Silicon and Mechanisms of Plant Resistance to Insect Pests
Abstract
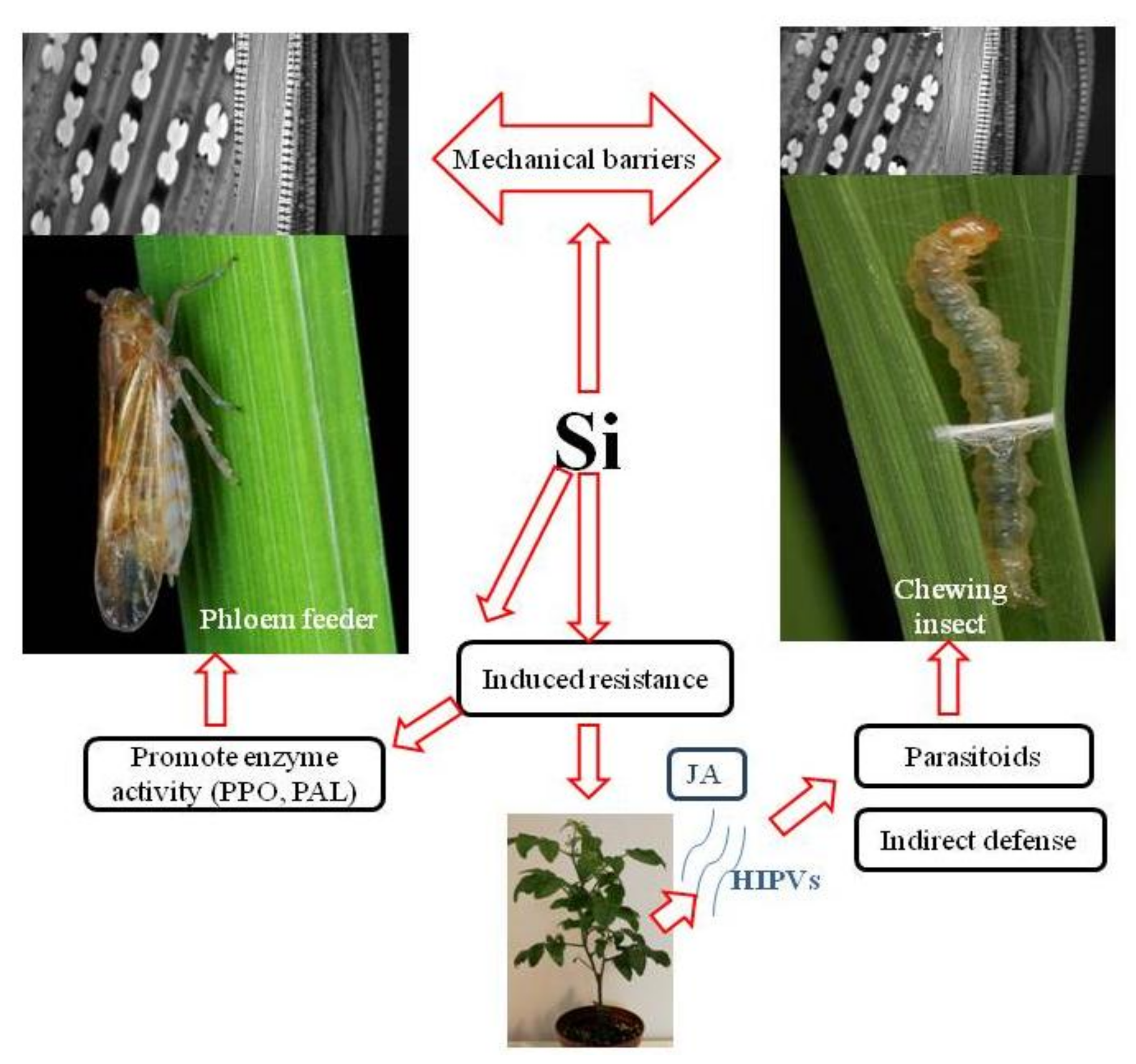
:1. Introduction
2. Formation of Physical Barriers to Insects
3. Silicon-Mediated Induced Resistance to Insects
3.1. Si and Chewing Insect Pests
3.2. Si and Phloem Feeders
4. Si-Induced Resistance below Ground
5. Summary and Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kogan, M.; Lattin, J.D. Agricultural systems as ecosystems. In Handbook of Pest Management; Ruberson, J., Ed.; M. Dekker: New York, NY, USA, 1999; pp. 1–34. ISBN 0824794338. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997; ISBN 0 226 42495 2/0 226 42496 0. [Google Scholar]
- Van Poecke, R.M.P.; Dicke, M. Induced parasitoid attraction by Arabidopsis thaliana: Involvement of the octadecanoid and the salicylic acid pathway. J. Exp. Bot. 2002, 53, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Michael, G.P.; Tariq, A.; Abdul, A.B.; Barkat, H.; Savarimuthu, I.; Hari, C.S. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 10, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Pappas, M.L.; Broekgaarden, C.; Broufas, G.D.; Kant, M.R.; Messelink, G.J.; Steppuhn, A.; Wäckersf, F.; van Dam, N.M. Induced plant defences in biological control of arthropod pests: A double-edged sword. Pest Manag. Sci. 2017, 73, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Han, Y.Q.; Li, P.; Li, F.; Ali, S.; Hou, M.L. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef]
- Han, Y.; Lei, W.; Wen, L.; Hou, M. Silicon-mediated resistance in a susceptible rice variety to the rice leaf folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0120557. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Silicon in life: A bioinorganic solution to bioorganic essentiality. J. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Episten, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Takahashi, E. Silicon uptake and accumulation in plants. In Soil, Fertilizer and Plant Silicon Research in Japan, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 73–106. ISBN 0 444 51166 0. [Google Scholar]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Liang, Y.; Miroslav, N.; Haijun, G.; Alin, S. Silicon and insect pest resistance. In Silicon in Agriculture; Yongchao, L., Miroslav, N., Richard, B., Haijun, G., Alin, S., Eds.; Springer: Berlin, Germany, 2015; pp. 197–204. ISBN 978-94-017-9977-5. [Google Scholar]
- Kvedaras, O.L.; Keeping, M.G. Silicon impedes stalk penetration by the borer Eldana saccharina in sugarcane. Entomol. Exp. Appl. 2007, 125, 103–110. [Google Scholar] [CrossRef]
- Hou, M.L.; Han, Y.Q. Si-mediated rice plant resistance to the Asiatic rice borer: Effects of silicon amendment and rice varietal resistance. J. Econ. Entomol. 2010, 103, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.A.S.; Sampaio, M.V.; Rodrigues, M.P.; Korndorfer, A.P.; Oliveira, R.S.; Ferreira, S.E.; Korndörfer, G.H. Induction of resistance by silicon in wheat plants to alate and apterous morphs of Sitobion avenae (Hemiptera: Aphididae). Environ. Entomol. 2014, 43, 949–956. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yang, M.; Li, Z.; Qui, J.; Liu, F.; Qu, X.; Qiu, Y.; Li, R. High levels of silicon provided as a nutrient in hydroponic culture enhances rice plant resistance to brown planthopper. Crop Prot. 2015, 67, 20–25. [Google Scholar] [CrossRef]
