Functional Amyloids in Reproduction
Abstract
:1. Introduction
2. Gametogenesis
3. Germline Specification
4. Sperm Acrosome Reaction and Fertilization
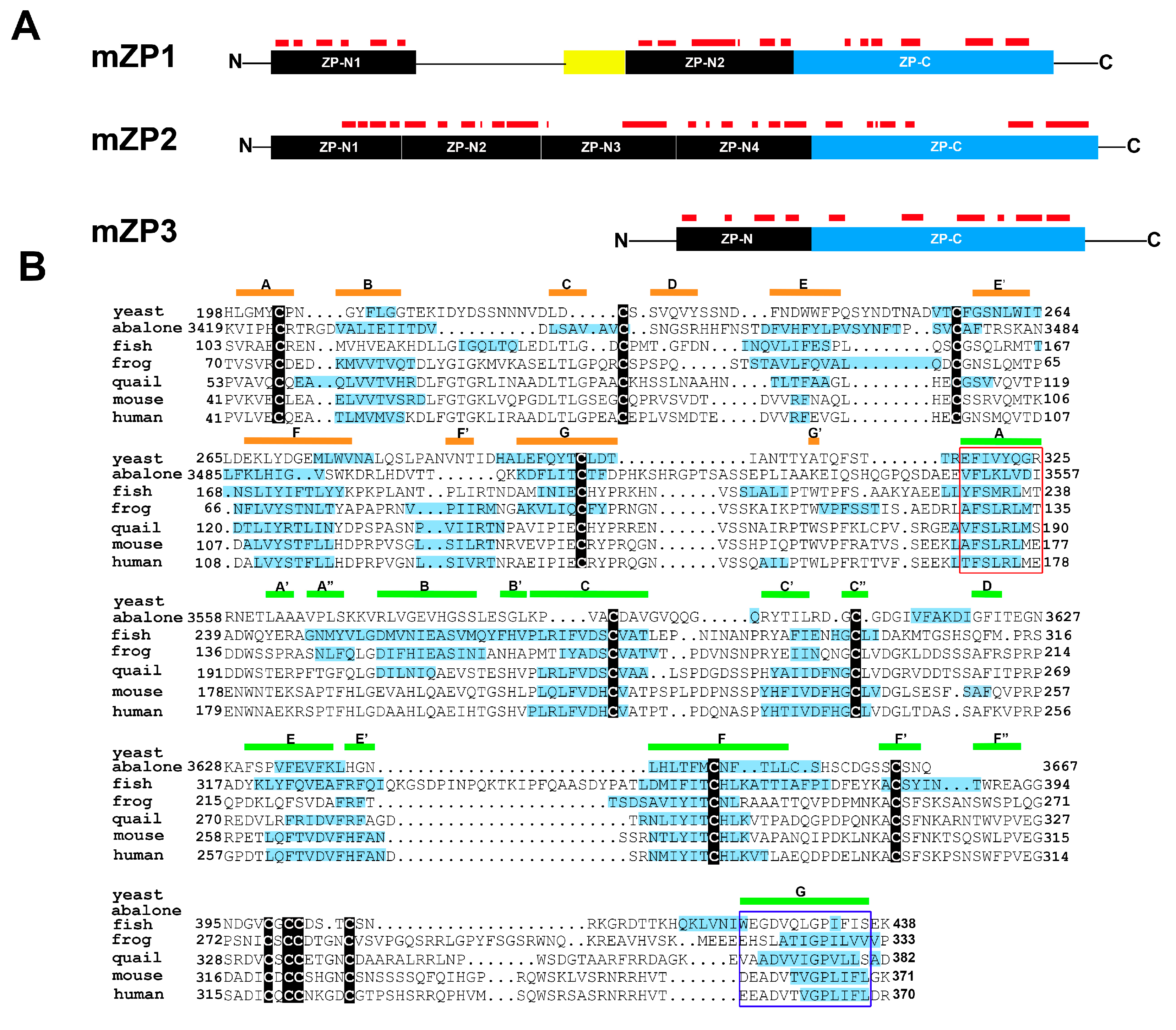
5. Egg Zona Pellucida and Fertilization
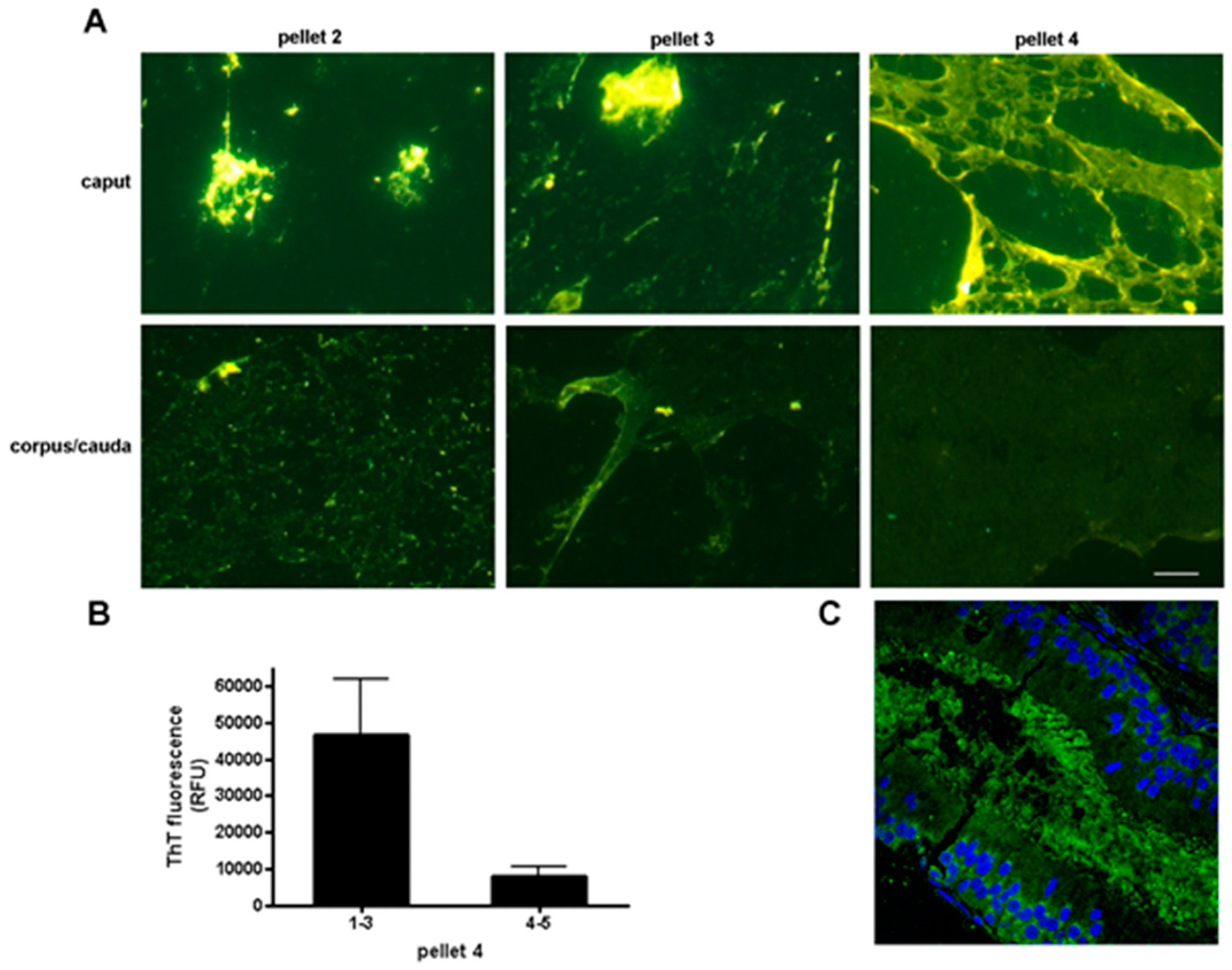
6. Epididymal Sperm Maturation
Cystatins and the Epididymal Amyloid Matrix
7. Semen Amyloids
8. Properties of Reproductive Functional Amyloids
8.1. Aggregation Properties In Vitro
8.2. Structural/Biophysical Properties In Vitro
8.3. Reproductive Functional Amyloids In Vivo
9. Reproductive Functional Amyloids May Become Pathological
10. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, Y.O. Amyloidogenic domains, prions and structural inheritance: Rudiments of early life or recent acquisition? Curr. Opin. Chem. Biol. 2004, 8, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, J.; Friedmann, M.P.; Riek, R. Amyloid aggregates arise from amino acid condensations under prebiotic conditions. Angew. Chem. Int. Ed. 2006, 55, 11609–11613. [Google Scholar] [CrossRef] [PubMed]
- Kronja, I.; Orr-Weaver, T.L. Translational regulation of the cell cycle: When, where, how and why? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Berchowitz, L.E.; Kabachinski, G.; Walker, M.R.; Carlile, T.M.; Gilbert, W.V.; Schwartz, T.U.; Amon, A. Regulated formation of an amyloid-like translational repressor governs gametogenesis. Cell 2015, 163, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.P.; Unal, E.; Brar, G.A.; Amon, A. Meiosis I chromosome segregation is established through regulation of microtubule-kinetochore interactions. eLife 2012, 1, e00117. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Halfmann, R.; King, O.; Kapila, A.; Lindquist, S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009, 137, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, R.; Lindquist, S. Epigenetics in the extreme: Prions and the inheritance of environmentally acquired traits. Science 2010, 330, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Collier, B.; Gorgoni, B.; Loveridge, C.; Cooke, H.J.; Gray, N.K. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005, 24, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; Speed, R.; Taggart, M.; McKay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997, 389, 73–77. [Google Scholar] [PubMed]
- King, O.D.; Gitler, A.D.; Shorter, J. The tip of the iceberg: RNA binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012, 1462, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Spalding, A.C. Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science 2016, 30, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.W.; King, M.L. Germ plasm and molecular determinants of germ cell fate. Curr. Top. Dev. Biol. 2000, 50, 155–181. [Google Scholar] [PubMed]
