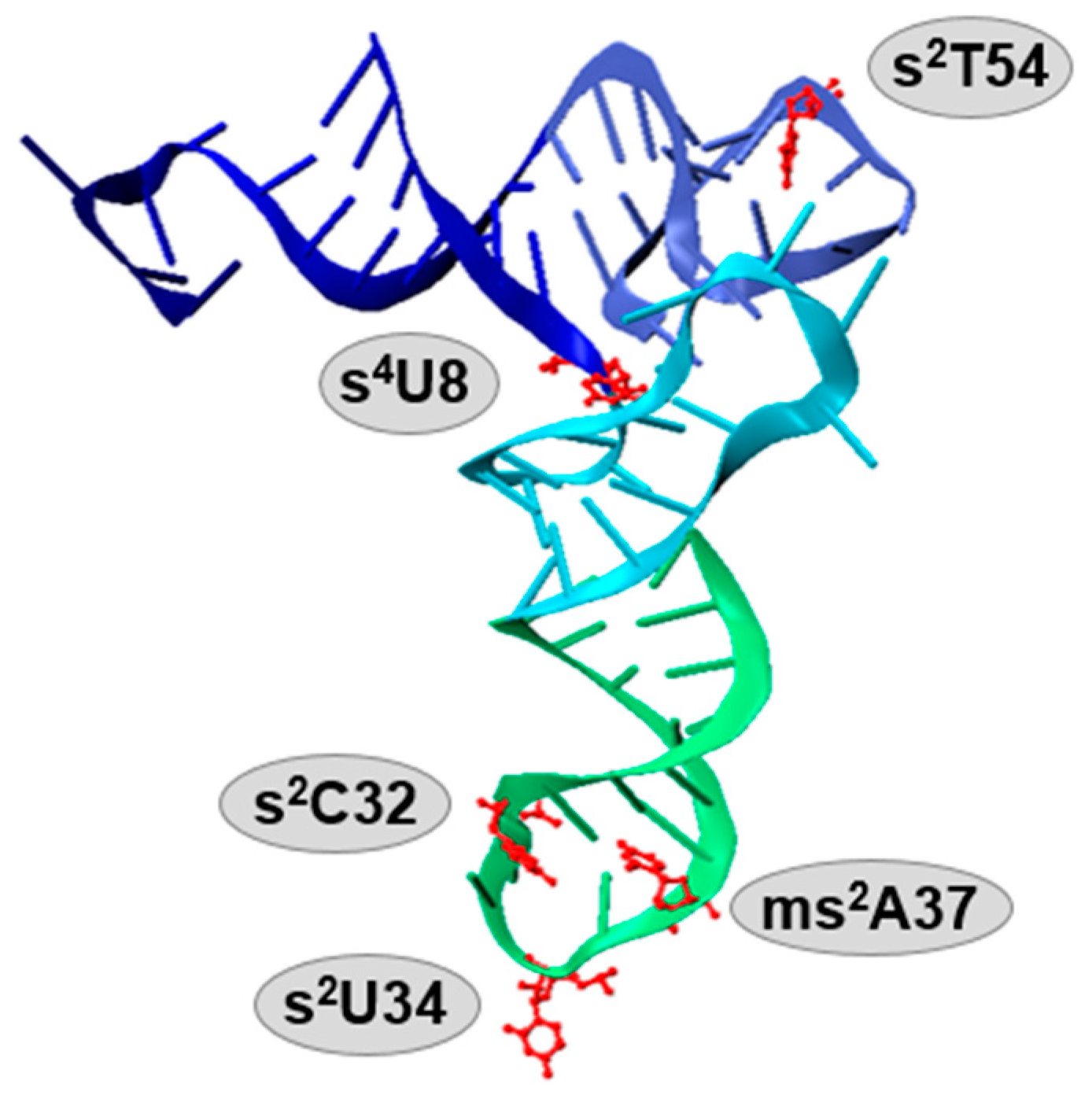
1.1. Anticodon Stem Loop Modifications
1.2. Modifications within tRNA Body and Acceptor Stem
2. Methods for Investigation and Quantification of tRNA Modifications
2.1. Isolation of tRNA from Biological Samples
2.1.1. Chemical Labeling
2.1.2. Enrichment of Modified Nucleoside
2.1.3. Northern Blot
2.2. Quantification of tRNA Modification Levels Using Liquid Chromatography
2.2.1. High Pressure Liquid Chromatography Separation Coupled to Ultraviolet-Visible Detection
2.2.2. Advances in Liquid Chromatography–Mass Spectrometry Methods for Detection of tRNA Modifications
3. Biosynthesis of Thionucleosides in Bacterial tRNA
3.1. 4-thiouridine (s4U)
3.2. 2-thiouridine (s2U)
3.2.1. E. coli 2-thiouridine Biosynthesis: Assembly Line of Sulfur Transfer
3.2.2. tRNA Binding and Adenylation by MnmA
3.2.3. Proposed Order for tRNA U34 Modification
3.3. 2-thiocytosine (s2C)
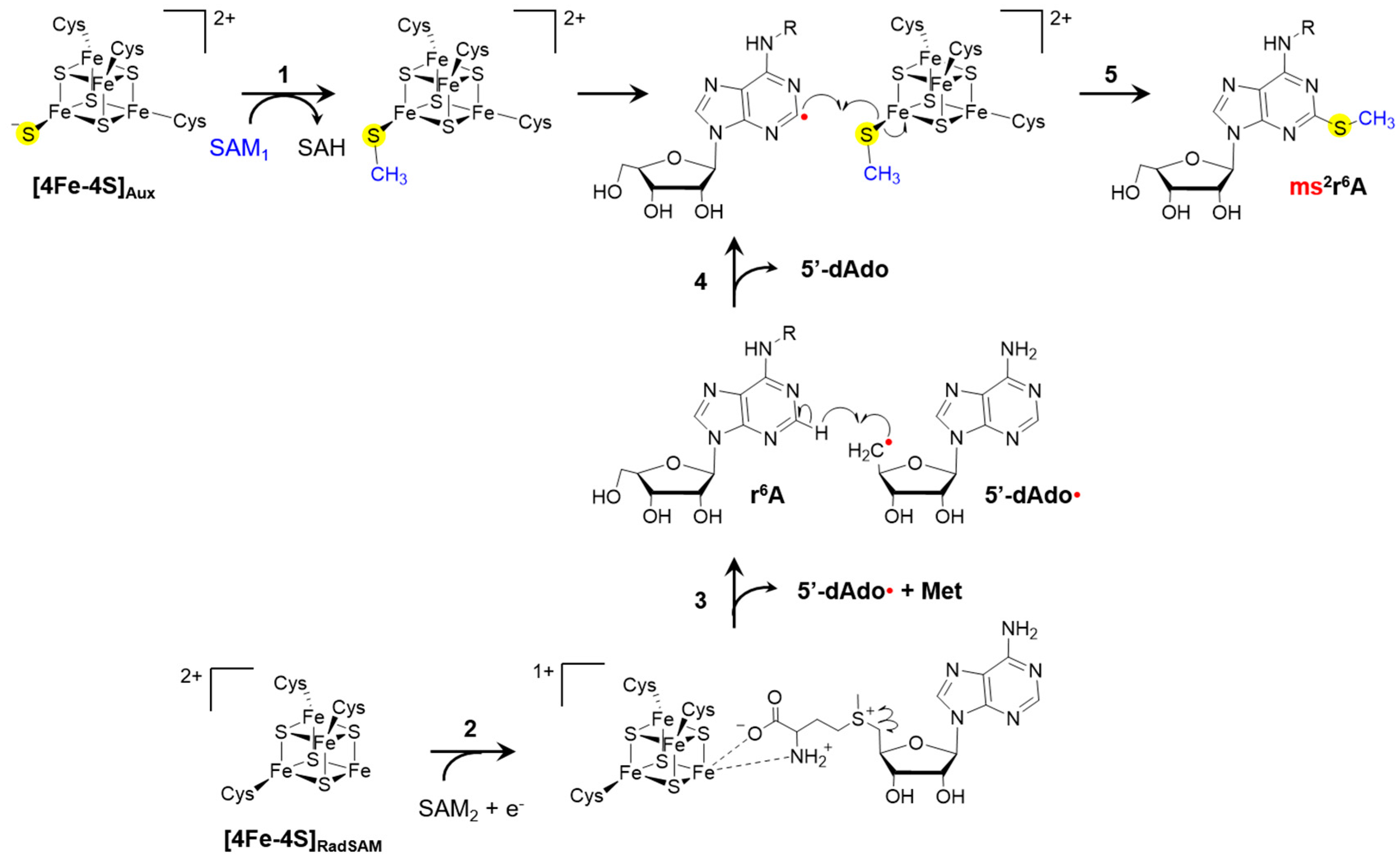
3.4. Modifications within Adenosine 37
4. Interconnectivity between tRNA Modification and Biosynthesis of Other Cofactors
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mccloskey, J.A.; Nishimura, S. Modified nucleosides in transfer-RNA. Acc. Chem. Res. 1977, 10, 403–410. [Google Scholar] [CrossRef]
- Marbaniang, C.N.; Vogel, J. Emerging roles of rna modifications in bacteria. Curr. Opin. Microbiol. 2016, 30, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Machnicka, M.A.; Milanowska, K.; Osman Oglou, O.; Purta, E.; Kurkowska, M.; Olchowik, A.; Januszewski, W.; Kalinowski, S.; Dunin-Horkawicz, S.; Rother, K.M.; et al. Modomics: A database of RNA modification pathways—2013 update. Nucleic Acids Res. 2013, 41, D262–D267. [Google Scholar] [CrossRef] [PubMed]
- Phizicky, E.M.; Alfonzo, J.D. Do all modifications benefit all tRNAs? FEBS Lett. 2010, 584, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Cantara, W.A.; Crain, P.F.; Rozenski, J.; McCloskey, J.A.; Harris, K.A.; Zhang, X.; Vendeix, F.A.; Fabris, D.; Agris, P.F. The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 2011, 39, D195–D201. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, A.; Spears, J.L.; Gaston, K.W.; Limbach, P.A.; Gamper, H.; Hou, Y.M.; Kaiser, R.; Agris, P.F.; Perona, J.J. Structural and mechanistic basis for enhanced translational efficiency by 2-thiouridine at the tRNA anticodon wobble position. J. Mol. Biol. 2013, 425, 3888–3906. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.E.; Alfonzo, J.D. Transfer RNA modifications: Nature’s combinatorial chemistry playground. Wiley Interdiscip. Rev. RNA 2013, 4, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Charette, M.; Gray, M.W. Pseudouridine in RNA: What, where, how, and why. IUBMB Life 2000, 49, 341–351. [Google Scholar] [PubMed]
- Shigi, N. Biosynthesis and functions of sulfur modifications in tRNA. Front. Genet. 2014, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Shigi, N.; Sakaguchi, Y.; Asai, S.; Suzuki, T.; Watanabe, K. Common thiolation mechanism in the biosynthesis of tRNA thiouridine and sulphur-containing cofactors. EMBO J. 2008, 27, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Lauhon, C.T. Requirement for IscS in biosynthesis of all thionucleosides in Escherichia coli. J. Bacteriol. 2002, 184, 6820–6829. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.G. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, B.J.; Arcinas, A.J.; Lee, K.H.; Booker, S.J. Identification of an intermediate methyl carrier in the radical S-adenosylmethionine methylthiotransferases RimO and MiaB. J. Am. Chem. Soc. 2013, 135, 15404–15416. [Google Scholar] [CrossRef] [PubMed]
- Maiocco, S.J.; Arcinas, A.J.; Landgraf, B.J.; Lee, K.H.; Booker, S.J.; Elliott, S.J. Transformations of the FeS clusters of the methylthiotransferases MiaB and RimO, detected by direct electrochemistry. Biochemistry 2016, 55, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Forouhar, F.; Arragain, S.; Atta, M.; Gambarelli, S.; Mouesca, J.M.; Hussain, M.; Xiao, R.; Kieffer-Jaquinod, S.; Seetharaman, J.; Acton, T.B.; et al. Two Fe-S clusters catalyze sulfur insertion by radical-SAM methylthiotransferases. Nat. Chem. Biol. 2013, 9, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.L.; Pierrel, F.; Elleingand, E.; Garcia-Serres, R.; Huynh, B.H.; Johnson, M.K.; Fontecave, M.; Atta, M. MiaB, a bifunctional radical-s-adenosylmethionine enzyme involved in the thiolation and methylation of tRNA, contains two essential [4Fe-4S] clusters. Biochemistry 2007, 46, 5140–5147. [Google Scholar] [CrossRef] [PubMed]
- Pierrel, F.; Douki, T.; Fontecave, M.; Atta, M. MiaB protein is a bifunctional radical-S-adenosylmethionine enzyme involved in thiolation and methylation of tRNA. J. Biol. Chem. 2004, 279, 47555–47563. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F. Bringing order to translation: The contributions of transfer RNA anticodon-domain modifications. EMBO Rep. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Vendeix, F.A.; Graham, W.D. tRNA’s wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007, 366, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.V.T.; Ramakrishnan, V.; Malkiewicz, A.; Agris, P.F. The role of modifications in codon discrimination by tRNALysUUU. Nat. Struct. Mol. Biol. 2004, 11, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.S.; Guenther, R.; Agris, P.F. Orientation of the tRNA anticodon in the ribosomal P-site: Quantitative footprinting with U33-modified, anticodon stem and loop domains. RNA 1999, 5, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.; Janjic, N.; Pribnow, D.; Zichi, D.A. Alignment editing and identification of consensus secondary structures for nucleic acid sequences: Interactive use of dot matrix representations. Nucleic Acids Res. 1995, 23, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Helm, M. tRNA stabilization by modified nucleotides. Biochemistry 2010, 49, 4934–4944. [Google Scholar] [CrossRef] [PubMed]
- El Yacoubi, B.; Bailly, M.; de Crecy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012, 46, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Nameki, N. Identity elements of tRNAThr towards Saccharomyces cerevisiae threonyl-tRNA synthetase. Nucleic Acids Res. 1995, 23, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Rudinger, J.; Florentz, C.; Giege, R. Histidylation by yeast HisRs of tRNA or tRNA-like structure relies on residues −1 and 73 but is dependent on the RNA context. Nucleic Acids Res. 1994, 22, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Shigi, N.; Suzuki, T.; Terada, T.; Shirouzu, M.; Yokoyama, S.; Watanabe, K. Temperature-dependent biosynthesis of 2-thioribothymidine of Thermus thermophilus tRNA. J. Biol. Chem. 2006, 281, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Pan, T. Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet. 2013, 47, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Dumelin, C.E.; Chen, Y.; Leconte, A.M.; Chen, Y.G.; Liu, D.R. Discovery and biological characterization of geranylated RNA in bacteria. Nat. Chem. Biol. 2012, 8, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Tukenmez, H.; Xu, H.; Esberg, A.; Bystrom, A.S. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucleic Acids Res. 2015, 43, 9489–9499. [Google Scholar] [CrossRef]