- Parrella, M.P.; Costamagna, T.P.; Kaspi, R. The addition of potassium silicate to the fertilizer mix to suppress Liriomyza leafminers attacking chrysanthemums. Acta Hortic. 2007, 747, 365–369. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Keeping, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Laing, M.D.; Gatarayiha, M.C.; Adandonon, A. Silicon use for pest control in agriculture. Proc. S. Afr. Sugar Technol. Assoc. 2006, 80, 278–286. [Google Scholar]
- Nikpay, A.; Nejadian, E.S. Field applications of silicon-based fertilizers against sugarcane yellow mite Oligonychus sacchari. Sugar Tech. 2014, 16, 319–324. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Song, Y.Y.; Long, J.; Wang, R.L.; Baeron, S.R.; Pan, Z.Q.; Salzman, K.Z.; Xie, J.F.; Cai, K.Z.; Luo, S.M.; et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Gregor, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 15, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Histochemistry of Si in rice tissues. III. The presence of cuticle–silica double layer in the epidermal tissue. Soil Sci. Plant Nutr. 1962, 8, 1–51. [Google Scholar]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.; Junqueira, A.R.; de Sá, V.M.; Zanúncio, J.; Serrão, J. Effect of silicon on the morphology of the midgut and mandible of tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) larvae. ISJ 2015, 12, 158–165. [Google Scholar]
- Sangster, A.G.; Hodson, M.J.; Tubb, H.J. Silicon deposition in higher plants. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 85–113. ISBN 978-0-444-50262-9. [Google Scholar]
- Smagghe, G.; Tirry, L. Insect midgut as a site for insecticide detoxification and resistance. In Biochemical Sites of Insecticide Action and Resistance; Ishaaya, I., Ed.; Springer: Berlin, Germany, 2001; pp. 293–321. ISBN 978-3-642-59549-3. [Google Scholar]
- Keeping, M.G.; Kvedaras, O.L.; Bruton, A.G. Epidermal silicon in sugarcane: Cultivar differences and role in resistance to sugarcane borer Eldana saccharina. Environ. Exp. Bot. 2009, 66, 54–60. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.A.A.; Alves, E.; Pozza, E.A.; Carvalho, J.G.; Montanari, M.; Guimarães, P.T.G.; Santos, D.M. Efeito do silício no controle da cercosporiose em três variedades de cafeeiro. Fitopatol. Bras. 2004, 29, 185–188. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Dorairaj, D.; Ismail, M.R. Distribution of silicified microstructures, regulation of cinnamyl alcohol dehydrogenase and lodging resistance in silicon and paclobutrazol mediated Oryza sativa. Front. Physiol. 2017, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Handley, R.; Ekbom, B.; Agren, J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol. Entomol. 2005, 30, 284–292. [Google Scholar] [CrossRef]