- Richardson, B.E.; Lehmann, R. Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat. Rev. Mol. Cell Biol. 2010, 11, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Boke, E.; Ruer, M.; Wuhr, M.; Coughlin, M.; Lemaitre, R.; Gygi, S.P.; Alberti, S.; Drechsel, D.; Hyman, A.A.; Mitchison, T.J. Amyloid-like self-assembly of a cellular compartment. Cell 2016, 166, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Dosch, R.; Wagner, D.S.; Mintzer, K.A.; Runke, G.; Wiemelt, A.P.; Mϋllins, M.C. Maternal control of vertebrate development before the midblastula transition: Mutants from the zebrafish I. Dev. Cell 2004, 6, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Ephrussi, A.; Dickinson, L.K.; Lehmann, R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 1991, 66, 37–50. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Berruti, G.; Paiardi, C. Acrosome biogenesis: Revisiting old questions to yield new insights. Spermatogenesis 2011, 1, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.M.; Oda, M.N.; Friend, D.S.; Huang, T.T. A mechanism for differential release of acrosomal enzymes during the acrosome reaction. Biochem. J. 1991, 285, 759–766. [Google Scholar] [CrossRef]
- Kim, K.-S.; Gerton, G.L. Differential release of soluble and matrix components: Evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev. Biol. 2003, 264, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kang-Decker, N.; Mantchev, G.T.; Juneja, S.C.; McNiven, M.A.; van Deursen, J.M. Lack of acrosome formation in Hrb-deficient mice. Science 2001, 294, 1531–1533. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-N.; Roy, A.; Yan, W.; Burns, K.H.; Matzuk, M.M. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol. Cell. Biol. 2007, 27, 6794–6805. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Egge, N.; Cornwall, G.A. Functional amyloids in the mouse sperm acrosome. Mol. Cell. Biol. 2014, 34, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.R.; Lindquist, S. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 1998, 94, 73–82. [Google Scholar] [CrossRef]
- Chau, K.M.; Cornwall, G.A. Reduced fertility in vitro in mice lacking the cystatin CRES (cystatin-related epididymal spermatogenic): Rescue by exposure of spermatozoa to dibutyryl cAMP and isobutylmethylxanthine. Biol. Reprod. 2011, 84, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Thaler, C.D.; Cardullo, R.A. The initial molecular interaction between mouse sperm and the zona pellucida is a complex binding event. J. Biol. Chem. 1996, 271, 23289–23297. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.M.; Garbers, D.L. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J. Biol. Chem. 1995, 270, 26025–26028. [Google Scholar]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Wassarman, P.M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J. Mol. Biol. 1985, 181, 253–264. [Google Scholar] [CrossRef]
