Clinical Outcomes Used in Clinical Pharmacy Intervention Studies in Secondary Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
- described primary research
- were published in English
- described interventions delivered by clinical pharmacists
- were not published as a research paper (e.g., reviews, books, congress abstracts, posters, reports, protocols)
- did not include outcome data
- presented data for a secondary study, where the original study had been published previously
- had been conducted in primary care
- included 100 patients or less
2.2. Assessment
3. Results
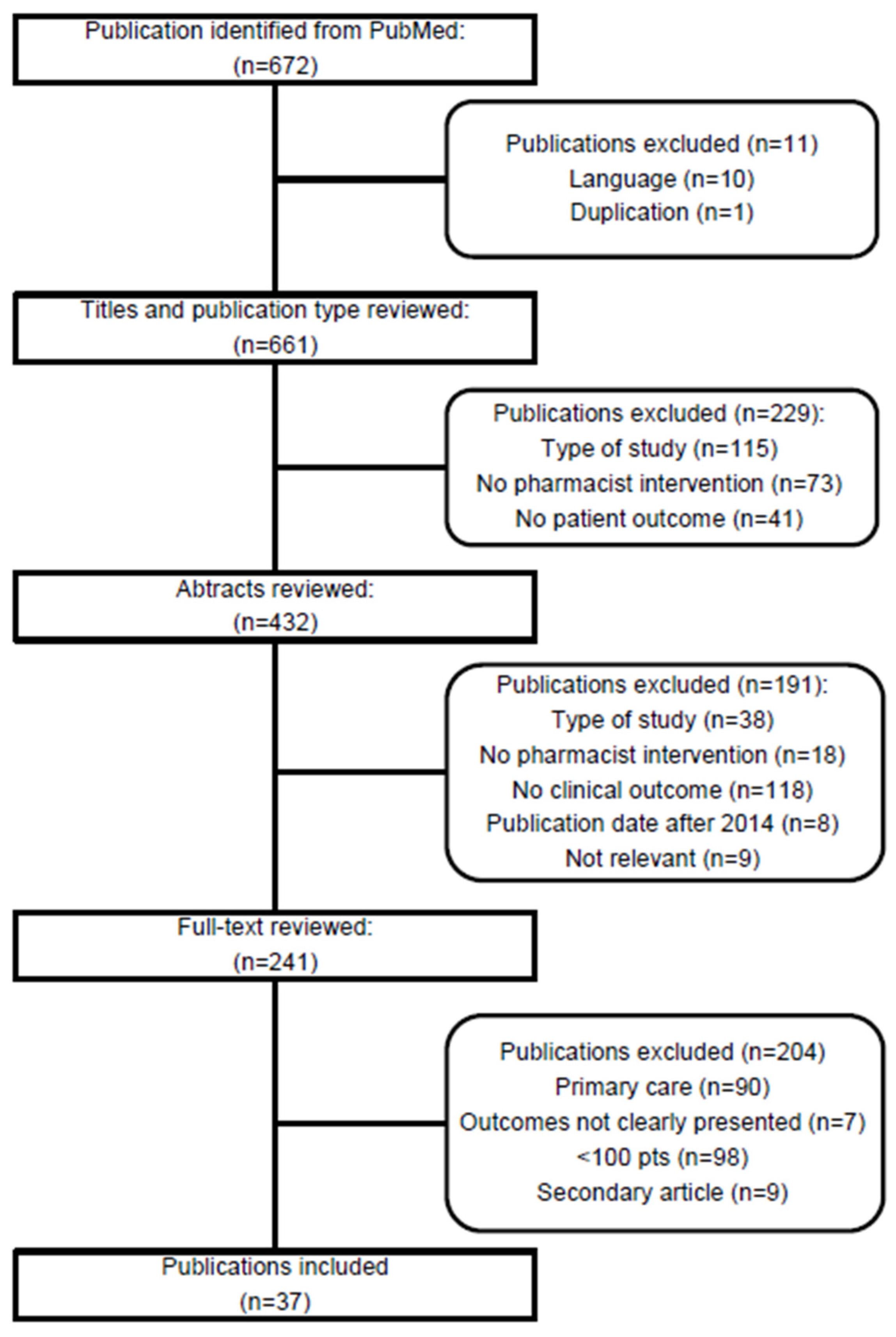
3.1. Study Selection
3.2. Description of Studies
3.3. Description of Outcome
4. Discussion
4.1. Outcome Measures
4.2. Generic Versus Disease Specific Tools
4.3. Primary Versus Secondary Outcomes
4.4. Target Groups for Results
4.5. Relevant Outcomes
4.6. Implementation Rate of the Clinical Pharmacy Intervention
4.7. Limitation
5. Conclusions
Author Contributions
Conflicts of interest
References
- Graabaek, T.; Kjeldsen, L.J. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: A systematic review. Basic Clin. Pharmacol. Toxicol. 2013, 112, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Lundh, A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst. Rev. 2016, 2, CD008986. [Google Scholar] [PubMed]
- Holland, R.; Desborough, J.; Goodyer, L.; Hall, S.; Wright, D.; Loke, Y.K. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2008, 65, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Hoth, A.B.; McClimon, B.J.; Schnipper, J.L. Clinical pharmacists and inpatient medical care: A systematic review. Arch. Intern. Med. 2006, 166, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.B.; McLachlan, A.J.; Brien, J.A. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: A systematic review and meta-analysis. BMJ Open 2016, 6, e010003. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.C.K.; RESPECT Team. Randomised controlled trials (RCTs) to evaluate complex healthcare interventions—A case study. Pharm. World Sci. 2004, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Krska, J.; Rowe, P.H. Outcome measures: A sensitive approach. Int. J. Pharm. Pract. 2010, 18, 125–127. [Google Scholar] [PubMed]
- Kjeldsen, L.J.; Nielsen, T.R.H.; Olesen, C. The challenges of outcome research. Int. J. Clin. Pharm. 2016, 38, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Frenk, J. Obituary of avedis donabedian. Bull. World Health Organ. 2000, 70, 1475. [Google Scholar]
- Donabedian, A. The quality of care: How can it be assessed? JAMA 1988, 121, 1145–1150. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Blazeby, J.M.; Clarke, M.; Devane, D.; Gargon, E.; Tugwell, P. Developing core outcome sets for clinical trials: Issues to consider. Trials 2012, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- The COMET Initiative. Available online: http://www.comet-initiative.org/ (accessed on 1 May 2016).