- Tissier, A. Glandular trichomes: What comes after expressed sequence tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Han, Y.; Li, P.; Wen, L.; Hou, M. Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiptera: Delphacidae). Sci. Rep. 2017, 7, 1101. Available online: http://www.nature.com/articles/s41598-017-01060-4 (accessed on 24 April 2017).
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.; Allsopp, P.G.; Gherlenda, A.N.; Johnson, S.N. Increased root herbivory under elevated atmospheric carbon dioxide concentrations is reversed by silicon-based plant defences. J. Appl. Ecol. 2016, 54, 1310–1319. [Google Scholar] [CrossRef]
- Frew, A.; Powel, J.R.; Hiltpold, I.; Allsopp, P.G.; Sallam, N.; Johnson, S.N. Host plant colonisation by arbuscular mycorrhizal fungi stimulates immune function whereas high root silicon concentrations diminish growth in a soil-dwelling herbivore. Soil Biol. Biochem. 2017, 112, 117–126. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Kindt, F.; Joosten, N.N.; Peters, D.; Tjallingii, W.F. Characterisation of the feeding behaviour of western flower thrips in terms of electrical penetration graph (EPG) waveforms. J. Insect Physiol. 2003, 49, 183–191. [Google Scholar] [CrossRef]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, J.; Zhang, P.; Han, L.; Reynolds, O.L.; Zeng, R.; Wu, J.; Shao, Y.; You, M.; Gurr, G.M. Silicon supplementation alters the composition of herbivore induced plant volatiles and enhances attraction of parasitoids to infested rice plants. Front. Plant Sci. 2017, 8, 1265. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322. [Google Scholar] [CrossRef]
- Ghareeb, H.; Bozso, Z.; Ott, P.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Vivancos, J.; Labbe, C.; Menzies, J.G.; Belanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the “cry for help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Van Poecke, R.M.P.; Dicke, M. Indirect defence of plants against herbivores: Using Arabidopsis thaliana as a model plant. Plant Biol. 2004, 6, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Van Oudenhove, L.; Mailleret, L.; Fauvergue, X. Infochemical use and dietary specialization in parasitoids: A meta-analysis. Ecol. Evol. 2017, 7, 4804–4811. [Google Scholar] [CrossRef] [PubMed]
- Kvedaras, O.L.; An, M.; Choi, Y.S.; Gurr, G.M. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bull. Entomol. Res. 2010, 100, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Connick, V.J. The Impact of Silicon Fertilisation on the Chemical Ecology of Grapevine, Vitis vinifera; Constitutive and Induced Chemical Defences against Arthropod Pests and Their Natural Enemies. Master’s Thesis, Charles Sturt University, Albury–Wodonga, NSW, Australia, 2011. [Google Scholar]
- Pereira, R.R.C.; Moraes, J.C.; Prado, E.; Dacosta, R.R. Resistance inducing agents on the biology and probing behaviour of the greenbug in wheat. Sci. Agric. 2010, 67, 430–434. [Google Scholar] [CrossRef]
