Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L.
Abstract
:1. Introduction
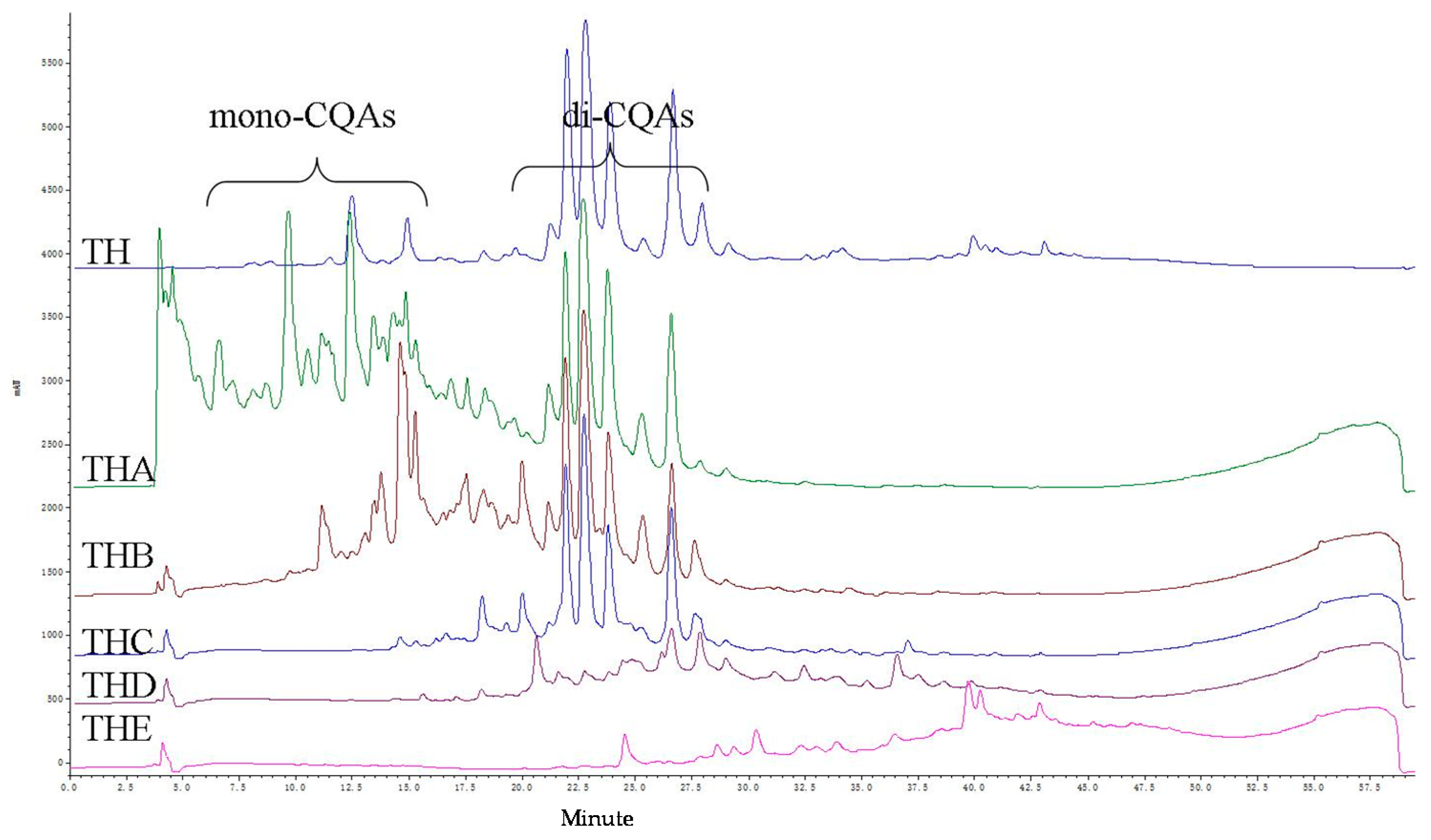
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Equipment and Reagents
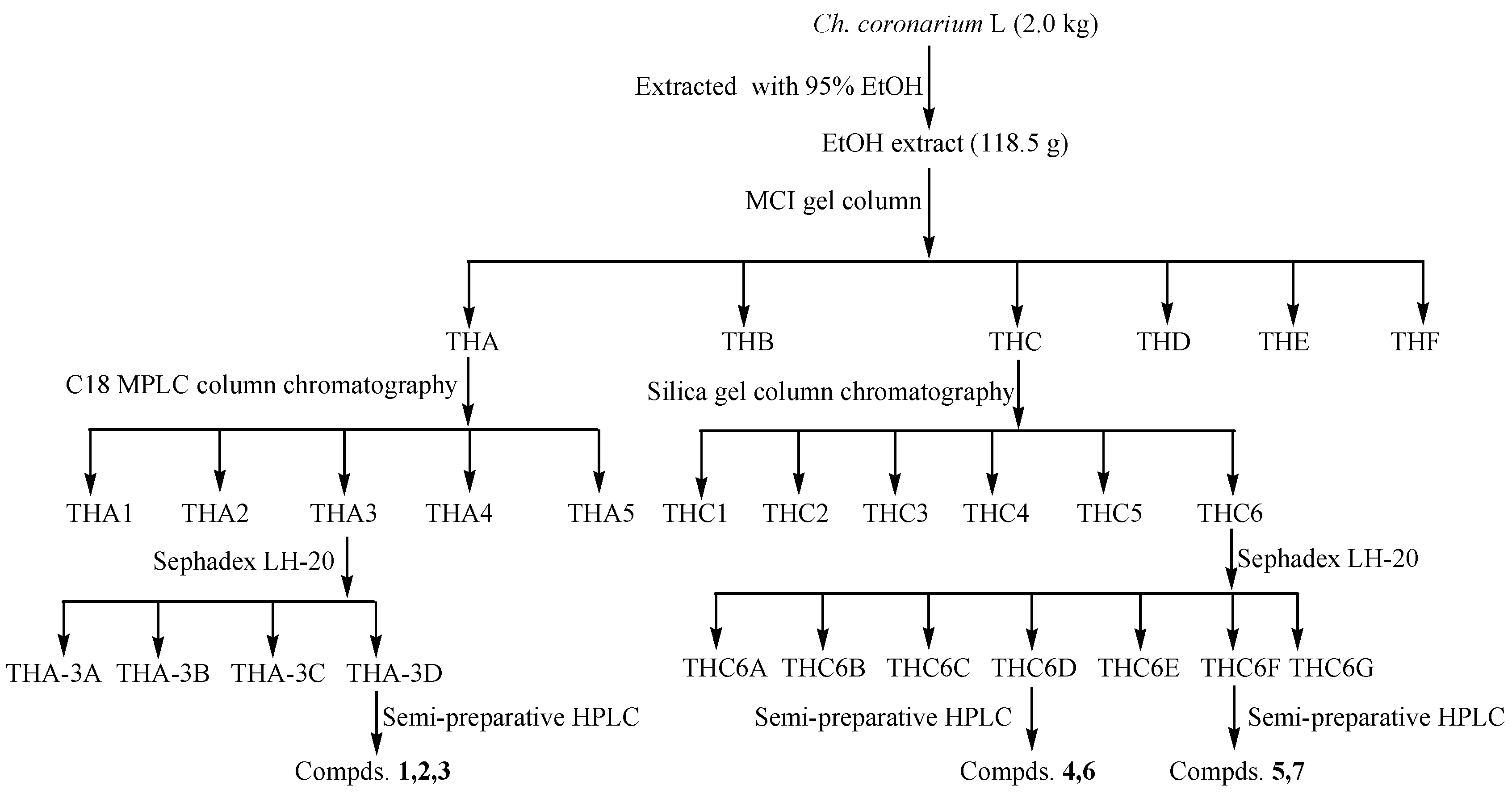
4.3. Extraction and Chromatography
4.4. Purification of the Caffeoylquinic Acid Derivatives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dokuparthi, S.K.; Manikanta, P. Phytochemical and pharmacological studies on Chrysanthemum coronarium L.: A review. J. Drug Discov. Ther. 2015, 3, 11–16. [Google Scholar]
- Ibrahim, L.F.; El-Senousy, W.M.; Hawas, U.W. NMR spectral analysis of flavonoids from Chrysanthemum coronarium. Chem. Nat. Compd. 2007, 43, 659–662. [Google Scholar] [CrossRef]
- Chuda, Y.; Suzuki, M.; Nagata, T.; Tsushida, T. Contents and cooking loss of three quinic acid derivatives from garland (Chrysanthemum coronarium L.). J. Agric. Food Chem. 1998, 46, 1437–1439. [Google Scholar] [CrossRef]
- El-Masry, S.; Abou-Donia, A.H.; Darwish, F.A.; Abou-Karam, M.A.; Grenz, M.; Bohlmann, F. Sesquiterpene lactones from Chrysanthemum coronarium. Phytochemistry 1984, 23, 2953–2954. [Google Scholar] [CrossRef]
- Song, M.C.; Kim, D.H.; Hong, Y.H.; Kim, D.K.; Chung, I.S.; Kim, S.H.; Baek, N.I. Terpenes from the aerial parts of Chrysanthemum coronarium L. Agric. Chem. Biotechnol. 2003, 46, 118–121. [Google Scholar]
- Ragasa, C.Y.; Natividad, G.M. An Antimicrobial Diterpene from Chrysanthemum coronarium. Kimica 1998, 14, 17–20. [Google Scholar]