- Bowling, A. Research Methods in Health: Investigating Health and Health Services, 2nd ed.; Open University Press: Buckingham, UK, 2002. [Google Scholar]
- Al Mazroui, N.R.; Kamal, M.M.; Ghabash, N.M.; Yacout, T.A.; Kole, P.L.; McElnay, J.C. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. Br. J. Clin. Pharmacol. 2009, 67, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Albsoul-Younes, A.M.; Hammad, E.A.; Yasein, N.A.; Tahaineh, L.M. Pharmacist-physician collaboration improves blood pressure control. Saudi Med. J. 2011, 32, 288–292. [Google Scholar] [PubMed]
- Barker, A.; Barlis, P.; Berlowitz, D.; Page, K.; Jackson, B.; Lim, W.K. Pharmacist directed home medication reviews in patients with chronic heart failure: A randomised clinical trial. Int. J. Cardiol. 2012, 159, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bladh, L.; Ottosson, E.; Karlsson, J.; Klintberg, L.; Wallerstedt, S.M. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: A randomised controlled trial. BMJ Qual. Saf. 2011, 20, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Siu, S.C.; Wong, C.K.; Lee, V.W. A pharmacist care program: Positive impact on cardiac risk in patients with type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Wu, S.S.; Lee, P.Y.; Huang, Y.C.; Tan, T.Y.; Chang, K.C. Control of modifiable risk factors in ischemic stroke outpatients by pharmacist intervention: An equal allocation stratified randomized study. J. Clin. Pharm. Ther. 2008, 33, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Lee, K.K.; Tomlinson, B.; Lee, V.W. Clinical and economic impact of clinical pharmacy service on hyperlipidemic management in Hong Kong. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Crotty, M.; Rowett, D.; Spurling, L.; Giles, L.C.; Phillips, P.A. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am. J. Geriatr. Pharmacother. 2004, 2, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, P.; Kravet, S.; Bulger, J.; Hinson, T.; Sridharan, A.; Kolodner, K.; Wright, S.; Howell, E. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J. Am. Geriatr. Soc. 2009, 57, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, U.; Alassaad, A.; Henrohn, D.; Garmo, H.; Hammarlund-Udenaes, M.; Toss, H.; Kettis-Lindblad, A.; Melhus, H.; Mörlin, C. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: A randomized controlled trial. Arch. Intern. Med. 2009, 169, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Hammad, E.A.; Yasein, N.; Tahaineh, L.; Albsoul-Younes, A.M. A randomized controlled trial to assess pharmacist-physician collaborative practice in the management of metabolic syndrome in a university medical clinic in Jordan. J. Manag. Care Pharm. 2011, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hellström, L.M.; Höglund, P.; Bondesson, A.; Petersson, G.; Eriksson, T. Clinical implementation of systematic medication reconciliation and review as part of the Lund Integrated Medicines Management model—Impact on all-cause emergency department revisits. J. Clin. Pharm. Ther. 2012, 37, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.W.; Chetty, V.K.; Anthony, D.; Greenwald, J.L.; Sanchez, G.M.; Johnson, A.E.; Forsythe, S.R.; O’Donnell, J.K.; Paasche-Orlow, M.K.; Manasseh, C.; et al. A reengineered hospital discharge program to decrease rehospitalization: A randomized trial. Ann. Intern. Med. 2009, 150, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Peterson, G.M.; Vial, J.H.; Jupe, D.M. Improving the outcomes of anticoagulation: An evaluation of home follow-up of warfarin initiation. J. Intern. Med. 2004, 256, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; Sherry, P.S.; Taylor, L.M.; Amato, M.; Tataronis, G.R.; Cushing, G. Pharmacist Assisted Medication Program Enhancing the Regulation of Diabetes (PAMPERED) study. J. Am. Pharm. Assoc. 2012, 52, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Jarab, A.S.; Alqudah, S.G.; Khdour, M.; Shamssain, M.; Mukattash, T.L. Impact of pharmaceutical care on health outcomes in patients with COPD. Int. J. Clin. Pharm. 2012, 34, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jarab, A.S.; Alqudah, S.G.; Mukattash, T.L.; Shattat, G.; Al-Qirim, T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J. Manag. Care Pharm. 2012, 18, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Kirwin, J.L.; Cunningham, R.J.; Sequist, T.D. Pharmacist recommendations to improve the quality of diabetes care: A randomized controlled trial. J. Manag. Care Pharm. 2010, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kripalani, S.; Roumie, C.L.; Dalal, A.K.; Cawthon, C.; Businger, A.; Eden, S.K.; Shintani, A.; Sponsler, K.C.; Harris, L.J.; Theobald, C.; et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: A randomized trial. Ann. Intern. Med. 2012, 157, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.S.; Chua, S.S.; Chan, S.P. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int. J. Clin. Pharm. 2013, 35, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.S.; Chua, S.S.; Chew, Y.Y.; Chan, S.P. Effects of pharmaceutical care on adherence and persistence to bisphosphonates in postmenopausal osteoporotic women. J. Clin. Pharm. Ther. 2011, 36, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.W.; Fan, C.S.; Li, A.W.; Chau, A.C. Clinical impact of a pharmacist-physician co-managed programme on hyperlipidaemia management in Hong Kong. J. Clin. Pharm. Ther. 2009, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Low, H.N.; Chan, S.P.; Chen, H.N.; Ding, Y.Y.; Tan, T.L. Impact of a pharmacist consult clinic on a hospital-based geriatric outpatient clinic in Singapore. Ann. Acad. Med. Singap. 2004, 33, 220–227. [Google Scholar] [PubMed]
- Magid, D.J.; Ho, P.M.; Olson, K.L.; Brand, D.W.; Welch, L.K.; Snow, K.E.; Lambert-Kerzner, A.C.; Plomondon, M.E.; Havranek, E.P. A multimodal blood pressure control intervention in 3 healthcare systems. Am. J. Manag. Care 2011, 17, e96–e103. [Google Scholar] [PubMed]
- McCoy, A.B.; Cox, Z.L.; Neal, E.B.; Waitman, L.R.; Peterson, N.B.; Bhave, G.; Siew, E.D.; DanciuI, L.; Lewis, J.B.; Peterson, J.F. Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: A randomized, controlled trial. Appl. Clin. Inform. 2012, 3, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, K.A.; Blum, S.S.; Kugler, A.; Livote, E.E.; Nebeker, J.R.; Ott, M.C.; Signor, D.; Sung, S.; Yeh, J.; Boockvar, K.S. Pharmacist-versus physician-initiated admission medication reconciliation: Impact on adverse drug events. Am. J. Geriatr. Pharmacother. 2012, 10, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Morgado, M.; Rolo, S.; Castelo-Branco, M. Pharmacist intervention program to enhance hypertension control: A randomised controlled trial. Int. J. Clin. Pharm. 2011, 33, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.D.; Young, J.; Hoke, S.; Tu, W.; Weiner, M.; Morrow, D.; Stroupe, K.T.; Wu, J.; Clark, D.; Smith, F.; et al. Pharmacist intervention to improve medication adherence in heart failure: A randomizedtrial. Ann. Intern. Med. 2007, 146, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Sadik, A.; Yousif, M.; McElnay, J.C. Pharmaceutical care of patients with heartfailure. Br. J. Clin. Pharmacol. 2005, 60, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Schnipper, J.L.; Kirwin, J.L.; Cotugno, M.C.; Wahlstrom, S.A.; Brown, B.A.; Tarvin, E.; Kachalia, A.; Horng, M.; Roy, C.L.; McKean, S.C.; et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch. Intern. Med. 2006, 166, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Spinewine, A.; Swine, C.; Dhillon, S.; Lambert, P.; Nachega, J.B.; Wilmotte, L.; Tulkens, P.M. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: A randomized, controlled trial. J. Am. Geriatr. Soc. 2007, 55, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Stange, D.; Kriston, L.; von-Wolff, A.; Baehr, M.; Dartsch, D.C. Reducing cardiovascular medication complexity in a German university hospital: Effects of a structured pharmaceutical management intervention on adherence. J. Manag. Care Pharm. 2013, 19, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Suppapitiporn, S.; Chindavijak, B.; Onsanit, S. Effect of diabetes drug counseling by pharmacist, diabetic disease booklet and special medication containers on glycemic control of type 2 diabetes mellitus: A randomized controlled trial. J. Med. Assoc. Thail. 2005, 88 (Suppl. S4), 134–141. [Google Scholar]
- Tsuyuki, R.T.; Fradette, M.; Johnson, J.A.; Bungard, T.J.; Eurich, D.T.; Ashton, T.; Gordon, W.; Ikuta, R.; Kornder, J.; Mackay, E.; et al. A multicenter disease management program for hospitalized patients with heart failure. J. Card. Fail. 2004, 10, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, V.; Troillet, N.; Beney, J.; Boubaker, K.; Lüthi, J.C.; Taffé, P.; Reymond, J.P. Impact of an interdisciplinary strategy on antibiotic use: A prospective controlled study in three hospitals. J. Antimicrob. Chemother. 2005, 55, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Leung, W.Y.; Chang, S.; Lee, B.; Zee, B.; Tong, P.C.; Chan, J.C. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receivingpolypharmacy: Randomised controlled trial. BMJ 2006, 333, 522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, L.; Huang, L.; Luo, R.; Wen, J. Clinical pharmacists on medical care of pediatric inpatients: A single-center randomized controlled trial. PLoS ONE 2012, 7, e30856. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Absence of evidence is not evidence of absence. BMJ 1995, 311, 485. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Effective Practice and Organisation of Care Review Group. EPOC Resources. Data Collection Checklist and Risk of Bias—EPOC Specific. Available online: http://epoc.cochrane.org/epoc-resources (accessed on 30 April 2017).