- Urbonavicius, J.; Qian, Q.; Durand, J.M.; Hagervall, T.G.; Bjork, G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001, 20, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Esberg, B.; Bjork, G.R. The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J. Bacteriol. 1995, 177, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Esberg, B.; Leung, H.C.; Tsui, H.C.; Bjork, G.R.; Winkler, M.E. Identification of the MiaB gene, involved in methylthiolation of isopentenylated A37 derivatives in the tRNA of Salmonella typhimurium and Escherichia coli. J. Bacteriol. 1999, 181, 7256–7265. [Google Scholar] [PubMed]
- Connolly, D.M.; Winkler, M.E. Structure of Escherichia coli K-12 MiaA and characterization of the mutator phenotype caused by MiaA insertion mutations. J. Bacteriol. 1991, 173, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F. Wobble position modified nucleosides evolved to select transfer RNA codon recognition: A modified-wobble hypothesis. Biochimie 1991, 73, 1345–1349. [Google Scholar] [CrossRef]
- Crick, F.H. The genetic code: III. Sci. Am. 1966, 215, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Shigi, N.; Kato, J.; Nishimura, A.; Suzuki, T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 2006, 21, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Ogle, J.M.; Brodersen, D.E.; Clemons, W.M., Jr.; Tarry, M.J.; Carter, A.P.; Ramakrishnan, V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 2001, 292, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Yarian, C.; Townsend, H.; Czestkowski, W.; Sochacka, E.; Malkiewicz, A.J.; Guenther, R.; Miskiewicz, A.; Agris, P.F. Accurate translation of the genetic code depends on tRNA modified nucleosides. J. Biol. Chem. 2002, 277, 16391–16395. [Google Scholar] [CrossRef]
- McKenney, K.M.; Alfonzo, J.D. From prebiotics to probiotics: The evolution and functions of tRNA modifications. Life (Basel) 2016, 6, 13. [Google Scholar] [CrossRef]
- Laxman, S.; Sutter, B.M.; Wu, X.; Kumar, S.; Guo, X.; Trudgian, D.C.; Mirzaei, H.; Tu, B.P. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell 2013, 154, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tuck, S.; Bystrom, A.S. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 2009, 5, e1000561. [Google Scholar] [CrossRef]
- Walker, J.; Kwon, S.Y.; Badenhorst, P.; East, P.; McNeill, H.; Svejstrup, J.Q. Role of elongator subunit Elp3 in Drosophila melanogaster larval development and immunity. Genetics 2011, 187, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.X.; Yan, Q.; Li, X.; Bykhovskaya, Y.; Gallo-Teran, J.; Hajek, P.; Umeda, N.; Zhao, H.; Garrido, G.; Mengesha, E.; et al. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am. J. Hum. Genet. 2006, 79, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Yasukawa, T.; Ohta, S.; Akira, S.; Ishihara, K.; Watanabe, K.; Suzuki, T. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl. Acad. Sci. USA 2004, 101, 15070–15075. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Suzuki, T. Human mitochondrial diseases associated with tRNA wobble modification deficiency. RNA Biol. 2005, 2, 41–44. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, T.; Ishii, N.; Ueda, T.; Ohta, S.; Watanabe, K. Defect in modification at the anticodon wobble nucleotide of mitochondrial tRNALys)with the MERRF encephalomyopathy pathogenic mutation. FEBS Lett. 2000, 467, 175–178. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, T.; Ishii, N.; Ohta, S.; Watanabe, K. Wobble modification defect in tRNA disturbs codon-anticodon interaction in a mitochondrial disease. EMBO J. 2001, 20, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Kirino, Y.; Ishii, N.; Holt, I.J.; Jacobs, H.T.; Makifuchi, T.; Fukuhara, N.; Ohta, S.; Suzuki, T.; Watanabe, K. Wobble modification deficiency in mutant tRNAs in patients with mitochondrial diseases. FEBS Lett. 2005, 579, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Alfonzo, J.D. Posttranscriptional RNA modifications: Playing metabolic games in a cell’s chemical legoland. Chem. Biol. 2014, 21, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Noma, A.; Sakaguchi, Y.; Suzuki, T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009, 37, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Lundgren, H.K.; Hagervall, T.G.; Bjork, G.R. The cysteine desulfurase IscS is required for synthesis of all five thiolated nucleosides present in tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002, 184, 6830–6835. [Google Scholar] [CrossRef] [PubMed]
- Lauhon, C.T.; Kambampati, R. The IscS gene in Escherichia coli is required for the biosynthesis of 4-thiouridine, thiamin, and NAD. J. Biol. Chem. 2000, 275, 20096–20103. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.J.; Djaman, O.; Imlay, J.A.; Kiley, P.J. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 9009–9014. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Batlle, E.; Ribas de Pouplana, L. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014, 20, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Suzuki, T.; Watanabe, S.; Kimura, S.; Kaitsuka, T.; Fujimura, A.; Matsui, H.; Atta, M.; Michiue, H.; Fontecave, M.; et al. Deficit of tRNALys modification by CDKAL1 causes the development of type 2 diabetes in mice. J. Clin. Investig. 2011, 121, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Zhou, B.; Suzuki, T.; Miyata, K.; Ujihara, Y.; Horiguchi, H.; Takahashi, N.; Xie, P.; Michiue, H.; Fujimura, A.; et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 2015, 21, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Giege, R. Toward a more complete view of tRNA biology. Nat. Struct. Mol. Biol. 2008, 15, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, K.; Kimura, S.; Suzuki, T. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 2013, 9, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Favre, A.; Michelson, A.M.; Yaniv, M. Photochemistry of 4-thiouridine in Escherichia coli transfer RNA1Val. J. Mol. Biol. 1971, 58, 367–379. [Google Scholar] [CrossRef]
- Thomas, G.; Favre, A. 4-thiouridine as the target for near-ultraviolet light induced growth delay in Escherichia coli. Biochem. Biophys. Res. Commun. 1975, 66, 1454–1461. [Google Scholar] [CrossRef]
- Mueller, E.G.; Buck, C.J.; Palenchar, P.M.; Barnhart, L.E.; Paulson, J.L. Identification of a gene involved in the generation of 4-thiouridine in tRNA. Nucleic Acids Res. 1998, 26, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Jager, G.; Leipuviene, R.; Pollard, M.G.; Qian, Q.; Bjork, G.R. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 2004, 186, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.G.; Palenchar, P.M.; Buck, C.J. The role of the cysteine residues of ThiI in the generation of 4-thiouridine in tRNA. J. Biol. Chem. 2001, 276, 33588–33595. [Google Scholar] [CrossRef]