- Song, M.C.; Yang, H.J.; Lee, D.G.; Kim, D.K.; Ahn, E.M.; Woo, Y.M.; Baek, N.I. Glycosyldiglycerides from the Aerial Parts of Garland (Chrysanthemum coronarium). J. Korean Soc. Appl. Biol. Chem. 2009, 52, 88–91. [Google Scholar] [CrossRef]
- Song, M.C.; Yang, H.J.; Jeong, T.S.; Kim, K.T.; Baek, N.I. Heterocyclic compounds from Chrysanthemum coronarium L. and their inhibitory activity on hACAT-1, hACAT-2, and LDL-oxidation. Arch. Pharm. Res. 2008, 31, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Kim, D.H.; Hong, Y.H.; Yang, H.J.; Chung, I.S.; Kim, S.H.; Baek, N.I. Polyacetylenes and Sterols from the Aerial Parts of Chrysanthemum coronarium L. (Garland). Front. Nat. Prod. Chem. 2005, 1, 163–168. [Google Scholar] [CrossRef]
- Alvarez-Castellanos, P.P.; Bishop, C.D.; Pascual-Villalobos, M.J. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 2001, 57, 99–102. [Google Scholar] [CrossRef]
- Basta, A.; Pavlović, M.; Couladis, M.; Tzakou, O. Essential oil composition of the flowerheads of Chrysanthemum coronarium L. from Greece. Flavour Fragr. J. 2007, 22, 197–200. [Google Scholar] [CrossRef]
- Mahahiro, T.; Kazuhiro, C. Novel plant growth inhibitors and an insect antifeedant from Chrysanthemum coronarium L. Agric. Biol. Chem. 1984, 48, 1367–1369. [Google Scholar]
- Lee, K.D.; Park, K.H.; Kim, H.; Kim, J.H.; Rim, Y.S.; Yang, M.S. Cytotoxic Activity and Structural Analogues of Guaianolide Derivatives from the Flower of Chrysanthemum coronarium L. Agric. Chem. Biotechnol. 2003, 46, 29–32. [Google Scholar]