| Author | Setting and Country | Patient Population | No. of Included Patients | No. of Patients Analysed/at Endpoint | Mean Age (Years) IG | Mean Age (Years) CG | Gender, Male (%) IG | Gender, Male (%) CG |
|---|---|---|---|---|---|---|---|---|
| Al Mazroui et al. (2009) [14] | General medical wards, endocrinology and medical outpatient clinics, 1 Hospital, UAE | Pts with type 2 diabetes | 240 pts: IG: 120 pts CG: 120 pts | 234 pts: IG: 117 CG: 117 | 48.7, n = 120 | 49.9, n = 120 | 84 (70), n = 120 | 82 (68.3), n = 120 |
| Albsoul-Younes et al. (2011) [15] | 1 family medicine clinic, 1 hospital, Jordan | Pts with uncontrolled hypertension | 266 pts: IG: 136 pts CG: 130 pts | 253 pts: IG: 130 pts CG: 123 | 56.3, n = 130 | 57.5, n = 123 | 61 (47), n = 130 | 59 (48), n = 123 |
| Barker et al. (2012) [16] | 1 hospital, Australia | Pts with chronic heart failure | 120 pts: IG = 64 pts CG = 56 pts | 87 pts: IG: 48 pts CG: 39 pts | 73.0, n = 64 | 72.0, n = 56 | 32 (50), n = 64 | 23 (41), n = 56 |
| Bladh et al. (2011) [17] | 2 internal medicine wards, 1 hospital, Sweden | All patients admitted to the wards on week days | 400 pts: IG: 199 pts CG: 201 pts | 345 pts: IG: 164 CG: 181 | Median: ITT: 81, n = 164 PP: 84, n = 87 | Median: ITT/PP: 82, n = 181 | ITT: 66 (40), n = 164 PP: 30 (34), n = 87 | ITT/PP: 71 (39), n = 181 |
| Chan et al. (2012) [18] | 1 diabetics clinic, 1 hospital, Hong Kong | Pts with type 2 diabetes | 105 pts: IG: 51 pts CG: 54 pts | 105 pts: IG: 51 pts CG: 54 pts | 63.2, n = 51 | 61.7, n = 54 | 30 (59), n = 51 | 28 (52), n = 54 |
| Chiu et al. (2008) [19] | Outpatients, 1 hospital, Taiwan | Pts with ischemic stroke | 160 pts: IG: 80 pts CG: 80 pts | Missing | 65.7, n = 80 | 64.8, n = 80 | 40 (50), n = 80 | 40 (50), n = 80 |
| Chung et al. (2011) [20] | 1 lipid clinic (medical outpatient), 1 hospital, Hong Kong | Pts with chronic dyslipidaemia | 300 pts: IG: 150 pts CG: 150 pts | 300 pts: IG: 150 pts CG: 150 pts | 56.2, n = 150 | 57.9, n = 150 | 68 (45), n = 150 | 60 (40), n = 150 |
| Crotty et al. (2004) [21] | 3 hospitals, Australia | Elderly pts awaiting transfer from hospital to a long term residential care facility for the first time | 110 pts: IG: 56 pts CG: 54 pts | 88 pts: IG: 44 pts CG: 44 | 82.0 | 83.4 | 41% | 37% |
| Dedhia et al. (2009) [22] | General medicine wards, 3 hospitals, USA | Pts aged ≥65 years | 422 pts: IG: 185 pts CG: 237 pts | 422 pts: IG: 185 pts CG: 237 pts | 76.7 | 77.3 | 72 (39), n = 185 | 94 (40), n = 237 |
| Gillespie et al. (2009) [23] | 2 acute internal medicine wards, 1 hospital, Sweden | Pts admitted to the wards | 400 pts: IG: 199 pts CG: 201 pts | 368 pts: IG: 182 pts CG: 186 pts | 86.4, n = 182 | 87.1, n = 186 | 77 (42), n = 182 | 75 (40) n = 186 |
| Hammad et al. (2011) [24] | 6 family medicine outpatient clinics, 1 Hospital, Jordan | Pts with metabolic syndrome | 202 pts: IG: 112 pts CG: 90 pts | 199 pts: IG: 110 pt CG: 89 pts | 56.0, n = 110 | 57.4, n = 89 | 44 (40), n = 110 | 32 (36), n = 89 |
| Hellström et al. (2012) [25] | 3 internal medicine wards, 1 hospital, Sweden | All patients hospitalised at the three study wards | 4290 pts: IG: 1325 CG: 2965 | 3974 pts: IG: 1216 pts CG: 2758 | 78.3 | 79.5 | 46% | 45% |
| Jack et al. (2009) [26] | 1 hospital, USA (entire hospital) | Pts admitted to the hospital, ≥18 years and English speaking | 749 pts: IG: 373 pts CG: 376 pts | 738 pts: IG: 370 pts CG: 368 pts | 50.1, n = 373 | 49.6, n = 376 | 195 (52), n = 373 | 176 (47), n = 376 |
| Jackson et al. (2004) [27] | 1 hospital, Australia (entire hospital) | Pts initiated on warfarin in hospital | 128 pts: IG: 60 pts CG: 68 pts | 127 pts: IG: 59 pts CG: 68 pts | Median: 70, n = 60 | Median: 72.5, n = 68 | 53%, n = 60 | 53%, n = 68 |
| Jacobs et al. (2012) [28] | An ambulatory general internal medicine setting, 1 Clinic, USA | Pts with type 2 diabetes | 396 pts: IG: 195 pts CG: 201 pts | 164 pts: IG: 72 pts CG: 92 pts | 62.7, n = 72 | 63.0, n = 92 | 49 (68), n = 72 | 51 (55), n = 92 |
| Jarab et al. (2012a) [29] | 1 outpatient COPD Clinic, 1 Hospital, Jordan | Pts with COPD | 133 pts: IG: 66 pts CG: 67 pts | 127 pts: IG: 63 pts CG: 64 pts | Median: 61, n = 66 | Median: 64, n = 67 | 26 (39), n = 66 | 28 (42), n = 67 |
| Jarab et al. (2012b) [30] | outpatient diabetes clinic, 1 hospital, Jordan | Pts with type 2 diabetes | 171 pts: IG: 85 pts CG: 86 pts | IG: 77 pts, CG: 79 pts | 63.4, n = 85 | 65.3, n = 86 | 68%, n = 85 | 56%, n = 86 |
| Kirwin et al. (2010) [31] | 1 hospital-based, primary care practice, 1 hospital, USA | Pts with diabetes (type 1 and 2) | 346 pts: IG: 171 pts CG: 175 pts | 301 pts: IG: 150 pts CG: 151 pts | 62.9, n = 150 | 62.8, n = 151 | 29% n = 150 | 39% n = 151 |
| Kripalani et al. (2012) [32] | 2 medical centers, 2 hospitals, USA | Pts with acute coronary syndromes or acute decompensated heart failure | 862 pts: IG: 430 pts CG: 432 pts | 851 pts: IG: 423 pts CG: 428 pts | 61, n = 423 | 59, n = 428 | 250 (59), n = 423 | 249 (58), n = 428 |