- You, D.; Xu, T.; Yao, F.; Zhou, X.; Deng, Z. Direct evidence that ThiI is an ATP pyrophosphatase for the adenylation of uridine in 4-thiouridine biosynthesis. Chembiochem 2008, 9, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gomez, N.C.; Palmer, L.D.; Vivas, E.; Roach, P.L.; Downs, D.M. The rhodanese domain of ThiI is both necessary and sufficient for synthesis of the thiazole moiety of thiamine in Salmonella enterica. J. Bacteriol. 2011, 193, 4582–4587. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Lakomek, K.; Naumann, P.T.; Erwin, W.M.; Lauhon, C.T.; Ficner, R. Crystal structure of a 4-thiouridine synthetase-RNA complex reveals specificity of tRNA U8 modification. Nucleic Acids Res. 2014, 42, 6673–6685. [Google Scholar] [CrossRef] [PubMed]
- Rajakovich, L.J.; Tomlinson, J.; Dos Santos, P.C. Functional analysis of Bacillus subtilis genes involved in the biosynthesis of 4-thiouridine in tRNA. J. Bacteriol. 2012, 194, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Waterman, D.G.; Ortiz-Lombardia, M.; Fogg, M.J.; Koonin, E.V.; Antson, A.A. Crystal structure of Bacillus anthracis ThiI, a tRNA-modifying enzyme containing the predicted RNA-binding thump domain. J. Mol. Biol. 2006, 356, 97–110. [Google Scholar] [CrossRef]
- Kambampati, R.; Lauhon, C.T. Mnma and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 2003, 42, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Fukai, S.; Ikeuchi, Y.; Suzuki, T.; Nureki, O. Structural basis for sulfur relay to RNA mediated by heterohexameric TusBCD complex. Structure 2006, 14, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Dos Santos, P.C. An abreviated pathway for the biosynthesis of 2-thiouridine in Bacillus subtilis. J. Bacteriol. 2015, 197, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Bujnicki, J.M.; Oudjama, Y.; Roovers, M.; Owczarek, S.; Caillet, J.; Droogmans, L. Identification of a bifunctional enzyme MnmC involved in the biosynthesis of a hypermodified uridine in the wobble position of tRNA. RNA 2004, 10, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Nishimoto, M.; Sengoku, T.; Shibata, R.; Jager, G.; Bjork, G.R.; Grosjean, H.; Yokoyama, S.; Bessho, Y. Characterization and structure of the Aquifex aeolicus protein DUF752: A bacterial tRNA-methyltransferase (MnmC2) functioning without the usually fused oxidase domain (MnmC1). J. Biol. Chem. 2012, 287, 43950–43960. [Google Scholar] [CrossRef] [PubMed]
- Yim, L.; Moukadiri, I.; Bjork, G.R.; Armengod, M.E. Further insights into the tRNA modification process controlled by proteins MnmE and GidA of Escherichia coli. Nucleic Acids Res. 2006, 34, 5892–5905. [Google Scholar] [CrossRef] [PubMed]
- Moukadiri, I.; Prado, S.; Piera, J.; Velazquez-Campoy, A.; Bjork, G.R.; Armengod, M.E. Evolutionarily conserved proteins MnmE and GidA catalyze the formation of two methyluridine derivatives at tRNA wobble positions. Nucleic Acids Res. 2009, 37, 7177–7193. [Google Scholar] [CrossRef] [PubMed]
- Cabedo, H.; Macian, F.; Villarroya, M.; Escudero, J.C.; Martinez-Vicente, M.; Knecht, E.; Armengod, M.E. The Escherichia coli TrmE (MnmE) gene, involved in tRNA modification, codes for an evolutionarily conserved GTPase with unusual biochemical properties. EMBO J. 1999, 18, 7063–7076. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Yim, L.; Villarroya, M.; Mellado, M.; Perez-Paya, E.; Bjork, G.R.; Armengod, M.E. Effects of mutagenesis in the switch I region and conserved arginines of Escherichia coli MnmE protein, a gtpase involved in tRNA modification. J. Biol. Chem. 2005, 280, 30660–30670. [Google Scholar] [CrossRef] [PubMed]
- Veres, Z.; Stadtman, T.C. A purified selenophosphate-dependent enzyme from Salmonella typhimurium catalyzes the replacement of sulfur in 2-thiouridine residues in tRNAs with selenium. Proc. Natl. Acad. Sci. USA 1994, 91, 8092–8096. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.D.; Ahmed, F.; Lacourciere, G.M.; Lauhon, C.T.; Stadtman, T.C.; Larson, T.J. Functional diversity of the rhodanese homology domain—The Escherichia coli YbbB gene encodes a selenophosphate-dependent tRNA 2-selenouridine synthase. J. Biol. Chem. 2004, 279, 1801–1809. [Google Scholar] [CrossRef]
- Jager, G.; Chen, P.; Bjork, G.R. Transfer RNA bound to mnmh protein is enriched with geranylated tRNA—A possible intermediate in its selenation? PLoS ONE 2016, 11, e0153488. [Google Scholar] [CrossRef] [PubMed]
- Veres, Z.; Tsai, L.; Scholz, T.D.; Politino, M.; Balaban, R.S.; Stadtman, T.C. Synthesis of 5-methylaminomethyl-2-selenouridine in tRNAs: 31P NMR studies show the labile selenium donor synthesized by the seld gene product contains selenium bonded to phosphorus. Proc. Natl. Acad. Sci. USA 1992, 89, 2975–2979. [Google Scholar] [CrossRef] [PubMed]
- Sierant, M.; Leszczynska, G.; Sadowska, K.; Dziergowska, A.; Rozanski, M.; Sochacka, E.; Nawrot, B. S-geranyl-2-thiouridine wobble nucleosides of bacterial tRNAs; chemical and enzymatic synthesis of S-geranylated-RNAs and their physicochemical characterization. Nucleic Acids Res. 2016, 44, 10986–10998. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Estepa, M.; Arda, A.; Savko, M.; Round, A.; Shepard, W.E.; Bruix, M.; Coll, M.; Fernandez, F.J.; Jimenez-Barbero, J.; Vega, M.C. The crystal structure and small-angle X-ray analysis of CsdL/TcdA reveal a new tRNA binding motif in the MoeB/E1 superfamily. PLoS One 2015, 10, e0118606. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Park, S. The structure of Escherichia coli TcdA (also known as CsdL) reveals a novel topology and provides insight into the tRNA binding surface required for N6-threonylcarbamoyladenosine dehydratase activity. J. Mol. Biol. 2015, 427, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.Y.; Park, J.K.; Lee, B.I.; Kim, Y.G.; Park, S. Overproduction, crystallization and preliminary X-ray crystallographic analysis of Escherichia coli tRNA N6-threonylcarbamoyladenosine dehydratase. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, M.; Wojciechowski, J.; Miyauchi, K.; Gdaniec, Z.; Wolf, W.M.; Suzuki, T.; Sochacka, E. A hydantoin isoform of cyclic N6-threonylcarbamoyladenosine (ct6A) is present in tRNAs. Nucleic Acids Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.I.; Miyauchi, K.; Matuszewski, M.; D’Almeida, G.S.; Rubio, M.A.; Alfonzo, J.D.; Inoue, K.; Sakaguchi, Y.; Suzuki, T.; Sochacka, E.; et al. Identification of 2-methylthio cyclic N6-threonylcarbamoyladenosine (ms2ct6A) as a novel RNA modification at position 37 of tRNAs. Nucleic Acids Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shigi, N.; Sakaguchi, Y.; Suzuki, T.; Watanabe, K. Identification of two tRNA thiolation genes required for cell growth at extremely high temperatures. J. Biol. Chem. 2006, 281, 14296–14306. [Google Scholar] [CrossRef]