- Mondolot, L.; La, Fisca, P.; Buatois, B.; Talansier, E.; De Kochko, A.; Campa, C. Evolution in caffeoylquinic acid content and histolocalization during Coffea canephora leaf development. Ann. Bot. 2006, 98, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Yu, Y.; Zhou, S.; Tian, S.; Cao, S. Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacogn. Mag. 2011, 7, 101–108. [Google Scholar] [PubMed]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, Quantitative Determination, and Antioxidative Activities of Chlorogenic Acid Isomers in Prune (Prunus domestica L.). J. Agri. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012, 135, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, K.; Zhang, Y.; Zhao, Y.; Du, Q. Purification and identification of antiviral components from Laggera pterodonta by high-speed counter-current chromatography. J. Chromatogr. B 2007, 859, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.; Li, W.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carnat, A.; Heitz, A.; Fraisse, D.; Carnat, A.P.; Lamaison, J.L. Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia 2000, 71, 587–589. [Google Scholar] [CrossRef]
- Kodoma, M.; Wada, H.; Otani, H.; Kohmoto, K.; Kimura, Y. 3,5-Di-O-caffeoylquinic acid, an infection-inhibiting factor from Pyrus pyrifolia induced by infection with Alternaria alternata. Phytochemistry 1998, 47, 371–373. [Google Scholar] [CrossRef]
- Wan, C.; Liu, Q.; Zhang, X.; Fan, S. A Review of the Chemical Composition and Biological Activities of the Edible and Medicinal Plant Chrysanthemum coronarium L. Mod. Food Sci. Technol. 2014, 30, 282–288. [Google Scholar]
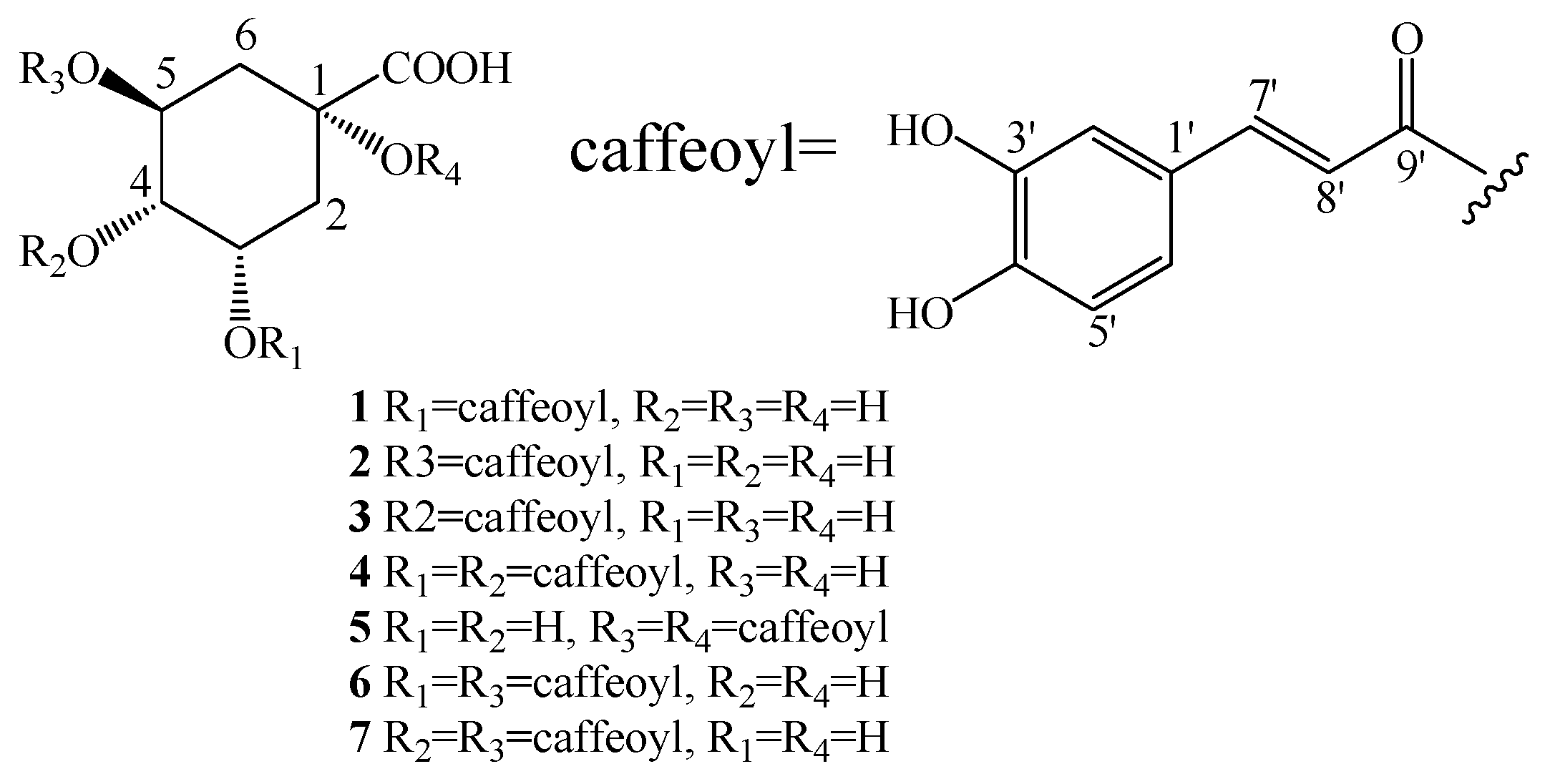