- Litscher, E.S.; Janssen, W.G.; Darie, C.C.; Wassarman, P.M. Purified mouse egg zona pellucida glycoproteins polymerize into homomeric fibrils under non-denaturing conditions. J. Cell. Physiol. 2008, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Boja, E.S.; Hoodbhoy, T.; Fales, H.M.; Dean, J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 2003, 278, 34189–34202. [Google Scholar] [CrossRef] [PubMed]
- Jovine, L.; Darie, C.C.; Litscher, E.S.; Wassarman, P.M. Zona pellucida domain proteins. Annu. Rev. Biochem. 2005, 74, 83–114. [Google Scholar] [CrossRef] [PubMed]
- Monne, M.; Han, L.; Schwend, T.; Burendahl, S.; Jovine, L. Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats. Nature 2008, 456, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, J.E.; Yi, X.; MacCoss, M.J.; Swanson, W.J. Rapidly evolving zona pellucida domain proteins are a major component of the vitelline envelope of abalone eggs. Proc. Natl. Acad. Sci. USA 2006, 103, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.J.; Aagaard, J.E.; Vacquier, V.D.; Monne, M.; Hosseini, H.A.S.; Jovine, L. The molecular basis of sex: Linking yeast to humans. Mol. Biol. Evol. 2011, 28, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Monne, M.; Jovine, L. A structural view of egg coat architecture and function in fertilization. Biol. Reprod. 2011, 85, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Podrabsky, J.E.; Carpenter, J.F.; Hand, S.C. Survival of water stress in annual fish embryos: Dehydration avoidance and egg envelope amyloid fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R123–R131. [Google Scholar] [PubMed]
- Louros, N.N.; Petronikolou, N.; Karamanos, T.; Cordopatis, P.; Iconomidou, V.A.; Hamodrakas, S.J. Structural studies of ‘aggregation-prone’ peptide-analogues of teleostean egg chorion ZPB proteins. Biopolymers 2014, 102, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Iconomidou, V.A.; Vriend, G.; Hamodrakas, S.J. Amyloids protect the silkmoth oocyte and embryo. FEBS Lett. 2000, 479, 141–145. [Google Scholar] [CrossRef]
- Egge, N.; Mutusubramanian, A.; Cornwall, G.A. Amyloid properties of the mouse egg zona pellucida. PLoS ONE 2015, 10, e0129907. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Monne, M.; Okumura, H.; Schwend, T.; Cherry, A.L.; Flot, D.; Matsuda, T.; Jovine, L. Insights into egg coat assembly and egg-sperm interaction from the X-ray structure of full-length ZP3. Cell 2010, 143, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, D.; Lu, C.-F.; Kurjan, J.; Lipke, P.N. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein a-agglutinin, a member of the immunoglobulin superfamily. Mol. Cell. Biol. 1993, 13, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Tsubamoto, H.; Hasegawa, A.; Nakata, Y.; Naito, S.; Yamasaki, N.; Koyama, K. Expression of recombinant human zona pellucida protein 2 and its binding capacity to spermatozoa. Biol. Reprod. 1999, 61, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.A.; Baibakov, B.; Dean, J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J. Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Louros, N.N.; Evangelia, D.C.; Baltatzis, G.E.; Patsouris, E.S.; Hamodrakas, S.J.; Iconomidou, V.A. A common “aggregation-prone” interface possibly participates in the self-assembly of human zona pellucida proteins. FEBS Lett. 2016, 590, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Louros, N.N.; Iconomidou, V.A.; Giannelou, P.; Hamodrakas, S.J. Structural analysis of peptide-analogues of human Zona Pellucida ZP1 protein with amyloidogenic properties: Insights into mammalian Zona Pellucida formation. PLoS ONE 2013, 8, e73258. [Google Scholar] [CrossRef] [PubMed]
- Kurotaki, Y.; Hatta, K.; Nakao, K.; Nabeshima, Y.; Fujimori, T. Blastocyst axis is specified independently of early cell lineage but aligns with the ZP shape. Science 2007, 316, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.; McFarlane, H.T.; et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C.F. Common core structure of amyloid fibrils by synchroton X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Plaza, S.; Chanut-Delalande, H.; Fernandes, I.; Wassarman, P.M.; Payre, F. From A to Z: Apical structures and zona pellucida-domain proteins. Trends Cell Biol. 2010, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Whelly, S.; Johnson, S.; Powell, J.; Borchardt, C.; Hastert, M.C.; Cornwall, G.A. Nonpathological extracellular amyloid is present during normal epididymal sperm maturation. PLoS ONE 2012, 7, e36394. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Whelly, S.; Muthusubramanian, A.; Powell, J.; Johnson, S.; Hastert, M.C.; Cornwall, G.A. Cystatin-related epididymal spermatogenic subgroup members are part of an amyloid matrix and associated with extracellular vesicles in the mouse epididymal lumen. Mol. Hum. Reprod. 2016, 22, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Wahlbom, M.; Wang, X.; Lindström, V.; Carlemalm, E.; Jaskolski, M.; Grubb, A. Fibrillogenic oligomers of human cystatin C are formed by propagated domain swapping. J. Biol. Chem. 2007, 282, 18318–18326. [Google Scholar] [CrossRef] [PubMed]
- Hamil, K.G.; Liu, Q.; Sivashanmugam, P.; Yenugu, S.; Soundararajan, R.; Grossman, G.; Richardson, R.T.; Zhang, Y.L.; O’Rand, M.G.; Petrusz, P.; et al. Cystatin 11: A new member of the cystatin type 2 family. Endocrinology 2002, 143, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Wassler, M.; Syntin, P.; Sutton-Walsh, H.G.; Hsia, N.; Hardy, D.M.; Cornwall, G.A. Identification and characterization of cystatin-related epididymal spermatogenic protein in human spermatozoa: Localization in the equatorial segment. Biol. Reprod. 2002, 67, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.D.; Cornwall, G.A.; Liu, L.Y.; Smith, C.E.; Hermo, L. Alterations in the testis and epididymis associated with loss of function of the cystatin-related epididymal spermatogenic (CRES) protein. J. Androl. 2011, 32, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, Q.; Chen, S.; Cai, H.; Lu, M.; Liu, Y.; Xu, C. Antimicrobial activity and molecular mechanism of the CRES protein. PLoS ONE 2012, 7, e48368. [Google Scholar] [CrossRef] [PubMed]