- Hao, P.; Liu, C.; Wang, Y.; Chen, R.; Tang, M.; Du, B.; Zhu, L.; He, G. Herbivore-induced callose deposition on the sieve plates of rice: An important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.B.; Jair, C.D.M.; Custódio, D.D.S.; Márcio, M.G. Resistance induction in wheat plants by silicon and aphids. Sci. Agric. 2005, 62, 547–551. [Google Scholar] [CrossRef]
- Costa, R.R.; Moraes, J.C.; DaCosta, R.R. Feeding behaviour of the greenbug Schizaphis graminum on wheat plants treated with imidacloprid and/or silicon. J. Appl. Entomol. 2011, 135, 115–120. [Google Scholar] [CrossRef]
- Correa, R.S.B.; Moraes, J.C.; Auad, A.M.; Carvalho, G.A. Silicon and acibenzolar-S-methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Neotrop. Entomol. 2005, 34, 429–433. [Google Scholar] [CrossRef]
- Lu, J.; Robert, C.A.; Riemann, M.; Cosme, M.; Mène-Saffrané, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.A.E.; Jair, C.M.; Luis, C.P.S.; Jonas, F.; Amanda, M.N.; Cristiana, S.A. Inducers of resistance in potato and its effects on defoliators and predatory insects. Rev. Colomb. Entomol. 2012, 38, 30–34. [Google Scholar]
- Olli, S.; Kirti, P.B. Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J. Biochem. Mol. Biol. 2006, 39, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Kuai, J.; Sun, Y.; Guo, C.; Zhao, L.; Zuo, Q.; Wu, J.; Zhou, G. Root-applied silicon in the early bud stage increases the rapeseed yield and optimizes the mechanical harvesting characteristics. Field Crop. Res. 2017, 200, 88–97. [Google Scholar] [CrossRef]
- Han, Y.; Li, P.; Gong, S.; Yang, L.; Wen, L.; Hou, M. Defense responses in rice induced by silicon amendment against infestation by the leaf folder Cnaphalocrocis medinalis. PLoS ONE 2016, 11, e0153918. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Yang, L.; Han, Y.Q.; Li, P. Improved resistance to the brown planthopper in rice plants amended with silicon and the underlying mechanisms. In Proceedings of the 7th International Conference on Silicon in Agriculture, UAS, Bengaluru, India, 24–28 October 2017. [Google Scholar]
- Assis, F.A.; Moraes, J.C.; Auad, A.M.; Coelho, M. The effects of foliar spray application of silicon on plant damage levels and components of larval biology of the pest butterfly Chlosyne lacinia saundersii (Nymphalidae). Int. J. Pest Manag. 2013, 59, 128–134. [Google Scholar] [CrossRef]
- Peixtot, M.L.; Moraes, J.C.; Silva, A.A.; Assis, F.A. Effect of silicon on the oviposition preference of Bemisia tabaci Biotype B (GENN.) (Hemiptera: Aleyrodidae) on bean (Phaseolus vulgaris L.) plants. Cienc. Agrotec. 2011, 35, 478–481. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rasmann, S.; Hallett, P.D.; Gillespie, T.L.; Halpin, C. Below-ground herbivory and root toughness: A potential model system using lignin-modified tobacco. Physiol. Entomol. 2010, 35, 186–191. [Google Scholar] [CrossRef]