- Shigi, N. Posttranslational modification of cellular proteins by a ubiquitin-like protein in bacteria. J. Biol. Chem. 2012, 287, 17568–17577. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Miyauchi, K.; Kang, B.I.; Suzuki, T. Nucleoside analysis by hydrophilic interaction liquid chromatography coupled with mass spectrometry. Methods Enzymol. 2015, 560, 19–28. [Google Scholar]
- Watanabe, K.; Shinma, M.; Oshima, T.; Nishimura, S. Heat-induced stability of tRNA from an extreme thermophile, Thermus thermophilus. Biochem. Biophys. Res. Commun. 1976, 72, 1137–1144. [Google Scholar] [CrossRef]
- Kowalak, J.A.; Dalluge, J.J.; McCloskey, J.A.; Stetter, K.O. The role of posttranscriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry 1994, 33, 7869–7876. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Labessan, N.; Clemancey, M.; Latour, J.M.; Ravanat, J.L.; Fontecave, M.; Atta, M. TtcA a new tRNA-thioltransferase with an Fe-S cluster. Nucleic Acids Res. 2014, 42, 7960–7970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; McCloskey, J.; Kersten, W.; Kersten, H. New function of vitamin B12: Cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1988, 170, 2078–2082. [Google Scholar] [CrossRef] [PubMed]
- Miles, Z.D.; Roberts, S.A.; McCarty, R.M.; Bandarian, V. Biochemical and structural studies of 6-carboxy-5,6,7,8-tetrahydropterin synthase reveal the molecular basis of catalytic promiscuity within the tunnel-fold superfamily. J. Biol. Chem. 2014, 289, 23641–23652. [Google Scholar] [CrossRef] [PubMed]
- Van Lanen, S.G.; Reader, J.S.; Swairjo, M.A.; de Crecy-Lagard, V.; Lee, B.; Iwata-Reuyl, D. From cyclohydrolase to oxidoreductase: Discovery of nitrile reductase activity in a common fold. Proc. Natl. Acad. Sci. USA 2005, 102, 4264–4269. [Google Scholar] [CrossRef]
- Miles, Z.D.; McCarty, R.M.; Molnar, G.; Bandarian, V. Discovery of epoxyqueuosine (oQ) reductase reveals parallels between halorespiration and tRNA modification. Proc. Natl. Acad. Sci. USA 2011, 108, 7368–7372. [Google Scholar] [CrossRef] [PubMed]
- McCarty, R.M.; Somogyi, A.; Bandarian, V. Escherichia coli QueD is a 6-carboxy-5,6,7,8-tetrahydropterin synthase. Biochemistry 2009, 48, 2301–2303. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Noguchi, S.; Kasai, H.; Shindo-Okada, N.; Ohgi, T.; Goto, T.; Nishimura, S. Novel mechanism of post-transcriptional modification of tRNA. Insertion of bases of Q precursors into tRNA by a specific tRNA transglycosylase reaction. J. Biol. Chem. 1979, 254, 3067–3073. [Google Scholar] [PubMed]
- Phillips, G.; El Yacoubi, B.; Lyons, B.; Alvarez, S.; Iwata-Reuyl, D.; de Crecy-Lagard, V. Biosynthesis of 7-deazaguanosine-modified tRNA nucleosides: A new role for GTP cyclohydrolase I. J. Bacteriol. 2008, 190, 7876–7884. [Google Scholar] [CrossRef]
- Slany, R.K.; Bosl, M.; Kersten, H. Transfer and isomerization of the ribose moiety of adomet during the biosynthesis of queuosine tRNAs, a new unique reaction catalyzed by the QueA protein from Escherichia coli. Biochimie 1994, 76, 389–393. [Google Scholar] [CrossRef]
- Miles, Z.D.; Myers, W.K.; Kincannon, W.M.; Britt, R.D.; Bandarian, V. Biochemical and spectroscopic studies of epoxyqueuosine reductase: A novel iron-sulfur cluster- and cobalamin-containing protein involved in the biosynthesis of queuosine. Biochemistry 2015, 54, 4927–4935. [Google Scholar] [CrossRef]
- McCarty, R.M.; Krebs, C.; Bandarian, V. Spectroscopic, steady-state kinetic, and mechanistic characterization of the radical SAM enzyme QueE, which catalyzes a complex cyclization reaction in the biosynthesis of 7-deazapurines. Biochemistry 2013, 52, 188–198. [Google Scholar] [CrossRef] [PubMed]
- McCarty, R.M.; Somogyi, A.; Lin, G.; Jacobsen, N.E.; Bandarian, V. The deazapurine biosynthetic pathway revealed: In vitro enzymatic synthesis of PreQ0 from guanosine 5′-triphosphate in four steps. Biochemistry 2009, 48, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.S.; Metzgar, D.; Schimmel, P.; de Crecy-Lagard, V. Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. J. Biol. Chem. 2004, 279, 6280–6285. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Paez, A.; Villarroya, M.; Armengod, M.E. The Escherichia coli RlmN methyltransferase is a dual-specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy. RNA 2012, 18, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Saneyoshi, M.; Oashi, Z.; Harada, F.; Nishimura, S. Isolation and characterization of 2-methyladenosine from Escherichia coli tRNA Glu 2, tRNA Asp 1, tRNA His 1 and tRNA Arg. Biochim. Biophys. Acta 1972, 262, 1–10. [Google Scholar] [CrossRef]
- Pierrel, F.; Bjork, G.R.; Fontecave, M.; Atta, M. Enzymatic modification of tRNAs: MiaB is an iron-sulfur protein. J. Biol. Chem. 2002, 277, 13367–13370. [Google Scholar] [CrossRef]
- Durand, J.M.B.; Bjork, G.R.; Kuwae, A.; Yoshikawa, M.; Sasakawa, C. The modified nucleoside 2-methylthio-N6-isopentenyladenosine in tRNA of shigella flexneri is required for expression of virulence genes. J. Bacteriol. 1997, 179, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Anton, B.P.; Russell, S.P.; Vertrees, J.; Kasif, S.; Raleigh, E.A.; Limbach, P.A.; Roberts, R.J. Functional characterization of the YmcB and YqeV tRNA methylthiotransferases of Bacillus subtilis. Nucleic Acids Res. 2010, 38, 6195–6205. [Google Scholar] [CrossRef] [PubMed]
- Arragain, S.; Handelman, S.K.; Forouhar, F.; Wei, F.Y.; Tomizawa, K.; Hunt, J.F.; Douki, T.; Fontecave, M.; Mulliez, E.; Atta, M. Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010, 285, 28425–28433. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.C.; Bjork, G.R. Isolation of the gene (MiaE) encoding the hydroxylase involved in the synthesis of 2-methylthio-cis-ribozeatin in tRNA of Salmonella typhimurium and characterization of mutants. J. Bacteriol. 1993, 175, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Mathevon, C.; Pierrel, F.; Oddou, J.L.; Garcia-Serres, R.; Blondin, G.; Latour, J.M.; Menage, S.; Gambarelli, S.; Fontecave, M.; Atta, M. tRNA-modifying MiaE protein from Salmonella typhimurium is a nonheme diiron monooxygenase. Proc. Natl. Acad. Sci. USA 2007, 104, 13295–13300. [Google Scholar] [CrossRef] [PubMed]
- Lorsch, J. Methods in enzymology. Laboratory methods in enzymology: RNA. Preface. Methods Enzymol. 2013, 530, 21. [Google Scholar]
- Cai, W.M.; Chionh, Y.H.; Hia, F.; Gu, C.; Kellner, S.; McBee, M.E.; Ng, C.S.; Pang, Y.L.; Prestwich, E.G.; Lim, K.S.; et al. A platform for discovery and quantification of modified ribonucleosides in RNA: Application to stress-induced reprogramming of tRNA modifications. Methods Enzymol. 2015, 560, 29–71. [Google Scholar] [PubMed]