- Björck, L.; Grubb, A.; Kjellén, L. Cystatin C, a human proteinase inhibitor, blocks replication of herpes simplex virus. J. Virol. 1990, 64, 941–943. [Google Scholar] [PubMed]
- Blankenvoorde, M.F.; van’t Hof, W.; Walgreen-Weterings, E.; van Steenbergen, T.J.; Brand, H.S.; Veerman, E.C.; Nieuw Amerongen, A.V. Cystatin and cystatin-derived peptides have antibacterial activity against the pathogen Porphyromonas gingivalis. Biol. Chem. 1998, 379, 1371–1375. [Google Scholar] [PubMed]
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Moon, J.C.; Shin, S.Y.; Son, H.; Jung, Y.J.; Kim, N.H.; Kim, Y.M.; Jang, M.K.; Lee, J.R. Functional characterization of α-synuclein protein with antimicrobial activity. Biochem. Biophys. Res. Commun. 2016, 478, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Kagan, B.L.; Jang, H.; Capone, R.; Teran Arce, F.; Ramachandran, S.; Lal, R.; Nussinov, R. Antimicrobial properties of amyloid peptides. Mol. Pharm. 2012, 9, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Mϋnch, J.; Rϋcker, E.; Stӓndker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Roan, N.R.; Mϋller, J.A.; Liu, H.; Chu, S.; Arnold, F.; Stϋrzel, C.M.; Walther, P.; Dong, M.; Witkowska, H.W.; Kirchhoff, F.; et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe 2011, 10, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Easterhoff, D.; Ontiveros, F.; Brooks, L.R.; Kim, Y.; Ross, B.; Silva, J.N.; Olsen, J.S.; Feng, C.; Hardy, D.J.; Dunman, P.M.; et al. Semen-derived enhancer of viral infection (SEVI) binds bacteria, enhances bacterial phagocytosis by macrophages, and can protect against vaginal infection by a sexually transmitted bacterial pathogen. Antimicrob. Agents Chemother. 2013, 57, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J,L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.S.; Balch, W.E.; Kelly, J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Lee, S.-J.; Rochet, J.-C.; Ding, T.T.; Williamson, R.E.; Lansbury, P.T., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Vitrenko, Y.A.; Gracheva, E.O.; Richmond, J.E.; Liebman, S.W. Visualization of aggregation of the Rnq1 prion domain and cross-seeding interactions with Sup35NM. J. Biol. Chem. 2007, 282, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.S.; DiMaio, J.T.M.; Doran, T.M.; Brown, C.; Nilsson, B.L.; Dewhurst, S. Seminal plasma accelerates semen-derived enhancer viral infection (SEV1) fibril formation by the prostatic acid phosphatase (PAP 248–286) peptide. J. Biol. Chem. 2012, 287, 11842–11849. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, S.A.; Bajakian, T.H.; Soria, M.A.; Falk, A.S.; Service, R.J.; Langen, R.; Siemer, A.B. Identification and structural characterization of the N-terminal amyloid core of Orb2 isoform A. Sci. Rep. 2016, 6, 38265. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, R.P.; Shewmaker, F.; McPhie, P.; Monterroso, B.; Thurber, K.; Wickner, R.B. The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc. Natl. Acad. Sci. USA 2009, 106, 13731–13736. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.-H.; Hewetson, A.; Cornwall, G.A.; Wylie, B.J. Structural characterization of a functional amyloid along its unique amyloidogenic pathway. Manuscript in preparation.
- Raveendra, B.L.; Siemer, A.B.; Puthanveettil, S.V.; Hendrickson, W.A.; Kandel, E.R.; McDermott, A.E. Characterization of prion-like conformational changes of the neuronal isoform of Aplysia CPEB. Nat. Struct. Mol. Biol. 2013, 20, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.H.; Bockmann, A. The structure of fibrils from “misfolded” proteins. Curr. Opin. Struct. Biol. 2015, 30, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hewetson, A.; Cornwall, G.A.; Sutton, R.B. X-ray crystal structure of cystatin-related epididymal spermatogenic (CRES) to 1.8 Å. Manuscript in preparation.
- Horvath, I.; Wittung-Stafshede, P. Cross-talk between amyloidogenic proteins in type-2 diabetes and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2016, 113, 12473–12477. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Brender, J.R.; Monde, K.; Ono, A.; Evans, M.L.; Popovych, N.; Chapman, M.R.; Ramamoorthy, A. Bacterial curli protein promotes the conversion of PAP248–286 into amyloid SEV1: Cross-seeding of dissimilar amyloid sequences. PeerJ 2013, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Pawlik, M.; Soto, C.; Castaño, E.M.; Sigurdsson, E.M.; Kumar, A.; Gallo, G.; Frangione, B.; Levy, E. Distinct properties of wild-type and the amyloidogenic human cystatin C variant of hereditary cerebral hemorrhage with amyloidosis, Icelandic type. J. Neurochem. 2001, 77, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Whelly, S.; Serobian, G.; Borchardt, C.; Powell, J.; Johnson, S.; Hakansson, K.; Lindstrom, V.; Abrahamson, M.; Grubb, A.; Cornwall, G.A. Fertility defects in mice expressing the L68Q variant of human cystatin C: A role for amyloid in male infertility. J. Biol. Chem. 2014, 289, 7718–7729. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewetson, A.; Do, H.Q.; Myers, C.; Muthusubramanian, A.; Sutton, R.B.; Wylie, B.J.; Cornwall, G.A. Functional Amyloids in Reproduction. Biomolecules 2017, 7, 46. https://doi.org/10.3390/biom7030046
Hewetson A, Do HQ, Myers C, Muthusubramanian A, Sutton RB, Wylie BJ, Cornwall GA. Functional Amyloids in Reproduction. Biomolecules. 2017; 7(3):46. https://doi.org/10.3390/biom7030046
Chicago/Turabian StyleHewetson, Aveline, Hoa Quynh Do, Caitlyn Myers, Archana Muthusubramanian, Roger Bryan Sutton, Benjamin J. Wylie, and Gail A. Cornwall. 2017. "Functional Amyloids in Reproduction" Biomolecules 7, no. 3: 46. https://doi.org/10.3390/biom7030046