| Lai et al. (2013) [33] | 1 osteoporosis clinic, 1 hospital, Malaysia | Pts with postmenopausal osteoporosis | 198 pts: IG: 100 pts CG: 98 pts | 177 IG:88 pts CG: 89 pts | 65.1, n = 100 | 67.1, n = 98 | Missing | Missing |
| Lai et al. (2011) [34] | 1 osteoporosis clinic, 1 hospital, Malaysia | Pts with postmenopausal osteoporosis | 198 pts: IG: 100 pts CG: 98 pts | 177 IG:88 pts CG: 89 pts | 65.1, n = 100 | 67.1, n = 98 | Missing | Missing |
| Lee et al. (2009) [35] | 3 Out-Patient Departments, 3 hospitals, Hong Kong | Pts with hyperlipidaemia | 119 pts: IG: 59 pts CG: 60 pts | 118 pts: IG: 58 pts CG: 60 pts | 63, n = 58 | 61, n = 60 | 34 (59), n = 58 | 26 (43), n = 60 |
| Lim et al. (2004) [36] | 1 geriatric outpatient clinic, 1 hospital, Singapore | Elderly outpatients with risk factors of non-compliance | 136 pts: IG: 68 pts CG: 68 pts | 126 pts IG: 64 pts CG: 62 pts | 79.6, n = 64 | 80.5, n = 62 | 39%, n = 64 | 31%, n = 62 |
| Magid et al. (2011) [37] | 3 healthcare systems, USA | Pts with uncontrolled BP | 338 pts: IG: 174 pts CG: 164 pts | 283 pts IG: 138 pts CG: 145 pts | 65.1, n = 138 | 66.7, n = 145 | 67%, n = 138 | 63%, n = 145 |
| McCoy et al. (2012) [38] | 1 hospital, USA (entire hospital) | Pts with an acute 0.5 mg/dL change in serum creatinine over 48 h and a nephrotoxic or renally cleared medication order | 540 pts: IG: 262 pts CG: 278 pts | 396 pts IG: 200 pts CG: 196 pts | 60.7, n = 200 | 58.3, n = 196 | 53%, n = 200 | 61%, n = 196 |
| Mergenhagen et al. (2012) [39] | 2 general medical units, 1 hospital, USA (entire hospital) | Pts admitted for at least 24 h to one of the study units | 359 ams: 111 ams (pharmacist) 248 ams (physician) | 218 ams: 102 ams (pharmacist) 116 ams (physician) | PharmG: 68, n = 102 | PhysG: 68, n = 116 | PharmG: 100%, n = 102 | PhysG: 98%, N = 116 |
| Morgado (2011) [40] | 1 hospital care hypertension/dyslipidemia outpatient clinic, 1 hospital, Portugal | Pts with essential hypertension | 197 pts: IG: 98 pts CG: 99 pts | Missing | 58.3, n = 99 | 60.7, n = 98 | 44 (45), n = 99 | 35 (35), n = 98 |
| Murray et al. (2007) [41] | 1 ambulatory care practice, USA | Pts with heart failure, low-income, ≥50 years | 314 pts: IG: 122 pts CG: 192 pts | 270 pts: IG: 106 pts CG: 164 pts | 61.4, n = 122 | 62.6, n = 192 | 39 (32), n = 122 | 65 (34), n = 192 |
| Sadik et al. (2005) [42] | General medical wards, cardiology and medical outpatient clinics, 1 hospital, UAE | Pts with heart failure | 221 pts IG: 109 pts CG: 112 pts | 208 pts IG: 104 pts CG: 104 pts | 58.6, n = 104 | 58.7, n = 104 | 52 (50), n = 104 | 52 (50), n = 104 |
| Schnipper et al. (2006) [43] | General medicine service, 1 hospital, USA | Pts discharged home | 178 pts: IG: 92 pts CG: 84 pts | IG: 79, CG: 73 pts | 60.7, n = 92 | 57.7, n = 84 | 33%, n = 92 | 35%, n = 84 |
| Spinewine et al. (2007) [44] | 1 acute Geriatric Evaluation and Management (GEM) unit, 1 hospital, Belgium | Pts aged ≥70 years | 203 pts | 186 pts IG: 96 pts CG: 90 pts | 82.4, n = 96 | 81.9, n = 90 | 28%, n = 96 | 33%, n = 90 |
| Stange et al. (2013) [45] | 1 medical Center, 1 hospital, Germany | Pts with chronic hypertension, diabetes, and/or dyslipidemia | 240 pts IG: 132 pts CG: 108 pts | 162 pts: IG:89 pts CG: 73 pts | 64.4, n = 129 | 63.2, n = 108 | 81 (63), n = 129 | 90 (83), n = 108 |
| Suppapitiporn et al. (2005) [46] | 1 endocrine Clinic, 1 hospital, Thailand | Pts with type 2 diabetes | 360 pts: IG: 180 IG 1 = 50 pts IG 2 = 50 pts IG 3 = 30 pts IG 4 = 50 pts CG: 180 | Missing | 61.4, n = 180 | 59.9, n = 180 | 59 (33), (n = 180) | 64 (36), n = 180 |
| Tsuyuki et al. (2004) [47] | 10 hospitals, Canada | Pts with heart failure | 276 pts: IG: 140 pts CG: 136 pts | Missing | 71, n = 140 | 72, n = 136 | 81 (58), n = 140 | 79 (58), n = 136 |
| von Gunten et al. (2005) [48] | General medical wards and intensive care units, 3 hospitals, Switzerland | Pts receiving antibiotic treatment | 1200 pts: IG; 600 pts, CG: 600 pts IG1: 200 + 200 pts IG2: 200 + 200 pts CG: 200 + 200 pts | Missing | Different categories | Different categories | Different categories | Different categories |
| Wu et al. (2006) [49] | Specialist medical clinics, 1 hospital, Hong Kong | Non-compliant pts with polypharmacy | 442 pts: IG: 219 pts CG:223 pts | Missing | 71.2, n = 219 | 70.5, n = 223 | 108 (49), n = 219 | 107 (48), n = 223 |
| Zhang et al. (2012) [50] | 1 pediatric unit, 1 hospital, China | Pediatric pts with nerve system disease, respiratory system disease or digestive system disease | 160 pts: IG: 80 pts CG: 80 pts | 150 pts: IG: 76 pts CG: 74 pts | Age groups | Age groups | 43 (54), n = 80 | 44 (55), n = 80 |