- Suzuki, T.; Ikeuchi, Y.; Noma, A.; Suzuki, T.; Sakaguchi, Y. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007, 425, 211–229. [Google Scholar] [PubMed]
- Chan, C.T.; Pang, Y.L.; Deng, W.; Babu, I.R.; Dyavaiah, M.; Begley, T.J.; Dedon, P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012, 3, 937. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Limbach, P.A. Mass spectrometry-based detection of transfer RNAs by their signature endonuclease digestion products. RNA 2007, 13, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Heiss, M.; Kellner, S. Detection of nucleic acid modifications by chemical reagents. RNA Biol. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Dugas, H. Specific spin-labeling of transfer ribonucleic acid molecules. Nucleic Acids Res. 1976, 3, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Soll, D. Covalent attachment of a fluorescent group to 4-thiouridine in transfer RNA. J. Biochem. 1973, 73, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Gornicki, P.; Judek, M.; Wolanski, A.; Krzyzosiak, W.J. Hypermodified nucleoside carboxyl group as a target site for specific tRNA modification. Eur. J. Biochem. 1986, 155, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Pingoud, A.; Kownatzki, R.; Maass, G. Fluoresceinylthiocarbamyl-tRNATyr: A useful derivative of tRNATyr (E. coli) for physicochemical studies. Nucleic Acids Res. 1977, 4, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Soll, D. Covalent attachment of fluorescent groups to transfer ribonucleic acid. Reactions with 4-bromomethyl-7-methoxy-2-oxo-2H-benzopyran. Biochemistry 1974, 13, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Kellner, S.; Seidu-Larry, S.; Burhenne, J.; Motorin, Y.; Helm, M. A multifunctional bioconjugate module for versatile photoaffinity labeling and click chemistry of RNA. Nucleic Acids Res. 2011, 39, 7348–7360. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.L. Interaction of tRNAs and of phosphorothioate-substituted nucleic acids with an organomercurial. Probing the chemical environment of thiolated residues by affinity electrophoresis. Biochemistry 1988, 27, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Biondi, E.; Burke, D.H. Separating and analyzing sulfur-containing RNAs with organomercury gels. Methods Mol. Biol. 2012, 883, 111–120. [Google Scholar] [PubMed]
- Kellner, S.; Burhenne, J.; Helm, M. Detection of RNA modifications. RNA Biol. 2010, 7, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.L.; Kossel, H. Affinity electrophoresis for monitoring terminal phosphorylation and the presence of queuosine in RNA. Application of polyacrylamide containing a covalently bound boronic acid. Nucleic Acids Res. 1985, 13, 6881–6898. [Google Scholar] [CrossRef] [PubMed]
- Zaborske, J.M.; DuMont, V.L.; Wallace, E.W.; Pan, T.; Aquadro, C.F.; Drummond, D.A. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biol. 2014, 12, e1002015. [Google Scholar] [CrossRef] [PubMed]
- Cahova, H.; Winz, M.L.; Hofer, K.; Nubel, G.; Jaschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Damon, J.R.; Pincus, D.; Ploegh, H.L. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol. Biol. Cell 2015, 26, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Pedrioli, P.G. Protein degradation and dynamic tRNA thiolation fine-tune translation at elevated temperatures. Nucleic Acids Res. 2015, 43, 4701–4712. [Google Scholar] [CrossRef] [PubMed]
- Leidel, S.; Pedrioli, P.G.; Bucher, T.; Brost, R.; Costanzo, M.; Schmidt, A.; Aebersold, R.; Boone, C.; Hofmann, K.; Peter, M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 2009, 458, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wohlgamuth-Benedum, J.M.; Rubio, M.A.; Paris, Z.; Long, S.; Poliak, P.; Lukes, J.; Alfonzo, J.D. Thiolation controls cytoplasmic tRNA stability and acts as a negative determinant for tRNA editing in mitochondria. J. Biol. Chem. 2009, 284, 23947–23953. [Google Scholar] [CrossRef] [PubMed]
- Paris, Z.; Changmai, P.; Rubio, M.A.; Zikova, A.; Stuart, K.D.; Alfonzo, J.D.; Lukes, J. The Fe-S cluster assembly protein Isd11 is essential for tRNA thiolation in Trypanosoma brucei. J. Biol. Chem. 2010, 285, 22394–22402. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Jinno, D.; Akiyama, Y.; Itoh, K.; Nankumo, S.; Shima, H.; Kikuchi, K.; Takeuchi, Y.; Elkordy, A.; Suzuki, T.; et al. Immuno-northern blotting: Detection of RNA modifications by using antibodies against modified nucleosides. PLoS ONE 2015, 10, e0143756. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, C.W.; Kuo, K.C.; McCune, R.A.; Gerhardt, K.O.; Agris, P.F. Quantitative enzymatic hydrolysis of tRNAs: Reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 1982, 230, 297–308. [Google Scholar] [CrossRef]
- Gehrke, C.W.; Kuo, K.C. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1989, 471, 3–36. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Wang, X.; Li, H.M.; Yang, B.; Yang, H.J.; Huang, L. Characterization of nucleosides and nucleobases in natural cordyceps by HILIC-ESI/TOF/MS and HILIC-ESI/MS. Molecules 2013, 18, 9755–9769. [Google Scholar] [CrossRef] [PubMed]
- Dubin, D.T.; Gunalp, A. Minor nucleotide composition of ribosomal precursor, and ribosomal, ribonucleic acid in Escherichia coli. Biochim. Biophys. Acta 1967, 134, 106–123. [Google Scholar] [CrossRef]
- Kowalak, J.A.; Bruenger, E.; McCloskey, J.A. Posttranscriptional modification of the central loop of domain V in Escherichia coli 23 S ribosomal RNA. J. Biol. Chem. 1995, 270, 17758–17764. [Google Scholar] [PubMed]
- Johnson, J.D.; Horowitz, J. Characterization of ribosomes and RNAs from Mycoplasma hominis. Biochim. Biophys. Acta 1971, 247, 262–279. [Google Scholar] [CrossRef]
- Emmerechts, G.; Barbe, S.; Herdewijn, P.; Anne, J.; Rozenski, J. Post-transcriptional modification mapping in the Clostridium acetobutylicum 16S rRNA by mass spectrometry and reverse transcriptase assays. Nucleic Acids Res. 2007, 35, 3494–3503. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.L.; Fefferman, R. The occurrence of methylated bases in ribosomal ribonucleic acid of Escherichia coli K12 W-6. J. Biol. Chem. 1964, 239, 3457–3461. [Google Scholar] [PubMed]
- Hayashi, Y.; Osawa, S.; Miura, K. The methyl groups in ribosomal RNA from Escherichia coli. Biochim. Biophys. Acta 1966, 129, 519–531. [Google Scholar] [CrossRef]
- Morse, D.P.; Bass, B.L. Detection of inosine in messenger RNA by inosine-specific cleavage. Biochemistry 1997, 36, 8429–8434. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef] [PubMed]