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| δH (J Hz) | δH (J Hz) | δH (J Hz) | δH (J Hz) | δH (J Hz) | δH (J Hz) | δH (J Hz) | |
| 2 | 1.93–2.22 (2H, m) | 2.04–2.25 (2H, m) | 1.98–2.23 (2H, m) | 2.07–2.35 (2H, m) | 2.54 (1H, dd, 3.5, 10.2) 2.43 (1H, m) | 2.12–2.32 (2H, m) | 2.14–2.30 (2H, m) |
| 3 | 5.36 (1H, brd, 2.9) | 4.18 (1H, brd, 2.9) | 4.29 (1H, brs) | 5.61 (1H, brd, 3.4) | 4.28 (1H, brd, 3.5) | 5.36 (1H, brd, 5.6) | 4.40 (1H, brs) |
| 4 | 3.65 (1H, dd, 2.8, 8.5) | 3.75 (1H, dd, 2.3, 8.0) | 4.80 (1H, dd, 2.3, 9.0) | 4.96 (1H, dd, 3.1, 9.4) | 3.76 (1H, dd, 3.0, 8.1) | 3.96 (1H, dd, 3.2, 7.3) | 5.10 (1H, d, 8.5) |
| 5 | 4.14 (1H, ddd, 3.6, 8.5, 8.5) | 5.35 (1H, ddd, 3.5, 8.0, 8.0) | 4.27 (1H, ddd, 9.0, 9.0, 4.5) | 4.35 (1H, ddd, 4.2, 9.4, 9.4) | 5.37 (1H, ddd, 3.7, 8.1, 8.1) | 5.42 (1H, ddd, 3.2, 7.3, 7.3) | 5.63 (1H, ddd, 4.2, 8.5, 8.5) |
| 6 | 1.93–2.22 (2H, m) | 2.04–2.25 (2H, m) | 1.98–2.23 (2H, m) | 2.07–2.35 (2H, m) | 2.05 (1H, dd, 11.1, 13.8) 2.43 (1H, m) | 2.12–2.32 (2H, m) | 2.14–2.30 (2H, m) |
| 2′/2″ | 7.04 (1H, d, 1.4) | 7.05 (1H, brs) | 7.06 (1H, d, 1.4) | 7.02/7.00 (each 1H, d, 1.8) | 7.04 (each 1H, brs) | 7.05 (each 1H, s) | 7.00/6.97 (each 1H, s) |
| 5′/5″ | 6.78 (1H, d, 8.0) | 6.78 (1H, d, 8.0) | 6.78 (1H, d, 8.0) | 6.76/6.72 (each 1H, d, 7.9) | 6.78/6.76 (each 1H, d, 8.1) | 6.77/6.75 (each 1H, d, 8.1) | 6.73/6.71 (each 1H, d, 8.1) |
| 6′/6″ | 6.94 (1H, dd, 1.4, 8.0) | 6.95 (1H, d, 8.0) | 6.97 (1H, dd, 1.4, 8.0) | 6.90/6.85 (each 1H, dd, 1.8, 7.9) | 6.96/6.94 (each 1H, d, 8.1) | 6.96/6.94 (each 1H, m) | 6.89/6.87 (each 1H, d, 8.1) |