| Author | Intervention Elements | Study Design | Duration of Study (Intervention Period)/Monitoring | Post Intervention Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient counselling/education * | Adherence assessment/intervention | Medication reconciliation | Medication review | Interdisciplinary collaboration in hospital | Therapeutic drug monitoring | Collaboration between primary acare and inpatient care | ||||
| Al Mazroui et al. (2009) [14] | X | X | X | RCT | Visits at 4 months, 8 months and 12 months | No further follow-up | ||||
| Albsoul-Younes et al. (2011) [15] | X | X | X | X | RCT | Regular monthly visits to the clinic during 6 months | No further follow-up | |||
| Barker et al. (2012) [16] | X | X | X | X | X | RCT | Home visits within 96 h of discharge, at 1 and 6 months | No further follow-up | ||
| Bladh et al. (2011) [17] | X | X | X | X | RCT | 6-month follow-up | ||||
| Chan et al. (2012) [18] | X | X | X | X | RCT | Intervention delivered at each clinic visit during 9 months after enrolment | No further follow-up | |||
| Chiu et al. (2008) [19] | X ** | X | Stratified RCT | The intervention was delivered monthly during 6 months | No further follow-up | |||||
| Chung et al. (2011) [20] | X | X | X | X | Prospective controlled trial | 3 clinic visits and monthly telephone follow-ups during 24 months | No further follow-up | |||
| Crotty et al. (2004) [21] | X | X | RCT | 1 interdisciplinary, cross-sectorial meeting at the long term care facility 14–28 days after discharge | 8-week follow-up | |||||
| Dedhia et al. (2009) [22] | X | X | X | X | Quasi-experimental pre–post study design | . | 1-week and 30-day follow-up | |||
| Gillespie et al. (2009) [23] | X | X | X | X | RCT | 1 follow-up telephone 2 months after discharge | 12-month follow-up | |||
| Hammad et al. (2011) [24] | X | X | X | X | RCT | The intervention was delivered monthly during 6 months | No further follow-up | |||
| Hellström et al. (2012) [25] | X | X | X | X | Prospective, controlled study | . | 6-month follow-up | |||
| Jack et al. (2009) [26] | X | X | X | X | RCT | 1 follow-up phone call by clinical pharmacist 2 to 4 days after discharge | 30-day follow-up | |||
| Jackson et al. (2004) [27] | X | X | X | Open-label RCT | 4 home visits by clinical pharmacist on alternate days after discharge | 90-day follow-up | ||||
| Jacobs et al. (2012) [28] | X | X | X | X | Prospective, randomized, clinical practice study | 12-month follow-up | ||||
| Jarab et al. (2012a) [29] | X | X | RCT | 6-month follow-up | ||||||
| Jarab et al. (2012b) [30] | X | X | X | RCT | 8-week telephone follow-up call by clinical pharmacist | 6-month follow-up | ||||
| Kirwin et al. (2010) [31] | X | X | RCT | 30-day follow-up | ||||||
| Kripalani et al. (2012) [32] | X | X | X | X | X | X | RCT | 1 telephone follow-up 1-4 days after discharge | 30-day follow-up | |
| Lai et al. (2013) [33] | X | X | X | RCT | Monthly follow-up via telephone calls for the first 6 months, then every 3 months until month 12 | No further follow-up | ||||
| Lai et al. (2011) [34] | X | X | X | RCT | Monthly follow-up via telephone calls for the first 6 months, then every 3 months until month 12 | No further follow-up | ||||
| Lee et al. (2009) [35] | X | X | X | X | RCT | A telephone follow-up every 4 weeks and a follow-up interview on the date of the following physician visit within 16 weeks. | No further follow-up | |||
| Lim et al. (2004) [36] | X | X | X | X | RCT | 2-month follow-up | ||||
| Magid et al. (2011) [37] | X | X | X | X | X | RCT | 6-month follow-up | No further follow-up | ||
| McCoy et al. (2012) [38] | X | X | Randomized clinical trial | No follow-up | ||||||
| Mergenhagen et al. (2012) [39] | X | Quasi-experimental study. Subgroup analysis of a prospective, nonrandom, analytic cohort study with concurrent controls | 1-month follow-up | |||||||
| Morgado (2011) [40] | X | X | X | RCT | 3, 6 and 9-month follow-up | No further follow-up | ||||
| Murray et al. (2007) [41] | X | X | X | X | RCT | A pharmacist provided a 9-month multilevel intervention | 3-month follow-up | |||
| Sadik et al. (2005) [42] | X | X | X | X | RCT | Clinic visits at 3, 6, 9 and 12 months | No further follow-up | |||
| Schnipper et al. (2006) [43] | X | X | X | X | X | X | RCT | A follow-up telephone call 3 to 5 days after discharge | 30-day follow-up | |
| Spinewine et al. (2007) [44] | X | X | X | X | RCT | 1 month, 3 months, and 1 year follow-up | ||||
| Stange et al. (2013) [45] | X | X | X | Prospective, semi-randomized study | 6-week follow-up | |||||
| Suppapitiporn et al. (2005) [46] | X | X | RCT | Follow-up visits at 3 and 6 months | No further follow-up | |||||
| Tsuyuki et al. (2004) [47] | X | X | Mixed design - partly RCT: Stage 1: In-hospital intervention in all patients Stage 2: randomized trial. | Follow-up at 2 weeks, 4 weeks, then monthly for 6 months after discharge | No further follow-up | |||||