- Chionh, Y.H.; McBee, M.; Babu, I.R.; Hia, F.; Lin, W.; Zhao, W.; Cao, J.; Dziergowska, A.; Malkiewicz, A.; Begley, T.J.; et al. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat. Commun. 2016, 7, 13302. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Ojo, T.T.; Soll, D.; Hohn, M.J. Selenomodification of tRNA in archaea requires a bipartite rhodanese enzyme. FEBS Lett. 2012, 586, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Basanta-Sanchez, M.; Temple, S.; Ansari, S.A.; D’Amico, A.; Agris, P.F. Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Res. 2016, 44, e26. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, C.M.; Limbach, P.A. Relative quantitation of transfer RNAs using liquid chromatography mass spectrometry and signature digestion products. Nucleic Acids Res. 2010, 38, e162. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Cao, X.; Yu, N.; Limbach, P.A. Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods 2016, 107, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.T.; Dyavaiah, M.; DeMott, M.S.; Taghizadeh, K.; Dedon, P.C.; Begley, T.J. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010, 6, e1001247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.T.; Deng, W.; Li, F.; DeMott, M.S.; Babu, I.R.; Begley, T.J.; Dedon, P.C. Highly predictive reprogramming of tRNA modifications is linked to selective expression of codon-biased genes. Chem. Res. Toxicol. 2015, 28, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C.; Begley, T.J. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem. Res. Toxicol. 2014, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Chan, C.T.; Gu, C.; Lim, K.S.; Chionh, Y.H.; McBee, M.E.; Russell, B.S.; Babu, I.R.; Begley, T.J.; Dedon, P.C. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 2014, 9, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.N. Isolation of 4-thiouridylic acid from soluble ribonucleic acid of Escherichia coli. J. Biol. Chem. 1965, 240, 3975. [Google Scholar] [PubMed]
- Cleary, M.D.; Meiering, C.D.; Jan, E.; Guymon, R.; Boothroyd, J.C. Biosynthetic labeling of RNA with uracil phosphoribosyltransferase allows cell-specific microarray analysis of mRNA synthesis and decay. Nat. Biotechnol. 2005, 23, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Palenchar, P.M.; Buck, C.J.; Cheng, H.; Larson, T.J.; Mueller, E.G. Evidence that ThiI, an enzyme shared between thiamin and 4-thiouridine biosynthesis, may be a sulfurtransferase that proceeds through a persulfide intermediate. J. Biol. Chem. 2000, 275, 8283–8286. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.G.; Palenchar, P.M. Using genomic information to investigate the function of ThiI, an enzyme shared between thiamin and 4-thiouridine biosynthesis. Protein Sci. 1999, 8, 2424–2427. [Google Scholar] [CrossRef] [PubMed]
- Kuratani, M.; Yoshikawa, Y.; Bessho, Y.; Higashijima, K.; Ishii, T.; Shibata, R.; Takahashi, S.; Yutani, K.; Yokoyama, S. Structural basis of the initial binding of tRNAIle lysidine synthetase TilS with ATP and l-lysine. Structure 2007, 15, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.A. The danger of annotation by analogy: Most “ThiI” genes play no role in thiamine biosynthesis. J. Bacteriol. 2011, 193, 4574–4575. [Google Scholar] [CrossRef] [PubMed]
- Selbach, B.P.; Chung, A.H.; Scott, A.D.; George, S.J.; Cramer, S.P.; Dos Santos, P.C. Fe-S cluster biogenesis in Gram-positive bacteria: SufU is a zinc-dependent sulfur transfer protein. Biochemistry 2014, 53, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Vinyard, D.J.; Reesbeck, M.E.; Suzuki, T.; Manakongtreecheep, K.; Holland, P.L.; Brudvig, G.W.; Soll, D. A [3Fe-4S] cluster is required for tRNA thiolation in archaea and eukaryotes. Proc. Natl Acad. Sci. USA 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, X.; Nakamura, A.; Orlando, R.; Soll, D.; Whitman, W.B. Biosynthesis of 4-thiouridine in tRNA in the methanogenic archaeon Methanococcus maripaludis. J. Biol. Chem. 2012, 287, 36683–36692. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.; Dosche, C.; Lohmannsroben, H.G.; Leimkuhler, S. Dual role of the molybdenum cofactor biosynthesis protein MOCS3 in tRNA thiolation and molybdenum cofactor biosynthesis in humans. J. Biol. Chem. 2012, 287, 17297–17307. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Chowdhury, M.M.; Hanzelmann, P.; Nimtz, M.; Lee, E.Y.; Schindelin, H.; Leimkuhler, S. The sulfurtransferase activity of Uba4 presents a link between ubiquitin-like protein conjugation and activation of sulfur carrier proteins. Biochemistry 2008, 47, 6479–6489. [Google Scholar] [CrossRef] [PubMed]
- Umeda, N.; Suzuki, T.; Yukawa, M.; Ohya, Y.; Shindo, H.; Watanabe, K.; Suzuki, T. Mitochondria-specific RNA-modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. J. Biol. Chem. 2005, 280, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Umeda, N.; Suzuki, T.; Nakai, M.; Hayashi, H.; Watanabe, K.; Kagamiyama, H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 2004, 279, 12363–12368. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Ikeuchi, Y.; Fukai, S.; Suzuki, T.; Nureki, O. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature 2006, 442, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Black, K.A.; Dos Santos, P.C. Functional investigation of Bacillus subtilis YrkF’s involvement in sulfur transfer reactions. Peptidomics 2015, 34, 54. [Google Scholar] [CrossRef]
- Dahl, J.U.; Radon, C.; Buhning, M.; Nimtz, M.; Leichert, L.I.; Denis, Y.; Jourlin-Castelli, C.; Iobbi-Nivol, C.; Mejean, V.; Leimkuhler, S. The sulfur carrier protein TusA has a pleiotropic role in Escherichia coli that also affects molybdenum cofactor biosynthesis. J. Biol. Chem. 2013, 288, 5426–5442. [Google Scholar] [CrossRef] [PubMed]
- Kozmin, S.G.; Stepchenkova, E.I.; Schaaper, R.M. Tusa (YhhP) and IscS are required for molybdenum cofactor-dependent base-analog detoxification. Microbiologyopen 2013, 2, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Nakayashiki, T.; Saito, N.; Takeuchi, R.; Kadokura, H.; Nakahigashi, K.; Wanner, B.L.; Mori, H. The tRNA thiolation pathway modulates the intracellular redox state in Escherichia coli. J. Bacteriol. 2013, 195, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Dos Santos, P.C. Shared-intermediates in the biosynthesis of thio-cofactors: Mechanism and functions of cysteine desulfurases and sulfur acceptors. Biochim. Biophys. Acta 2015, 1853, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Leimkuhler, S. The biosynthesis of the molybdenum cofactor in Escherichia coli and its connection to FeS cluster assembly and the thiolation of tRNA. Adv. Biol. 2014, 2014, 808569. [Google Scholar] [CrossRef]
- Kambampati, R.; Lauhon, C.T. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry 1999, 38, 16561–16568. [Google Scholar] [CrossRef] [PubMed]
- Armengod, M.E.; Moukadiri, I.; Prado, S.; Ruiz-Partida, R.; Benitez-Paez, A.; Villarroya, M.; Lomas, R.; Garzon, M.J.; Martinez-Zamora, A.; Meseguer, S.; et al. Enzymology of tRNA modification in the bacterial mnmeg pathway. Biochimie 2012, 94, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Moukadiri, I.; Garzon, M.J.; Bjork, G.R.; Armengod, M.E. The output of the tRNA modification pathways controlled by the Escherichia coli MnmEG and MnmC enzymes depends on the growth conditions and the tRNA species. Nucleic Acids Res. 2014, 42, 2602–2623. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Kato, S.; Lacourciere, G.M.; Stadtman, T.C.; Kennedy, R.A.; Kurihara, T.; Tokumoto, U.; Takahashi, Y.; Esaki, N. The IscS gene is essential for the biosynthesis of 2-selenouridine in tRNA and the selenocysteine-containing formate dehydrogenase H. Proc. Natl. Acad. Sci. USA 2002, 99, 6679–6683. [Google Scholar] [CrossRef]