| 7′/7″ | 7.58 (1H, d, 15.9) | 7.56 (1H, d, 15.9) | 7.63 (1H, d, 15.9) | 7.57/7.52 (each 1H, d, 15.9) | 7.58/7.55 (each 1H, d, 15.9) | 7.60/7.56 (each 1H, d, 15.9) | 7.57/7.49 (each 1H, d, 15.9) |
| 8′/8″ | 6.30 (1H, d, 15.9) | 6.27 (1H, d, 15.9) | 6.37 (1H, d, 15.9) | 6.27/6.23 (each 1H, d, 15.9) | 6.27/6.24 (each 1H, d, 15.9) | 6.33/6.24 (each 1H, d, 15.9) | 6.26/6.17 (each 1H, d, 15.9) |
| No. | Compounds | ||
|---|---|---|---|
| 2 | 5 | 6 | |
| 1 | 74.7 | 80.9 | 73.3 |
| 2 | 36.8 | 35.7 | 34.6 |
| 3 | 69.9 | 69.4 | 71.1 |
| 4 | 72.1 | 72.8 | 69.2 |
| 5 | 70.5 | 71.6 | 70.6 |
| 6 | 37.4 | 36.9 | 36.2 |
| 7 | 175.6 | 174.8 | 175.9 |
| 1′ | 126.4 | 127.8/127.8 | 126.5/126.4 |
| 2′ | 113.8 | 115.3/115.3 | 114.2/113.9 |
| 3′ | 145.3 | 147.6/147.4 | 145.3/145.3 |
| 4′ | 148.1 | 149.7/149.7 | 148.1/148.0 |
| 5′ | 115.1 | 116.5/116.5 | 115.1/115.1 |
| 6′ | 121.6 | 123.1/123.1 | 121.6/121.6 |
| 7′ | 145.7 | 147.4/146.9 | 145.9/145.6 |
| 8′ | 113.8 | 115.2/115.1 | 113.7/113.7 |
| 9′ | 167.3 | 168.7/168.0 | 167.4/167.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Li, S.; Liu, L.; Chen, C.; Fan, S. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants 2017, 6, 10. https://doi.org/10.3390/plants6010010
Wan C, Li S, Liu L, Chen C, Fan S. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants. 2017; 6(1):10. https://doi.org/10.3390/plants6010010
Chicago/Turabian StyleWan, Chunpeng, Shanshan Li, Lin Liu, Chuying Chen, and Shuying Fan. 2017. "Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L." Plants 6, no. 1: 10. https://doi.org/10.3390/plants6010010