| von Gunten et al. (2005) [48] | X | X | Pre-post study. Randomised at hospital level | No follow-up | ||||||
| Wu et al. (2006) [49] | X | X | RCT | 6-8 telephone calls and a finalizing visit during a 2-year follow-up | No further follow-up | |||||
| Zhang et al. (2012) [50] | X | X | X | RCT | Patients were usually interviewed on phone when discharge drugs were half finished | 2-week follow-up | ||||
| Measure | Primary Outcome | Secondary Outcome | Total | ||
|---|---|---|---|---|---|
| Statistical Difference in Favour of Intervention | No Statistical Difference in Favour of Intervention | Statistical Difference in Favour of Intervention | No Statistical Difference in Favour of Intervention | ||
| Medication regimen characteristics | |||||
| Unnecessary drug use | 44 | 1 | |||
| Duration of antibiotic treatment | 48 | 1 | |||
| Composite score (dose, frequency and indication) | 36 | 1 | |||
| Unplanned cessation of warfarin | 27 | 1 | |||
| Medication regimen intensity | 37 | 1 | |||
| Medication complexity | 45 B | 1 | |||
| Drug specific quality indicators | 17 | 1 | |||
| 72-h medication-prescribing risk score | 39 | 1 | |||
| Medication appropriateness index (MAI) | 19, 44 | 2 | |||
| Beers criteria | 44 | 1 | |||
| Assessing Care of Vulnerable Elders (ACOVE) underuse | 44 | 1 | |||
| Medication discrepancies | 43 | 1 | |||
| The number of clinically important medication errors per patient during the first 30 days after hospital discharge | 32 | 1 | |||
| Time to provider modification or discontinuation of targeted nephrotoxic or renally cleared medications | 38 | 1 | |||
| Medication beliefs | 29 | 1 | |||
| Adherence to medication | |||||
| Medication adherence/compliance self-reported (no validated tool) | 50 | 14, 36, 40, 42 | 5 | ||
| Medication adherence/compliance self-reported “Medication Adherence Rating Scale” (MARS-D) | 45 B | 1 | |||
| Medication adherence/compliance self-reported (4-item Morisky Scale) | 29, 30 | 2 | |||
| Medication adherence/compliance objectively assessed | 41 | 47 | 18 | 37 | 4 |
| Medication adherence/compliance self-reported and objectively assessed | 34 A | 49 | 43 | 3 | |
| Persistence | 34 A | 1 | |||
| Adherence to guidelines | |||||
| British National Formulary | 14 | 1 | |||
| Lifestyle advice adherence | 14, 42 | 2 | |||
| Adherence to guidelines | 48 | 1 | |||
| Adherence to screening for retinopathy, neropathy, and microalbuminuria | 28 | 1 | |||
| Annual (LDL-C) testing | 31 | 1 | |||
| Annual urine microalbumin testing | 31 | 1 | |||
| Rates of pneumococcal vaccination | 31 | 1 | |||
| Change in rates of semiannual A1c testing from baseline to 30-day follow-up | 31 B | 1 | |||
| Frequency of primary care providers’ follow-up within 30 days of discharge | 26 | 1 | |||
| Annual eye exam | 31 | 1 | |||
| Adverse drug events/reactions | |||||
| ADE (total) | 39 | 21, 43 | 3 | ||
| Potential adverse drug events | 32 | 1 | |||
| Potential Acute kidney injury (AKI) ADEs | 38 A | 1 | |||
| Acute kidney injury (AKI) related ADEs | 38 A | 1 | |||
| Preventable ADEs | 43 B | 1 | |||
| ADEs from admission prescribing errors | 39 | 1 | |||
| Clinically important ADEs | 32 | 1 | |||
| Adverse drug reactions | 50 | 1 | |||
| Residual ADRs at month 2 | 36 | 1 | |||
| Laboratory measures | |||||
| HbA1c | 14, 30 B | 18, 28, 46 | 19, 31 | 7 | |
| Fasting blood glucose | 30, 46 | 19, 24 | 4 | ||
| Postprandial blood glucose | 19 | 1 | |||
| Total cholesterol | 14, 20, 30, 35 | 19 | 5 | ||
| HDL | 14, 35 | 18, 20, 24, 30 | 6 | ||
| LDL | 35 B | 14, 18, 19, 20, 28, 30 | 31 | 8 | |
| Triglycerides | 14, 19, 20, 24, 30, 35 | 18 | 7 | ||
| The achievement of a therapeutic INR value on day 8 after discharge | 27 | 1 | |||
| % patients achieving the ATP III LCL-C goal at the end of the study | 20 | 1 | |||
| Urinary albumin-to-creatinine ratio (ACR) | 18 | 1 | |||
| Clinical measures/assessment by physicians | |||||
| BP | 14, 15, 19, 24, 30 | 18, 31, 42 | 8 | ||
| Systolic BP | 40 | 28 | 2 | ||
| Diastolic BP | 28, 40 | 2 | |||
| BP control | 40 | 1 | |||
| Achieving BP goals | 15 | 37 | 2 | ||
| Pulse | 42 | 1 | |||
| Waist circumference | 24 | 1 | |||
| Body weight | 24, 42 | 2 | |||
| BMI | 14 | 18, 30 | 3 | ||
| Symptoms | 42 | 1 | |||
| Bone turnover markers (BTMs) | 34 A | 1 | |||
| Clinical status according to primary physician | 36 | 1 | |||
| 2-min walk test | 42 | 1 | |||
| Forced vital capacity (FVC) measured by spirometer | 42 | 1 | |||
| Bleeding events 3 months after discharge | 27 | 1 | |||
| Falls | 21 | 1 | |||
| Framingham prediction scores | 14 | 1 | |||
| Change in coronary heart disease (CHD) risk | 18 | 1 | |||
| Changes in stroke risk | 18 | 1 | |||
| Shift from a status of MS to no MS | 24 | 1 | |||
| Worsening mobility | 21 | 1 | |||
| Worsening behaviours | 21 | 1 | |||
| Increased confusion | 21 | 1 | |||
| Worsening pain | 21 | 1 | |||
| Resource utilization | |||||