- Bartos, P.; Maciaszek, A.; Rosinska, A.; Sochacka, E.; Nawrot, B. Transformation of a wobble 2-thiouridine to 2-selenouridine via S-geranyl-2-thiouridine as a possible cellular pathway. Bioorg. Chem. 2014, 56, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Lacourciere, G.M.; Mihara, H.; Kurihara, T.; Esaki, N.; Stadtman, T.C. Escherichia coli NifS-like proteins provide selenium in the pathway for the biosynthesis of selenophosphate. J. Biol. Chem. 2000, 275, 23769–23773. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.G.; Crain, P.F.; Gupta, R.; Hashizume, T.; Hocart, C.H.; Kowalak, J.A.; Pomerantz, S.C.; Stetter, K.O.; McCloskey, J.A. Posttranscriptional modification of tRNA in thermophilic archaea (archaebacteria). J. Bacteriol. 1991, 173, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, J.A.; Graham, D.E.; Zhou, S.; Crain, P.F.; Ibba, M.; Konisky, J.; Soll, D.; Olsen, G.J. Post-transcriptional modification in archaeal tRNAs: Identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic methanococcales. Nucleic Acids Res. 2001, 29, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Villegas, J.; Winkler, M.E.; Nikonowicz, E.P. Solution conformations of unmodified and A37N6-dimethylallyl modified anticodon stem-loops of Escherichia coli tRNAPhe. J. Mol. Biol. 2002, 319, 1015–1034. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Mattijssen, S.; Maraia, R.J. Human cells have a limited set of tRNA anticodon loop substrates of the tRNA isopentenyltransferase Trit1 tumor suppressor. Mol. Cell. Biol. 2013, 33, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Blewett, N.H.; Maraia, R.J. Plasticity and diversity of tRNA anticodon determinants of substrate recognition by eukaryotic A37 isopentenyltransferases. RNA 2011, 17, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Ellis, S.R.; True, H.L. The Sua5 protein is essential for normal translational regulation in yeast. Mol. Cell. Biol. 2010, 30, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Suzuki, T. Iron-sulfur proteins responsible for RNA modifications. Biochim. Biophys. Acta 2015, 1853, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.; El Yacoubi, B.; de Crecy-Lagard, V.; Iwata-Reuyl, D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J. Biol. Chem. 2012, 287, 13666–13673. [Google Scholar] [CrossRef] [PubMed]
- Thiaville, P.C.; El Yacoubi, B.; Perrochia, L.; Hecker, A.; Prigent, M.; Thiaville, J.J.; Forterre, P.; Namy, O.; Basta, T.; de Crecy-Lagard, V. Cross kingdom functional conservation of the core universally conserved threonylcarbamoyladenosine tRNA synthesis enzymes. Eukaryot. Cell 2014, 13, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.A.; Bobay, B.G.; Sarachan, K.L.; Sims, A.F.; Bilbille, Y.; Deutsch, C.; Iwata-Reuyl, D.; Agris, P.F. NMR-based structural analysis of threonylcarbamoyl-AMP synthase and its substrate interactions. J. Biol. Chem. 2015, 290, 20032–20043. [Google Scholar] [CrossRef] [PubMed]
- Trotter, V.; Vinella, D.; Loiseau, L.; Ollagnier de Choudens, S.; Fontecave, M.; Barras, F. The CsdA cysteine desulphurase promotes Fe/S biogenesis by recruiting suf components and participates to a new sulphur transfer pathway by recruiting CsdL (ex-YgdL), a ubiquitin-modifying-like protein. Mol. Microbiol. 2009, 74, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, M.R.; Schwalm, E.L.; Booker, S.J. Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation. J. Biol. Chem. 2015, 290, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.J.; Grove, T.L. Mechanistic and functional versatility of radical SAM enzymes. F1000 Biol. Rep. 2010, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.J. Radical sam enzymes and radical enzymology. Biochim. Biophys. Acta 2012, 1824, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, B.J.; McCarthy, E.L.; Booker, S.J. Radical S-adenosylmethionine enzymes in human health and disease. Annu. Rev. Biochem. 2016, 85, 485–514. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, K.H.; Baraniak, U.; Boniecki, M.; Nowaczyk, K.; Czerwoniec, A.; Bujnicki, J.M. Structural bioinformatics analysis of enzymes involved in the biosynthesis pathway of the hypermodified nucleoside ms2io6A37 in tRNA. Proteins 2008, 70, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Anton, B.P.; Saleh, L.; Benner, J.S.; Raleigh, E.A.; Kasif, S.; Roberts, R.J. Rimo, a MiaB-like enzyme, methylthiolates the universally conserved Asp88 residue of ribosomal protein S12 in Escherichia coli. Proc. Natl. Acad. Sci. USA 2008, 105, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Saleh, L.; Anton, B.P.; Madinger, C.L.; Benner, J.S.; Iwig, D.F.; Roberts, R.J.; Krebs, C.; Booker, S.J. Characterization of RimO, a new member of the methylthiotransferase subclass of the radical SAM superfamily. Biochemistry 2009, 48, 10162–10174. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Armstrong, D.J.; Schafer, K.P.; Soll, D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975, 2, 691–698. [Google Scholar] [CrossRef]
- Lanz, N.D.; Booker, S.J. Auxiliary iron-sulfur cofactors in radical SAM enzymes. Biochim. Biophys. Acta 2015, 1853, 1316–1334. [Google Scholar] [CrossRef] [PubMed]



| Fe-S Cluster Independent Modifications | ||||
|---|---|---|---|---|
| Modification | Name | Position | Biosynthetic Genes and Precursors | |
| s4U | 4-thiouridine | 8 | iscS-thiI (Escherichia coli [11,62,64,65], Salmonella enterica [66] and Thermatoga maritima [67]); or nifZ-thiI (Bacillus subtilis [68] and Bacillus anthracis [69]) | |
| s2U | 2-thiouridine | 34 | iscS-tusABCDE-mnmA (E. coli [11,37,70,71], S. enterica, [52]); or yrvO-mnmA (B. subtilis [72]) | |
| mnm5s2U | 5-methylaminomethyl-2-thiouridine | 34 | s2U, mnmEG/gidA, mnmC1-2 (E. coli [73]); or s2U, mnmEG/gidA, mnmC2 (Aquifex aeolicus [74]) | |
| cmnm5s2U | 5-carboxymethylaminomethyl-2-thiouridine | 34 | s2U, mnmEG/gidA (E. coli [75,76,77,78]) | |
| inm5s2U | 5-(isopentenylaminomethyl)-2-thiouridine | 34 | nm5s2U, unknown isopentenyltransferase (Thermodesulfobacterium commune) | |
| nm5s2U | 5-aminomethyl-2-thiouridine | 34 | s2U, mnmEG/gidA, mnmC1 (E. coli [73]) | |
| se2U | 2-selenouridine | 34 | s2U, selU/mnmH, selD (S. enterica [79] and E. coli [80]) | |
| mnm5se2U | 5-methylaminomethyl-2-selenouridine | 34 | mnm5s2U, selU/mnmH, selD (S. enterica [79,81,82], E. coli [80]) | |
| ges2U | 2-geranylthiouridine | 34 | s2U, selU/mnmH (E. coli [83]) | |