- Saona, C.R.; Musser, R.O.; Vogel, H.; Sue, M.H.M.; Jennifer, S.T. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J. Chem. Ecol. 2010, 36, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, L.; Xing, G.; Li, B. Constitutive and induced resistance in soybean interact to affect performance of a herbivore and its parasitoid. Biol. Control 2016, 101, 145–151. [Google Scholar] [CrossRef]
- Saona, C.R.; Crafts, S.J.; Cañas, L.A. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J. Chem. Ecol. 2003, 29, 2539–2550. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Marco, B.; Rinaldo, B.; Franz, B.; Genevieve, C.; Silvia, D. The induction of volatile emissions in maize by three herbivore species with different feeding habits: Possible consequences for their natural enemies. Biol. Control 1998, 11, 122–129. [Google Scholar] [CrossRef]

| Crop | Insect Species | Resistance Mechanism | Reference |
|---|---|---|---|
| Grasses Lolium perenne L. and Festuca ovina L. | Locust Schistocerca gregaria | Mechanical protection of resources in chlorenchyma cells | [32] |
| Rice | Rice leaf folder Cnaphalocrocis medinalis | Reduced insect food quality and food conversion efficiencies; priming defence-related enzymes | [8,65] |
| Rice | Rice leaf folder Cnaphalocrocis medinalis | Induced defence based on HIPV production | [47] |
| Rice | Asiatic rice borer Chilo suppressalis Walker | Impeded stalk penetration and prolonged penetration duration by early instar larvae | [16] |
| Rice | Brown planthopper Nilaparvata lugens Stål. | Modulation of callose deposition | [66] |
| Rice | Brown planthopper Nilaparvata lugens Stål. | Antibiotic and xenobiotic effects targeting insect physiological functions | [18] |
| Rice | Brown planthopper Nilaparvata lugens Stål. | Physical barrier and induced chemical defences | [7,38] |
| Corn | Armyworm Spodoptera frugiperda | Affected biological parameters (fecundity of females) | [39] |
| Sunflower | Sunflower caterpillar Chlosyne lacinia saundersii | Affected feeding behaviour due to leaf palatability | [67] |
| Potato | Beetle Diabrotica speciosa | Negatively affected oviposition and feeding behaviour | [62] |
| Wheat | Green bug Schizaphis graminum Rondani | Induced defences affecting preference and suppressing population increase | [58,59] |
| Cucumber | Whitefly Bemisia tabaci | Induced defences (synthesis of defensive chemicals) reducing the whitefly population | [60] |
| Bean | Whitefly Bemisia tabaci | Negatively affected oviposition preference development of nymphs | [68] |
| Sugarcane | Greyback canegrub Dermolepida albohirtum | Increased lignin accumulation | [41] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhousari, F.; Greger, M. Silicon and Mechanisms of Plant Resistance to Insect Pests. Plants 2018, 7, 33. https://doi.org/10.3390/plants7020033
Alhousari F, Greger M. Silicon and Mechanisms of Plant Resistance to Insect Pests. Plants. 2018; 7(2):33. https://doi.org/10.3390/plants7020033
Chicago/Turabian StyleAlhousari, Fadi, and Maria Greger. 2018. "Silicon and Mechanisms of Plant Resistance to Insect Pests" Plants 7, no. 2: 33. https://doi.org/10.3390/plants7020033