| Length of stay (LOS) in hospital | 47, 49, 50 | 48 | 4 | ||
| Cardiovascular-related LOS | 47 | 1 | |||
| Physician visits | 47 | 1 | |||
| Cardiovascular-related Physician visits | 47 | 1 | |||
| Emergency department visits/casual department visits | 23 | 47, 49 | 3 | ||
| Emergency department visits (within 3 days) | 22 | 1 | |||
| Emergency department visits (within 30 days) | 22 | 1 | |||
| Emergency visits up to 12 months after discharge | 44 | 1 | |||
| Cardiovascular-related Emergency room visits | 47 | 1 | |||
| Time to emergency department revisits after discharge | 25 A | 1 | |||
| Hospital readmission/hospital admission | 23 | 49 | 44, 47, 50 | 6 | |
| 30 day readmission rate | 22 B | 1 | |||
| Drug-related readmissions | 23 | 1 | |||
| Unplanned readmission | 27 | 1 | |||
| Cardiovascular-related Hospital readmissions | 47 | 1 | |||
| Readmissions to hospital due to anticoagulant-related complications within 90 days of initial discharge | 27 | 1 | |||
| Number of all cause and CHF hospitalization within 6 months of enrolment | 16 A | 1 | |||
| Number of CHF hospitalization within 6 months of enrolment | 16 A | 1 | |||
| Days of all cause and CHF hospitalization within 6 months of enrolment | 16 A,C | 1 | |||
| Days of non-CHF-hospitalization within 6 months of enrolment | 16 | 1 | |||
| Combination of emergency department visits and hospital readmissions | 21 | 1 | |||
| Emergency department visits and hospitalizations within 30 days of discharge | 26 | 1 | |||
| Preventable medication related emergency department visits or readmissions | 43 | 1 | |||
| Exacerbations requiring emergency department care or hospital admission | 41 | 1 | |||
| The combined rate of post-discharge hospital revisits or death (ED visit, hospitalization or death) | 25 | 1 | |||
| Health care utilization (scheduled and unscheduled office visits, urgent care and ED visits, and hospital admissions) | 43 | 1 | |||
| Costs | |||||
| Costs | 23, 26, 47 | 3 | |||
| Total direct costs | 41 | 1 | |||
| Cost of antibiotic treatment | 48 | 1 | |||
| Cost of drugs and hospitalization | 50 | 1 | |||
| Cardiovascular-related Cost | 47 | 1 | |||
| Cost-effectiveness | 18 | 1 | |||
| Cost avoidance | 36 | 1 | |||
| Mortality | |||||
| Mortality (general) | 23, 27, 44 | 3 | |||
| Mortality within 6 months of enrolment | 16 A | 1 | |||
| Time from randomisation to death from any causes | 49 | 1 | |||
| Event-free survival | 25 | 1 | |||
| Quality of Life/Health related quality of life | |||||
| Short form 36 (SF 36) | 14, 16, 42 | 16, 42 | 5 | ||
| Short form 12 (SF 12) | 45 | 1 | |||
| EuroQol 5 dimension (EQ-5D) | 17 B | 1 | |||
| Self-rated global health | 17 | 17 | 2 | ||
| Assessment of quality of life (AQoL) | 16 | 1 | |||
| Minnesota living with heart failure questionnaire (MLHF) | 42 | 1 | |||
| St George Respiratory Questionnaire (SGRQ) | 29 B | 1 | |||
| Chronic Heart Failure Questionnaire | 41 | 1 | |||
| Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) | 33 | 1 | |||
| Patient knowledge | |||||
| Patient medication knowledge | 36 | 14, 18 | 42 | 4 | |
| COPD knowledge | 29 | 1 | |||
| Patients’ knowledge of target BP values and of hypertension risks | 40 | 1 | |||
| Malaysian Osteoporosis Knowledge Tool (MOKT) | 33 | 1 | |||
| Satisfaction and perception | |||||
| Satisfaction with information about medications | 44, 45 | 2 | |||
| Patient satisfaction with pharmacy services | 41 | 1 | |||
| Osteoporosis Patient Satisfaction Questionnaire (OPSQ) | 33 | 1 | |||
| Satisfaction with hospitalization and discharge processes | 43 | 1 | |||
| Coleman’s Care Transition Measures | 22 | 1 | |||
| Patient perception (perception of severity of illness, usefulness of treatment and appropriateness of the number of medications) | 36 | 1 | |||
| Other | |||||
| Self-perceived health status | 22 | 1 | |||
| Identification of index discharge diagnosis | 26 | 1 | |||
| Identification of primary care provider name | 26 | 1 | |||
| Self-reported preparedness for discharge | 26 | 1 | |||
| Self-care activities (Diabetes Self-Care Activities questionnaire) | 30 | 1 | |||
| Total | 26 | 16 | 96 | 78 | 216 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjeldsen, L.J.; Olesen, C.; Hansen, M.K.; Nielsen, T.R.H. Clinical Outcomes Used in Clinical Pharmacy Intervention Studies in Secondary Care. Pharmacy 2017, 5, 28. https://doi.org/10.3390/pharmacy5020028
Kjeldsen LJ, Olesen C, Hansen MK, Nielsen TRH. Clinical Outcomes Used in Clinical Pharmacy Intervention Studies in Secondary Care. Pharmacy. 2017; 5(2):28. https://doi.org/10.3390/pharmacy5020028
Chicago/Turabian StyleKjeldsen, Lene Juel, Charlotte Olesen, Merete Kjær Hansen, and Trine Rune Høgh Nielsen. 2017. "Clinical Outcomes Used in Clinical Pharmacy Intervention Studies in Secondary Care" Pharmacy 5, no. 2: 28. https://doi.org/10.3390/pharmacy5020028