| cmnm5ges2U | 5-carboxymethylaminomethyl-2-geranylthiouridine | 34 | cmnm5s2U, selU/mnmH (S. enterica [29,81], E. coli [29,83], Enterobacter aerogenes, and Pseudomonas aeruginosa [29]) | |
| mnm5ges2U | 5-methylaminomethy-2-geranylthiouridine | 34 | mnm5s2U, selU/mnmH (S. enterica [29,81], E. coli [29,83], E. aerogenes and P. aeruginosa [29]) | |
| nm5ges2U | 5-aminomethyl-2-geranylthiouridine | 34 | nm5s2U, proposed selU/mnmH (S. enterica and E. coli) | |
| ct6A | cyclic N6-threonylcarbamoyladenosine | 37 | t6A, tcdA/csdL (E. coli [59,84,85,86,87], B. subtilis [88]) | |
| m5s2U/s2T | 5-methyl-2-thiouridine/2-thioribothymidine | 54 | ttuBCA (Thermus thermophilus [10,89,90,91,92], Pyrococcus furiosus [93]) | |
| Fe-S Cluster Dependent Modifications | ||||
| Modification | Name | Position | Biosynthetic Genes and Precursors | |
| s2C | 2-thiocytidine | 32 | ttcA (E. coli [63,94]) | |
| Q | queuosine | 34 | folE, queACDEFG, tgt, (E. coli [95,96,97,98,99,100,101,102], S. enterica [95], B. subtilis [97,98,103,104], Streptomyces rimosus [105], Acinetobacter sp. and Zymomonas mobilis [106]) | |
| m2A | 2-methyladenosine | 37 | trmG/rlmN (E. coli [107,108]) | |
| ms2i6A | 2-methylthio-N6-isopentenyladenosine | 37 | i6A, miaB (T. maritima [13,15,16,17], E. coli [33,109], S. enterica [33], Shigella flexneri [110] and B. subtilis [111,112]) | |
| ms2io6A | 2-methylthio-cis-ribozeatin | 37 | ms2i6A, miaE (S. enterica [113,114]) | |
| ms2t6A | 2-methylthio-N6-threonyl carbamoyladenosine | 37 | t6A, yqeV/mtaB (B. subtilis [111,112]) | |
| ms2ct6A | 2-methylthio-cyclic-N6-threonyl carbamoyladenosine | 37 | ms2t6A, tcdA/csdL (B. subtilis [88]) | |
| Method | Target Modification | Advantage | Disadvantage |
|---|---|---|---|
| Chemical labeling | s2U, s4U, mnm5s2U, pseudouridine | Labeling only occurs in the modified species; high selectivity | Detection method varies with modification and potential side reactions |
| Northern Blot | all | High sensitivity towards specific tRNA sequences | Unable to differentiate modified and canonical nucleotides; requires RNA probe for each cognate tRNA; may require radioactive probe |
| Immuno-Northern Blot | m1A, m6A, m5C | Antibodies bind specifically to the modified nucleoside | Limited antibodies |
| APM-gel | s4U, s2U and derivatives | Detect polynucleotides and single nucleosides; simple analysis | Hazardous mercury compound involved; varied sensitivity toward different thionucleotides |
| APB-gel | Q | Specific to Q modification | Reactive with cis-diol functional groups |
| HPLC-UV/vis | all | Simple sample preparation and data quantification. Certain nucleosides have unique absorbance λ max | Nuclease and phosphatase treatments |
| HPLC-MS | all | High sensitivity and accuracy | Detection may result of fragmentation of certain modifications |
| S-Dependent Modifications | |||||
|---|---|---|---|---|---|
| Modification | Name | Monoisotopic Mass (amu) | Observed Molecular Ion (m/z+) | Major Fragment (m/z+) | Standard ** |
| s2C | 2-thiocytidine | 259.063 | 260.071 | 128.028 | 1 |
| s2U | 2-thiouridine | 260.047 | 261.055 | 129.009 * | 1 |
| s4U | 4-thiouridine | 260.047 | 261.055 | 129.009 * | 2 |
| s2Um | 2-thio-2′-O-methyluridine | 274.062 | 275.067 | 129.008 * | |
| m5s2U/s2T | 5-methyl-2-thiouridine/2-thioribothymidine | 274.062 | 275.062 | 143.019 | 1, 2 |
| m2A † | N2-methyladenosine † | 281.112 | 282.114 | 150.071 | |
| nm5s2U | 5-methylaminomethyl-2-thiouridine | 289.073 | 290.081 | 158.038 | |
| mnm5s2U | 5-methylaminomethyl-2-thiouridine | 303.089 | 304.097 | 172.057 * | |
| se2U | 2-selenouridine | 305.992 | 306.999 | 174.956 | |
| ms2m6A | 2-methylthio-N6-methyladenosine | 327.100 | 328.108 | 196.065 | |
| cmnm5s2U | 5-carboxymethylaminomethyl-2-thiouridine | 347.079 | 348.082 | 216.047 * | |
| mnm5se2U | 5-methylaminomethyl-2-selenouridine | 349.034 | 350.034 | 217.991 | |
| inm5s2U | 5-(isopentenylaminomethyl)-2-thiouridine | 357.136 | 358.144 | 226.101 | |
| ms2i6A | 2-methylthio-N6-isopentenyladenosine | 381.147 | 382.155 | 250.108 * | 1 |
| cmnm5se2U | 5-carboxymethylaminomethyl-2-selenouridine | 393.024 | 394.032 | 261.989 | |
| ct6A | cyclic N6-threonylcarbamoyladenosine | 394.124 | 395.128 | 263.089 * | |
| ges2U | 2-geranylthiouridine | 396.172 | 397.180 | 265.137 | |
| ms2io6A | 2-methylthio-N6-(cis-hydroxyisopentenyl) adenosine | 397.142 | 398.150 | 266.107 | |
| Q | queuosine | 409.160 | 410.168 | 278.125 | |
| nm5ges2U | 5-methylaminomethyl-2-geranylthiouridine | 425.198 | 426.206 | 294.163 | |
| mnm5ges2U | 5-methylaminomethyl-2-geranylthiouridine | 439.214 | 440.222 | 308.179 | |
| ms2ct6A | 2-methylthio-cyclic-N6-threonyl carbamoyladenosine | 440.111 | 441.119 | 309.076 | |
| ms2t6A | 2-methylthio-N6-threonyl carbamoyladenosine | 458.112 | 459.130 | 182.049 * | |
| ms2hn6A | 2-methylthio-N6-hydroxynorvalylcarbamoyladenosine | 472.138 | 473.146 | 341.103 | |
| cmnm5ges2U | 5-carboxymethylaminomethyl-2-geranylthiouridine | 483.204 | 484.212 | 352.169 | |
| Precursor and Related Modifications | |||||
| Modification | Name | Monoisotopic Mass (amu) | Observed Molecular Ion (m/z+) | Major Fragment (m/z+) | Standard ** |
| cmnm5Um | 5-carboxymethylaminomethyl-2′-O-methyluridine | 345.117 | 346.117 | 200.058 * | |
| m6A | N6-methyladenosine †,‡ | 281.112 | 282.114 | 150.071 * | 1 |
| mnm5U | 5-methylaminomethyluridine | 287.112 | 288.120 | 156.074 * | |
| i6A | N6-isopentenyladenosine | 335.159 | 336.167 | 204.119 * | 1 |
| inm5U | 5-(isopentenylaminomethyl) uridine | 341.159 | 342.167 | 210.135 | |
| io6A | N6-(cis-hydroxyisopentenyl) adenosine | 351.154 | 352.156 | 220.115 * | |
| inm5Um | 5-(isopentenylaminomethyl)-2′-O-methyluridine | 355.174 | 356.182 | 224.139 | |
| t6A | N6-threonylcarbamoyladenosine | 412.134 | 413.142 | 136.062 * | 3 |
| oQ | epoxyqueuosine | 425.155 | 426.163 | 294.120 | |
| hn6A | N6-hydroxynorvalylcarbamoyl adenosine | 426.150 | 427.158 | 295.115 | |
| Common Internal Standards | |||||
| Modification | Name | Monoisotopic Mass (amu) | Observed Molecular Ion (m/z+) | Major Fragment (m/z+) | Standard ** |
| I | Inosine ‡ | 268.081 | 269.088 | 137.047 * | 2 |
| Ψ | Pseudouridine † | 244.070 | 245.078 | 209.052 * | 2 |
| D | Dihydrouridine † | 246.085 | 247.092 | 115.050 * | 4, 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Black, K.A.; Dos Santos, P.C. Diverse Mechanisms of Sulfur Decoration in Bacterial tRNA and Their Cellular Functions. Biomolecules 2017, 7, 33. https://doi.org/10.3390/biom7010033
Zheng C, Black KA, Dos Santos PC. Diverse Mechanisms of Sulfur Decoration in Bacterial tRNA and Their Cellular Functions. Biomolecules. 2017; 7(1):33. https://doi.org/10.3390/biom7010033
Chicago/Turabian StyleZheng, Chenkang, Katherine A. Black, and Patricia C. Dos Santos. 2017. "Diverse Mechanisms of Sulfur Decoration in Bacterial tRNA and Their Cellular Functions" Biomolecules 7, no. 1: 33. https://doi.org/10.3390/biom7010033






